Abstract
QUESTION
Increasing numbers of pregnant patients are treated with valproic acid, not just for epilepsy, but also for psychiatric conditions. Are there teratogenic risks other than the risk of spina bifida?
ANSWER
It has now become evident that valproic acid might cause more than just neural tube defects (NTDs). In a systematic review of all cohort studies intended to answer this question, higher rates of major malformations (and not just NTDs) were found in most studies. The calculated relative risk was 2.59 when compared with other antiepileptic drugs and was 3.77 when compared with risk in the general population. There is compelling evidence that the risk is dose dependent. The risks appear to begin increasing at doses of 600 mg/d and to become more prominent at doses above 1000 mg/d.
Abstract
QUESTION
Un nombre grandissant de patientes enceintes suivent un traitement à l’acide valproïque, non seulement pour l’épilepsie, mais aussi pour des problèmes psychiatriques. Y a-t-il des risques tératogènes autres que celui du spina bifida?
RÉPONSE
Il est maintenant devenu évident que l’acide valproïque peut causer plus que des défauts du tube médullaire. Dans une synthèse critique de toutes les études de cohortes conçues pour répondre à cette question, des taux plus élevés de malformations majeures (ne se limitant pas au tube médullaire) ont été observés dans la plupart des études. Le risque relatif calculé se situait à 2,59 en comparaison de celui observé avec d’autres médicaments contre l’épilepsie et à 3,77 par rapport au risque dans la population en général. Des données scientifiques convaincantes indiquent que le risque dépend de la dose. Le risque semble commencer à augmenter avec des doses de 600 mg/j et à devenir plus prépondérant à des doses de plus de 1 000 mg/j.
Soon after valproic acid was introduced to clinical use for epilepsy, cases emerged suggesting an increased risk of neural tube defects (NTDs), particularly of spina bifida, among offspring exposed to the drug in early gestation.1,2 The experimental animal work by Nau and colleagues was very important in establishing that valproic acid caused development of NTDs.3 The overall risk for NTDs has been estimated at 2%. While this looks like a low rate, it virtually doubles the overall risk for major malformations in the general population from its usual 1% to 3%. Because NTDs can be detected in utero in most cases by detailed ultrasonography and by measuring levels of alpha-fetoproteins in maternal serum or amniotic fluid, women treated with valproic acid in early gestation should be informed of these diagnostic options.
Over the last 15 years, an increasing number of anecdotal reports and case series have suggested that valproic acid causes malformations other than NTDs, including limb and cardiac anomalies. Until recently, a lack of controlled or large-scale studies precluded corroboration of these impressions. To complicate the situation, increasing numbers of women are now receiving valproic acid as part of treatment for a variety of psychiatric conditions.4
Over the last few years, larger cohort studies of pregnancy outcome among women exposed to valproic acid in pregnancy have been published, now allowing us to evaluate the overall rates of teratogenic risk of valproic acid.
We searched the MEDLINE, EMBASE, and Cochrane databases from 1978, when reports on valproic acid use in pregnancy began to emerge, to December 31, 2005. We selected controlled cohort studies that reported the use of valproic acid during the first trimester of pregnancy and that had a comparison group of women treated with other antiepileptic drugs, untreated epileptic women, or healthy women representing the general population of pregnant women. To be included in our analysis, the studies had to describe rates of major malformations among the study and comparison groups. Several studies had comparison groups, but the papers did not allow extraction of these numbers. Individual and summary relative risks were calculated with the Mantel-Haenszel random effect test using Cochrane’s Review Manager (version 4.2). We calculated the relative risk for major malformations among babies exposed to valproic acid alone or in combination with other anticonvulsant drugs during embryogenesis as compared with babies exposed to other anticonvulsants, or babies of unexposed healthy control subjects.
Malformation rates
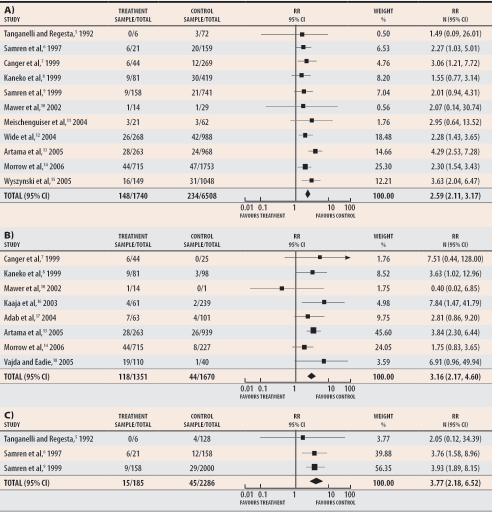
Based on more than 1700 exposed babies reported in 11 cohort studies, exposure to monotherapy with valproic acid was associated with a relative risk of 2.59 for major malformation (95% confidence interval [CI] 2.11 to 3.17) when compared with monotherapy using other anticonvulsant drugs (Figure 1A).5-15 Based on more than 1300 exposed babies, the relative risk was 3.16 (95% CI 2.17 to 4.60) when compared with untreated epileptic patients (Figure 1B).7,8,10,13,14,16-18 Although less frequently reported, when compared with the general population of healthy control subjects the relative risk of major congenital malformations among patients receiving monotherapy with valproic acid was 3.77 (95% CI 2.18 to 6.52) (Figure 1C).5,6,9 This means that women exposed to valproic acid monotherapy during embryogenesis have more than 2.5 times the risk of having babies with malformations and that this trend is highly significant (P < .001).
Figure 1. Meta-analysis of major congenital malformations in women treated during pregnancy with valproic acid monotherapy.
Rates are compared with those of A) