Abstract
We report here the results of a chemical genetic screen using small molecules with known pharmacologies coupled with a cortical brain slice-based model for ischemic stroke. We identified a small-molecule compound not previously appreciated to have neuroprotective action in ischemic stroke, the cardiac glycoside neriifolin, and demonstrated that its properties in the brain slice assay included delayed therapeutic potential exceeding 6 h. Neriifolin is structurally related to the digitalis class of cardiac glycosides, and its putative target is the Na+/K+-ATPase. Other cardiac glycoside compounds tested also showed neuroprotective activity, although with lower apparent potencies. In subsequent whole-animal studies, we found that neriifolin provided significant neuroprotection in a neonatal model of hypoxia/ischemia and in a middle cerebral artery occlusion model of transient focal ischemia. The neuroprotective potential of Na+/K+-ATPase is of particular interest because of its known “druggability”; indeed, Food and Drug Administration-approved, small-molecule compounds such as digitoxin and digoxin have been in clinical usage for congestive heart failure and arrhythmias for several decades. Thus, an existing cardiac glycoside or closely related compound could provide an accelerated path toward clinical trial testing for ischemic stroke. Our findings underscore the important role that hypothesis-neutral, high-content, tissue-based screens can play in the identification of new candidate drugs and drug targets for the treatment of diseases for which validated therapeutic pathways are not currently available.
Keywords: biotechnology, drug discovery, high-content screening, translational medicine
Stroke is the third leading cause of death in the United States, with >700,000 new cases diagnosed each year (1). By the year 2020 stroke is expected to be the fourth leading disease burden worldwide (2). Stroke-related healthcare costs are tremendous, with nearly 1 million hospitalizations each year in the U.S. and costs associated with the care of stroke victims exceeding an estimated $50 billion for the year 2005 (50).
Although dramatic improvements have been made in the last decade in the prevention of stroke, there remains a need for drugs that provide direct neuroprotection and/or neuroresuscitation to neural tissues that are functionally impaired after stroke but whose damage is potentially reversible. In fact, some 50 neuroprotective drug candidates have now been tested in >100 stroke clinical trials, with none as yet proven to be efficacious, despite promising results having been seen in preclinical models of ischemic stroke for many of these drug candidates (2). Thus, the only drug that has been approved by the Food and Drug Administration for stroke therapy remains i.v. recombinant tissue plasminogen activator, currently given to <5–10% of acute ischemic stroke patients because of its limited time window for drug administration of only 3 h beyond stroke onset (3).
The large number of failures of neuroprotective drug trials suggests that the molecular drug targets that have been addressed to date, such as the NMDA-type glutamate receptor (4), may be intrinsically problematic for a number of reasons, including inherent limitations on therapeutic time window and clinically limiting side-effect profiles. Consequently, much attention has been focused in recent years on using genomic, proteomic, and other systems biology approaches in identifying new drug target candidates for stroke drug intervention (see review in ref. 5).
In this context we developed a tissue-based, high-content assay model for ischemic stroke based on biolistic transfection of visual reporter proteins into cortical brain slice explants (6, 7). We used this screening platform to survey a chemical genetic collection of small molecules with known pharmacologies similar to the National Institute of Neurological Disorders and Stroke Custom Collection for neuroprotective action after oxygen and glucose deprivation (OGD) in these brain slices.
A key advantage of this approach was that no preselection of targets was made, allowing a “hypothesis-neutral” approach to be taken to maximize the range of pathways that would be tested. Moreover, such a chemical genetic approach using small-molecule organic compounds to probe cellular pathways increased the likelihood that any newly discovered pathways would be “druggable” (i.e., targetable by specific small-molecule drug compounds).
Finally, maintaining the three-dimensional tissue architecture surrounding the neurons being assayed increased the likelihood that mechanisms of pathogenesis occurring in this model would be physiologically and clinically relevant and representative of the in vivo situation of neural tissue degeneration after ischemic stroke. In contrast, substantial changes in the nature and balance of cell signaling pathways occur when neurons are dissociated from their native tissue context and either immortalized or placed into dissociated primary culture, potentially deemphasizing mechanisms and targets that would otherwise be clinically important.
We thus hypothesized that the “ultra”-high content of this screening approach could identify not only well characterized and previously drugged signaling pathways, but also drug-like compounds and biochemical pathways whose relevance to ischemic stroke may not have previously been detectable by using simpler screening platforms.
We report here the experimental findings for one of the neuroprotective “hits” identified in this chemical genetic survey, neriifolin, a member of the cardiac glycoside family of drugs that have been used extensively as inotropic agents in the treatment of congestive heart failure and atrial arrhythmias. We show the effectiveness of neriifolin in providing neuroprotection in two ex vivo brain explant-based experimental models of ischemic damage, as well as in two independent whole-animal rodent models for clinical stroke. Taken together, these findings suggest that the target of action of cardiac glycoside drugs such as digitoxin and digoxin in the heart, the sodium/potassium adenosine triphosphatase (Na+/K+-ATPase), may represent a favorable drug target for the treatment of ischemic stroke.
Results
Neuroprotective Activity and Delayed Therapeutic Potential of Neriifolin.
The potential efficacy of small-molecule compounds was compared against positive controls including cotransfection with the neuroprotective gene bcl-xL (Fig. 1b) (8, 9) and the neuroprotective compound MK-801 (Fig. 2a). Pretreatment with MK-801 before and during OGD provided levels of neuroprotection comparable to that afforded by cotransfection with bcl-xL. However, as has previously been documented in several experimental systems (10, 11), short delays in MK-801 administration (e.g., 10 min after OGD) resulted in a nearly complete loss of its neuroprotective activity (Fig. 2a). Based on this observation and the known clinical importance of a delayed treatment paradigm, we designed the primary screen such that the neuroprotective potential of small-molecule compounds was assessed after a minimum post-OGD delay of administration of 30 min.
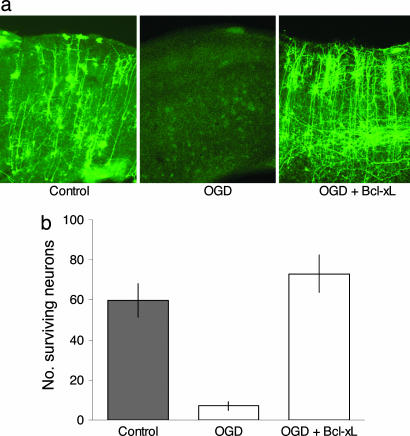
Fig. 1.
Cortical brain slice explant model for ischemic stroke. (a) Cortical brain slices biolistically transfected with an expression plasmid for yellow fluorescent protein (YFP) under normal conditions (Left) and 24 h after 7.5 min of OGD (Center). Cotransfection with the antiapoptotic gene bcl-xL rescues against the neuronal cell death induced by OGD (Right); note that neurons in these slices often appear healthier than control neurons in non-OGD slices, presumably because bcl-xL also protects against trauma induced in the process of slice preparation. (b) In this explant model, ≈90% of cortical neurons degenerate and die over the first 24 h after transient OGD; most or all of this neuronal cell death can be prevented by cotransfection with bcl-xL.
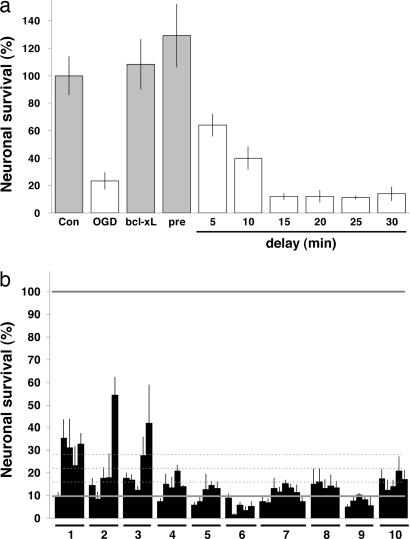
Fig. 2.
Survey of stroke drug clinical candidates in the brain-slice model. (a) Neuroprotective activity of MK-801 is transient. Although pretreatment of brain slices with the NMDA receptor antagonist MK-801 at 1 μM provided quantitative neuroprotection compared with controls (Con, 100%, vehicle-treated brain slices not subjected to OGD; bcl-xL, brain slices subjected to OGD but cotransfected with bcl-xL), even delays of 5–10 min in administration resulted in substantial loss of neuroprotective activity. Gray bars denote P < 0.05 with respect to OGD plus vehicle (second bar) using ANOVA followed by Dunnett’s post hoc comparison test. (b) Slices were treated 30 min after 7.5 min of OGD with 10 different stroke drug candidates studied in clinical trials, all of which, except atenolol, also have been reported to reduce cerebral infarct size resulting from experimental MCAO. Group 1, NXY-059 (tert-butyl-phenylnitrone); group 2, lubeluzole; group 3, repinotan; group 4, traxoprodil; group 5, aptiganel; group 6, MK-801; group 7, Maxipost; group 8, citicoline; group 9, gavestinel analog; group 10, atenolol. Each compound was tested at five concentrations between 1 and 100 μM (shown high to low from the left for each block), except for Maxipost, which was tested at eight concentrations between 0.8 and 100 μM. The lower solid gray line shows average OGD plus vehicle level for this experiment, with 1, 2, and 3 SDs above negative control shown as dotted lines. The upper solid gray line shows average positive control level (100%) for bcl-xL-transfected brain slices in this experiment.
When a set of 10 previous candidate stroke drugs was assayed in this manner, only modest neuroprotective effects were observed (Fig. 2b); indeed, these effects were only somewhat higher than fluctuations in neuronal survival that were seen in a panel of known pharmacologically active small molecules that was surveyed to gauge the “noisiness” of the assay system (see Fig. 6, which is published as supporting information on the PNAS web site). However, three of these stroke drug candidates, NXY-059, lubeluzole, and repinotan, exhibited neuroprotective effects strong enough to have been flagged as preliminary hits and, as such, indicated that this assay platform can be predictive of efficacy in in vivo models for focal ischemia, because all three of these compounds have been found to be efficacious in the middle cerebral artery occlusion (MCAO) model for ischemic stroke (12–14).
By using this delayed neuroprotection assay, the chemical genetic compound collection described was screened on a blinded basis, yielding a total of 84 primary hits (see Fig. 7, which is published as supporting information on the PNAS web site). Each of these hits was then assayed secondarily for preliminary concentration-response properties. Those hits that showed dose-dependent properties and good levels of efficacy were then assayed for delayed therapeutic potential by determining the extent of delay of drug administration (with respect to OGD) that could be tolerated without loss of neuroprotective activity. Of these initial hits, two compounds exhibited superior efficacy, reproducibility, and delayed therapeutic time windows of 5–10 h or more in providing increased neuronal survival after OGD.
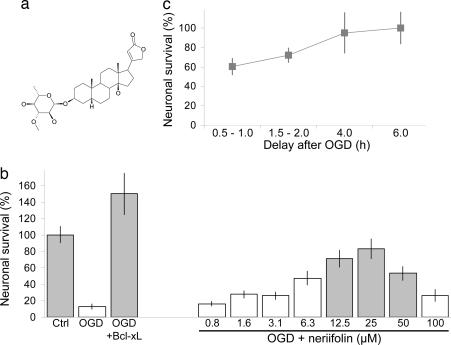
One of these compounds was uncoded and identified as neriifolin (Fig. 3a), a secondary plant metabolite derived from the yellow oleander (Thevetia peruviana, also known as Thevetia neriifolia) (15). Structurally, neriifolin is related to the cardenolide steroid subclass of the cardiac glycosides, which also includes digitoxin, digoxin, and ouabain (16). Neriifolin is known to be bioactive and is listed as one of the ≈450 compounds collected by the National Cancer Institute under the Molecular Targets Development Program. As can be seen in Fig. 3b, neriifolin showed significant rescue of cortical pyramidal neurons in a dose-dependent manner that otherwise would have degenerated and died subsequent to transient OGD in these brain slices.
Fig. 3.
Neuroprotective activity of the cardiac glycoside neriifolin. (a) Chemical structure of neriifolin. (b) Neriifolin provided dose-dependent rescue of cortical neurons when given 30 min after transient OGD in this biolistic transfection-based brain slice model for ischemic stroke. Ctrl indicates brain slices that were treated with vehicle but not subjected to OGD, and OGD+bcl-xL refers to brain slices that were subjected to OGD but cotransfected with bcl-xL. Means ± SEM are shown; n = 12 brain slices per condition. Gray bars denote P < 0.05 with respect to OGD plus vehicle (second bar) using ANOVA followed by Dunnett’s post hoc comparison test. (c) Neriifolin showed good delayed therapeutic potential, providing as much or more neuroprotection even after 6 h of application delay compared to no delay. Values denote percentage increase in survival compared with untreated controls ± SEM.
Importantly, neriifolin also exhibited good therapeutic potential for delayed drug administration; in fact, neriifolin appeared to show enhanced neuroprotection at delays of several hours after OGD (Fig. 3c). The delayed enhancement may be due to toxic aspects of cardiac glycoside action being more significant during the initial biochemical and signal transduction events triggered by OGD (e.g., refs. 17–19).
Confirmation of the Neuroprotective Activity of Neriifolin in a Second ex Vivo Model for Ischemic Injury.
Next we confirmed the neuroprotective activity of neriifolin in a second brain-tissue explant model for ischemic injury. In this model we used transgenic mice expressing GFP driven by the Thy-1 promoter in which incomplete “penetrance” of the transgene leads to expression of GFP in only a subset of neurons, including pyramidal neurons in the cortex (20). Explantation of cortical brain slices from these GFP mice at postnatal day (PND) 21 led to neuronal degeneration and cell death over the course of several days even in the absence of additional OGD treatment.
Although the dynamic range of this transgenic mouse-based model was not as robust as for the transfection-based primary screen, dose-dependent neuroprotection of cortical pyramidal neurons by neriifolin could be clearly observed (Fig. 4a). This neuroprotective activity was in a similar concentration range as was originally observed in the primary screen (compare Fig. 3b), although neriifolin’s apparent potency was somewhat greater, perhaps because of the milder ischemic insult that was used in this model.
Fig. 4.
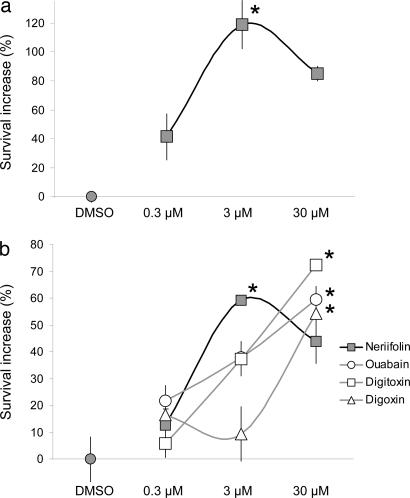
Confirmation of the neuroprotective activity of neriifolin in a GFP-transgenic mouse brain slice model for ischemic stroke. (a) Application of neriifolin to GFP-transgenic mouse cortical brain slice explants provided neuroprotection at an overlapping dose range to what was observed in the rat brain slice biolistic transfection-based model shown in Figs. 1–3. The greater apparent potency of neriifolin in this assay may have arisen from differences in species (mouse versus rat), in tissue age (PND21 versus PND7), in the ischemic injury protocols used, and in compound access and stability in these different tissues. (b) Although all four cardiac glycosides provided neuroprotection at 30 μM, only neriifolin showed significant rescue of cortical neurons at 3 μM at the P < 0.05 confidence level. Mean levels of neuroprotection ± SEM are shown relative to vehicle-treated controls (DMSO); n = 4 brain slices per treatment condition. ∗, P < 0.05 with respect to untreated control brain slices using ANOVA followed by Dunnett’s post hoc comparison test.
Neuroprotective Activity of Other Cardiac Glycosides.
Interestingly, three other cardiac glycoside drugs, digitoxin, digoxin, and ouabain, were also included in this compound set but had not shown significant levels of neuroprotection in the original primary screen (see Fig. 7). We hypothesized that if these compounds were less potent and/or efficacious than neriifolin in providing neuroprotection, they may not have emerged from the primary screen because of the harsh OGD conditions that were used and the high threshold that was taken to detect primary hits.
We thus used this milder transgenic mouse brain slice assay to reexamine the potential neuroprotective effects of these cardiac glycosides, of which digoxin is of particular interest because it is in current clinical usage for congestive heart failure and atrial arrhythmias. As would be predicted if the neuroprotective effects of neriifolin are mediated through its presumptive target, Na+/K+-ATPase, these additional cardiac glycosides also provided rescue of cortical neurons, although with reduced apparent potency compared with neriifolin (Fig. 4b). Digoxin appeared to be least potent, with ouabain and digitoxin having intermediate potencies relative to neriifolin.
Evaluation of Neriifolin in a Perinatal Whole-Animal Stroke Model.
The data from two independent brain slice-based models for ischemic injury suggested favorable neuroprotective properties for neriifolin in terms of efficacy, potential therapeutic index, and delayed therapeutic potential. Moreover, neriifolin was previously reported to have a plasma half-life of 5 days and to cross the blood–brain barrier to a significant extent (21). Neriifolin was thus advanced for testing in whole-animal rodent models for ischemic stroke to ask whether the neuroprotective effects we observed in vitro would also be relevant in in vivo injury paradigms.
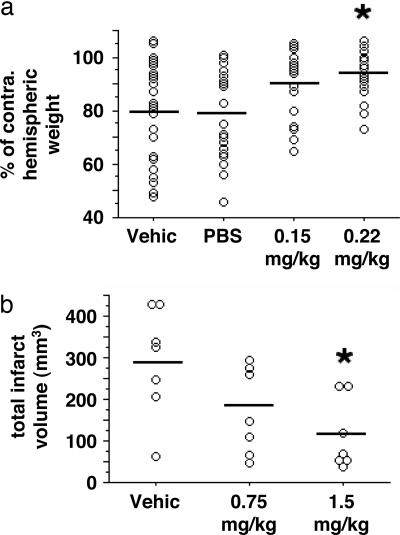
We first used the Rice–Vannucci model of perinatal hypoxia–ischemia as a preliminary in vivo test for neuroprotective action because of its simplicity and rapidity and because of the similarity of tissue maturation in this model to the brain slice-based OGD assays used in our primary and secondary screens described here. The Duke University Institutional Animal Care and Use committee approved this study. A dose escalation study was first performed that defined an i.p. neriifolin dose of 0.22 mg/kg to be subtoxic when given during hypoxia–ischemia. In an unblinded but randomized trial, neriifolin (0.15 or 0.22 mg/kg) or vehicle [propylene glycol/EtOH/PBS (40:10:50 vol/vol)] was given as a single 100-μl bolus i.p. injection at the time of carotid occlusion in PND 7 rat pups. At PND 14 the ratio of ipsilateral to contralateral brain weights (×100) was increased with 0.15 mg/kg neriifolin (86 ± 16%, n = 31, P = 0.0006) and 0.22 mg/kg neriifolin (97 ± 14%, n = 22, P < 0.0002) versus vehicle (69 ± 17%, n = 24). Assessment of neriifolin efficacy was repeated with a blinded randomized design, which also controlled for vehicle effects. There was no difference for interhemispheric weight ratios between vehicle (n = 28) and PBS (n = 25) injections, whereas brain weight differences were significantly reduced by 0.22 mg/kg neriifolin (n = 23, P ≤ 0.01; Fig. 5a).
Fig. 5.
In vivo analysis of the neuroprotective effect of neriifolin. (a) PND 7 rat pups were subjected to 90 min of hypoxia–ischemia. Pups were treated with vehicle [propylene glycol/EtOH/PBS (40:10:50 vol/vol)], PBS, or neriifolin (0.15 or 0.22 mg/kg i.p.) at the onset of right carotid occlusion. On PND 14 ipsilateral and contralateral hemispheric brain weights were measured. There was no difference between vehicle and PBS groups. Neriifolin reduced interhemispheric weight differences in a dose-dependent manner. ∗, difference from vehicle and PBS (P ≤ 0.01). (b) Ten- to 12-week-old rats were subjected to 90 min of temporary MCAO. Neriifolin, given at onset of ischemia, reduced cerebral infarct size measured 24 h after onset of reperfusion. Open circles, values for individual animals; horizontal bars, group mean values. ∗, difference from vehicle (P ≤ 0.02).
Evaluation of Neriifolin in an Adult MCAO Stroke Model.
Given the positive results for perinatal hypoxia–ischemia, neriifolin efficacy was next determined in adult rats subjected to 90 min of temporary MCAO. Pilot studies determined that up to 3 mg/kg neriifolin could be given i.v. without substantive changes in arterial blood pressure. Rats were then randomly assigned to receive vehicle (10% acetic acid) or neriifolin (0.75 mg/kg or 1.5 mg/kg i.v.) at MCAO onset (n = 7 per group). Cerebral infarct sizes measured at 24 h after MCAO were reduced in a dose-dependent manner by neriifolin (Fig. 5b).
Discussion
Taken together, these data from ex vivo and in vivo assay models for ischemic stroke suggest that modulation of biochemical pathways targeted by the cardiac glycoside neriifolin may be useful for the development of new therapies for stroke treatment. The neuroprotective activity of neriifolin was initially identified in the course of a hypothesis-neutral, chemical genetic screen we undertook to survey the widest possible range of potential biological drug targets in the shortest amount of time, and, in particular, to identify well characterized biochemical pathways not previously implicated in neuroprotection as the fastest path to clinical use. The presumptive target of neriifolin is the Na+/K+-ATPase, and, indeed, all four of the cardiac glycosides tested, including two that have been used clinically, showed neuroprotection in a transgenic brain slice model for ischemic damage (Fig. 4b).
Unexpected Neuroprotective Action of Cardiac Glycosides.
Nevertheless, we initially found the identification of neriifolin to be unexpected given the toxicity of ouabain and other related compounds (e.g., refs. 14–16). Moreover, compromised Na+/K+-ATPase activity has been suggested to play a role in a range of neuropathologic and apoptotic contexts (reviewed in refs. 22–24), and decreases in Na+/K+-ATPase activity have been reported in some model systems of focal ischemia and traumatic brain injury (e.g., refs. 25–27; but see refs. 28 and 29).
However, the cardiotonic action of digitalis cardiac glycosides provides an interesting precedent to this seeming paradox. Although the therapeutic use of digitalis dates back to the 18th century and is by now taken for granted, the intrinsic toxicity of these compounds must have originally appeared to be contrary to their clear positive inotropic effect in treating congestive heart failure.
By analogy, inhibition of Na+/K+-ATPase in the context of ischemic stroke could lead to a positive outcome: in this case as a “neurotonic” providing a neuroprotective/neuroresuscitative benefit as has been previously observed for kidney cells and neurons in some experimental settings (30–34). Indeed, neriifolin and other cardiac glycosides were recently found to provide neuroprotection against polyglutamine-mediated cytotoxicity (35).
Potential Neuroprotective Mechanisms of Cardiac Glycoside Action in Ischemic Stroke.
One possible mechanism for neriifolin’s apparent neurotonic action is the reduction of ATP consumption by the Na+/K+-ATPase through inhibition of its catalytic activity during ischemia–reperfusion injury, consistent with the concept framed by Hochachka (36) of combined metabolic and channel “arrest” as a defense strategy against hypoxia.
Second, although blocking excitotoxicity-induced Ca2+ overload of neurons after ischemic injury has been the focus of numerous therapeutic efforts, abnormally low levels of cytosolic Ca2+ can also lead to neuronal cell death, as proposed in the “Ca2+ set-point hypothesis” (37, 38). In fact, Lee et al. (39) suggest that Ca2+ starvation and apoptosis may be the predominant causes of cell death in the penumbra, particularly at later time intervals. The neuroprotective effects of neriifolin observed here may thus arise from increasing intracellular Ca2+ levels through inhibition of Na+/K+-ATPase activity and decreased extrusion of intracellular Ca2+ via the Na–Ca exchanger.
In support of this mechanism, Connor et al. (40) have reported that hippocampal CA1 neurons experience lower than normal levels of intracellular Ca2+ over 1–3 days after transient ischemia, and they propose that elevating Ca2+ levels in CA1 neurons could provide protection against delayed neuronal death across a wide postischemic therapeutic time window. Similarly, Biegon et al. (41) have shown that functional NMDA receptors fall below normal levels in the days after closed head injury and that administration of NMDA receptor agonists provides therapeutic benefit during this extended time period.
Finally, although the ionic transport function of the Na+/K+-ATPase has been extensively studied, a number of other signal transduction roles for Na+/K+-ATPase have emerged in recent years (e.g., refs. 42 and 43).
In summary, we have developed a brain slice-based, ultra-high-content chemical genetic screening assay that has allowed us to examine 1,200 small-molecule compounds with known pharmacologies for potential neuroprotective properties. Importantly, this assay approach is scalable and can be used to screen 5,000–10,000 or more compounds per year (at single concentrations each) even in an academic laboratory setting with a three- to four-person team. As such, this approach can be used to conduct meaningful screens of targeted compound libraries and to identify clinically relevant targets and pathways that otherwise might not be represented in simpler cell-based or enzyme-based drug screens.
The system was validated by use of an established neuroprotectant (MK-801), which offered a potent but narrow therapeutic window. Other known experimental neuroprotectants (e.g., NXY-059, lubeluzole, citicoline, and gavestinel) emerged from the screen but had limited potency in vitro when given at delayed intervals after OGD termination. In contrast, this assay identified an unexpected class of neuroprotectants, the cardiac glycosides, which were efficacious as late as 6 h after OGD, presumably through interactions with Na+/K+-ATPase. Extension of these findings into preliminary whole-animal stroke models confirmed efficacy in vivo. Taken together, these studies indicate that cardiac glycosides warrant further study to define potential clinical value as pharmacologic intervention for stroke and support the role of hypothesis-neutral drug screening as a discovery strategy for neuroprotective compounds.
Materials and Methods
Chemical Genetic Collection of Small Molecules with Elucidated Pharmacologies.
Approximately 1,200 drug-like compounds were selected for good pharmacologic properties, tractable biological targets, and likelihood of neural activity (from Microsource Discovery Systems, Prestwick Chemical, Tocris Cookson, and Sigma-RBI). This compound collection included the majority of Food and Drug Administration-approved compounds and was similar to that assembled by the National Institute of Neurological Disorders and Stroke Drug Screening Consortium (35, 44).
Preparation of Cortical Brain Slices and OGD.
Neocortical brain slices were prepared from PND 7 Sprague–Dawley rat pups, which were killed in accordance with National Institutes of Health guidelines. The cerebral cortex was dissected, cut into 400-μm-thick slices as described previously (6), and transferred into a container containing cold artificial cerebrospinal fluid with 1 μM MK-801 before plating; MK-801 was not included in any subsequent procedures unless otherwise specified.
To mimic ischemic injury using transient oxygen-glucose deprivation (OGD) (45), slices from one hemisphere of each brain were exposed to glucose-free, N2-bubbled artificial cerebrospinal fluid for 7.5 min in a low O2 (<0.5%) environment. The OGD slices were then plated side-by-side with control slices from the contralateral hemisphere on nitrocellulose or Millicell (Millipore) permeable membranes, which were prepared identically except for no OGD. Thirty minutes after plating, the brain slice pairs were transfected (see Biolistic Transfection), transferred to 24-well plates, and incubated at 37°C under 5% CO2 in humidified chambers.
Biolistic Transfection.
To assay the amount of neuronal cell degeneration and death after OGD, brain slice explants were transfected with yellow fluorescent protein (YFP) expression plasmids driven by the cytomegalovirus promoter (pEYFPN1, CLONTECH), via biolistic particle-mediated gene transfer, to create a subpopulation of “sentinel” neurons (Fig. 1a) as previously described (46) using a Helios Gene Gun (Bio-Rad). Gold particles (1.6 μm) were used as the DNA carrier (Bio-Rad or Strem Chemicals) with a DNA load of 2 μg of DNA per milligram of Au, with the Helios unit set at 95–105 psi at an aperture distance from the brain slices of ≈2.5 cm.
After transfection, brain slices were transferred to 24-well plates containing culture medium into which individual chemical compounds had been previously dispensed. For primary screening, compounds were tested at more than two concentrations and two to four slices were scored at each concentration. Primary hits were confirmed at least twice in follow-on retests. For subsequent characterization studies, numbers of test concentrations and brain slices assayed were increased as described in the figure legends.
Assessment of Neuronal Survival.
Neuronal survival was scored visually after 24 and 48 h by using Leica MZIIIFL fluorescence stereomicroscopes equipped with appropriate filter sets. Surviving pyramidal neurons in the cortical brain slice explants were identified based on their characteristic position, orientation, and morphology (for further details see Fig. 8, which is published as supporting information on the PNAS web site). All pyramidal neurons were counted in each cortical brain slice explant; therefore, values reported represent the total number of surviving pyramidal neurons per brain slice.
Advantages of this screening approach included the ability to assay several sequential time points on the same sets of slices by using the vital marker yellow fluorescent protein (YFP) and the ability to identify specific neuronal cell types such as cortical pyramidal neurons based on dendritic morphology as described here. Moreover, visual scoring of numbers of healthy neurons constituted a quantitative assay with good dynamic range (from zero to hundreds of sentinel neurons per cortical brain slice explant) that was linear throughout this range and had essentially no background noise.
Preparation of Transgenic Mouse Brain Slices.
Cortical brain slices were prepared from Thy-1–GFP transgenic mice (20) (line H) and placed into explant culture by using methods as described here for rat brain slices, except that PND 21 mice were used and no neuroprotectants (e.g., MK-801) were included in the artificial cerebrospinal fluid used during the slicing procedure. Under these conditions for older brain tissue, cortical neurons degenerated and died over the course of 1–3 days after explantation even in the absence of additional OGD.
Neonatal Hypoxia–Ischemia Model.
The preparation was a modification of the previously validated Rice–Vannucci method (47, 48). PND 7 Wistar rat pups of either sex were anesthetized with halothane. The right common carotid artery was exposed through a ventral skin incision. Without injuring the adjacent vagus nerve, the carotid was cauterized and transected, and the incision was closed with cyanoacrylate. After an 80-min feeding and recovery period with the dam, pups were placed in interconnected 450-ml Plexiglas chambers partially submerged in a 36.5–37°C water bath at one to two animals per chamber. Humidified 8% O2, balance N2 flowed through the chambers for 2.5 h. The animals were then returned to the dam. On PND 14 pups were given a halothane overdose and decapitated, and the brains were removed. After removal of the cerebellum and brainstem, the forebrain was sectioned at the interhemispheric fissure, and the hemispheres were weighed. Brain necrosis was determined by comparing hemispheric weight ipsilateral to the carotid transection against contralateral hemispheric weight. Values were compared with one-way ANOVA and the Scheffé post hoc test.
MCAO.
MCAO experiments were done through a contract with Skeletech (Bothell, WA). This study was conducted in compliance with the U.S. Department of Agriculture Animal Welfare Act. Briefly, normothermia male Sprague–Dawley rats (10 weeks of age) were anesthetized with halothane and surgically prepared for MCAO by using modifications of the technique described by Zea Longa et al. (49). Through midline ventral cervical skin incision, the right common carotid artery was isolated. The external carotid artery was ligated and divided. A blunted 3-0 nylon filament was passed 20 mm into the internal carotid through the external carotid artery stump to occlude the MCA for 90 min, after which the filament was removed, the wound was closed with sutures, and the animal was awakened. Body temperature was maintained at 37°C during the surgery and during the postoperative recovery period via a heating pad. Twenty-four hours later the rat was reanesthetized. The brain was removed, sectioned at 2-mm coronal intervals, and stained with triphenyltetrazolium chloride. Infarct size was computed by image analysis. Values were compared with one-way ANOVA and the Scheffé post hoc test.
Supplementary Material
Acknowledgments
We dedicate this paper to our phenomenal colleagues at Cogent Neuroscience; to Max Wallace, who made this work possible; and to the memory of Lawrence C. Katz. We give special thanks to D. Dunn, K. Evans, A. Jones, J. Michaux, H. Spalink, and J. Thorne for their superb experimental support. We are grateful to G. Feng and J. Sanes for access to their Thy-1–GFP mice.
Abbreviations
- PND
postnatal day
- OGD
oxygen and glucose deprivation
- MCAO
middle cerebral artery occlusion.
Footnotes
Conflict of interest statement: This work was funded by Cogent Neuroscience, Inc.; the authors declare no financial interests.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Marler J. R., Goldstein L. B. Science. 2003;301:1677. doi: 10.1126/science.1090270. [DOI] [PubMed] [Google Scholar]
- 2.Gladstone D. J., Black S. E., Hakim A. M. Stroke (Dallas) 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 3.Fisher M., Brott T. G. Stroke (Dallas) 2003;34:359–361. doi: 10.1161/01.str.0000054627.69159.c2. [DOI] [PubMed] [Google Scholar]
- 4.Lee J. M., Zipfel G. J., Choi D. W. Nature. 1999;399(Suppl.):A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 5.Read S. J., Parsons A. A., Harrison D. C., Philpott K., Kabnick K., O’Brien S., Clark S., Brawner M., Bates S., Gloger I., et al. J. Cereb. Blood Flow Metab. 2001;21:755–778. doi: 10.1097/00004647-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Lo D. C., McAllister A. K., Katz L. C. Neuron. 1994;13:1263–1268. doi: 10.1016/0896-6273(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 7.Arnold D., Feng L., Kim J., Heintz N. Proc. Natl. Acad. Sci. USA. 1994;91:9970–9974. doi: 10.1073/pnas.91.21.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinou J. C., Dubois-Dauphin M., Staple J. K., Rodriguez I., Frankowski H., Missotten M., Albertini P., Talabot D., Catsicas S., Pietra C. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 9.Willis S., Day C. L., Hinds M. G., Huang D. C. J. Cell Sci. 2003;116:4053–4056. doi: 10.1242/jcs.00754. [DOI] [PubMed] [Google Scholar]
- 10.Olsson T., Wieloch T., Smith M. L. Brain Res. 2003;982:260–269. doi: 10.1016/s0006-8993(03)03014-2. [DOI] [PubMed] [Google Scholar]
- 11.Margaill I., Parmentier S., Callebert J., Allix M., Boulu R. G., Plotkine M. J. Cereb. Blood Flow Metab. 1996;16:107–113. doi: 10.1097/00004647-199601000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Sydserff S. G., Borelli A. R., Green A. R., Cross A. J. Br. J. Pharmacol. 2002;135:103–112. doi: 10.1038/sj.bjp.0704449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronowski J., Strong R., Grotta J. C. Neuropharmacology. 1996;35:689–693. doi: 10.1016/0028-3908(96)84640-5. [DOI] [PubMed] [Google Scholar]
- 14.Mauler F., Horvath E. J. Cereb. Blood Flow Metab. 2005;25:451–459. doi: 10.1038/sj.jcbfm.9600038. [DOI] [PubMed] [Google Scholar]
- 15.Ghisalberti E. L., Pennacchio M., Alexander E. Pharm. Biol. 1998;36:237–279. [Google Scholar]
- 16.Hardman J. G., Limbird L. E., editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 9th Ed. New York: McGraw–Hill; 1996. [Google Scholar]
- 17.Hennion J. P., el-Masri M. A., Huff M. O., el-Mailakh R. S. Bipolar Disorders. 2002;4:201–206. doi: 10.1034/j.1399-5618.2002.01162.x. [DOI] [PubMed] [Google Scholar]
- 18.Xiao A. Y., Wei L., Xia S., Rothman S., Yu S. P. J. Neurosci. 2002;22:1350–1362. doi: 10.1523/JNEUROSCI.22-04-01350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldhuis W. B., van der Stelt M., Delmas F., Gillet B., Veldink G. A., Vliegenthart J. F., Nicolay K., Bar P. R. J. Cereb. Blood Flow Metab. 2003;23:62–74. doi: 10.1097/01.WCB.0000039287.37737.50. [DOI] [PubMed] [Google Scholar]
- 20.Feng G., Mellor R. H., Bernstein M., Keller-Peck C., Nguyen Q. T., Wallace M., Nerbonne J. M., Lichtman J. W., Sanes J. R. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhao K. C., Zhu X. Y., Yi M. G., Liu Z. M., Song Z. Y. Yao Xue Xue Bao. 1986;21:572–579. [PubMed] [Google Scholar]
- 22.Lees G. J. Brain Res. Brain Res. Rev. 1991;16:283–300. doi: 10.1016/0165-0173(91)90011-v. [DOI] [PubMed] [Google Scholar]
- 23.Yu S. P. Biochem. Pharmacol. 2003;66:1601–1609. doi: 10.1016/s0006-2952(03)00531-8. [DOI] [PubMed] [Google Scholar]
- 24.Yu S. P. Prog. Neurobiol. 2003;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 25.Mintorovitch J., Yang G. Y., Shimizu H., Kucharczyk J., Chan P. H., Weinstein P. R. J. Cereb. Blood Flow Metab. 1994;14:332–336. doi: 10.1038/jcbfm.1994.40. [DOI] [PubMed] [Google Scholar]
- 26.Jamme I., Petit E., Gerbi A., Maixent J. M., MacKenzie E. T., Nouvelot A. Brain Res. 1997;774:123–130. doi: 10.1016/s0006-8993(97)81695-2. [DOI] [PubMed] [Google Scholar]
- 27.Tavalin S. J., Ellis E. F., Satin L. S. J. Neurophysiol. 1997;77:632–638. doi: 10.1152/jn.1997.77.2.632. [DOI] [PubMed] [Google Scholar]
- 28.Kang T. C., Hwang I. K., Park S. K., An S. J., Nam Y. S., Kim D. H., Lee I. S., Won M. H. Brain Res. 2003;977:284–289. doi: 10.1016/s0006-8993(03)02681-7. [DOI] [PubMed] [Google Scholar]
- 29.Fuller W., Eaton P., Bell J. R., Shattock M. J. FASEB J. 2004;18:197–199. doi: 10.1096/fj.03-0213fje. [DOI] [PubMed] [Google Scholar]
- 30.Brezis M., Rosen S., Silva P., Epstein F. H. Kidney Int. 1984;25:65–72. doi: 10.1038/ki.1984.9. [DOI] [PubMed] [Google Scholar]
- 31.Brezis M., Rosen S., Spokes K., Silva P., Epstein F. H. Am. J. Pathol. 1984;116:327–341. [PMC free article] [PubMed] [Google Scholar]
- 32.Bruer U., Weih M. K., Isaev N. K., Meisel A., Ruscher K., Bergk A., Trendelenburg G., Wiegand F., Victorov I. V., Dirnagl U. FEBS Lett. 1997;414:117–121. doi: 10.1016/s0014-5793(97)00954-x. [DOI] [PubMed] [Google Scholar]
- 33.Isaev N. K., Stelmashook E. V., Halle A., Harms C., Lautenschlager M., Weih M., Dirnagl U., Victorov I. V., Zorov D. B. Neurosci. Lett. 2000;283:41–44. doi: 10.1016/s0304-3940(00)00903-4. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X., Jiang G., Zhao A., Bondeva T., Hirszel P., Balla T. Biochem. Biophys. Res. Commun. 2001;285:46–51. doi: 10.1006/bbrc.2001.5126. [DOI] [PubMed] [Google Scholar]
- 35.Piccioni F., Roman B. R., Fischbeck K. H., Taylor J. P. Hum. Mol. Genet. 2004;13:437–446. doi: 10.1093/hmg/ddh045. [DOI] [PubMed] [Google Scholar]
- 36.Hochachka P. W. Science. 1986;231:234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- 37.Johnson E. M., Jr, Koike T., Franklin J. Exp. Neurol. 1992;115:163–166. doi: 10.1016/0014-4886(92)90242-i. [DOI] [PubMed] [Google Scholar]
- 38.Johnson E. M., Jr, Deckwerth T. L. Annu. Rev. Neurosci. 1993;16:31–46. doi: 10.1146/annurev.ne.16.030193.000335. [DOI] [PubMed] [Google Scholar]
- 39.Lee J. M., Grabb M. C., Zipfel G. J., Choi D. W. J. Clin. Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connor J. A., Razani-Boroujerdi S., Greenwood A. C., Cormier R. J., Petrozzino J. J., Lin R. C. J. Neurophys. 1999;81:299–306. doi: 10.1152/jn.1999.81.1.299. [DOI] [PubMed] [Google Scholar]
- 41.Biegon A., Fry P. A., Paden C. M., Alexandrovich A., Tsenter J., Shohami E. Proc. Natl. Acad. Sci. USA. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Z., Askari A. Eur. J. Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 43.Xie Z., Cai T. Mol. Interventions. 2003;3:157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 44.Rothstein J. D., Patel S., Regan M. R., Haenggeli C., Huang Y. H., Bergles D. E., Jin L., Dykes Hoberg M., Vidensky S., Chung D. S., et al. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 45.Schurr A., Rigor B. M. In: Emerging Strategies in Neuroprotection. Marangos P. J., Lal H., editors. Boston: Birkhauser; 1992. pp. 24–43. [Google Scholar]
- 46.Lo D. C. In: Current Protocols in Neuroscience. Crawley J. N., Gerfen C. R., McKay R. D. G., Rogawski M. A., Sibley D. R., Skolnick P., editors. New York: Wiley; 1999. pp. 3.15.1–3.15.12. [Google Scholar]
- 47.Rice J. E. I., Vannucci R. C., Brierley J. B. Ann. Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 48.Gidday J. M., Fitzgibbons J. C., Shah A. R., Park T. S. Neurosci. Lett. 1994;168:221–224. doi: 10.1016/0304-3940(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 49.Zea Longa E., Weinstein P. R., Carlson S., Cummins R. W. Stroke (Dallas) 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 50.American Heart Association. Heart Disease and Stroke Statistics–2005 Update. 2005 Available at www.americanheart.org/downloadable/heart/1105390918119HDSStats2005Update.pdf. Accessed January 5, 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.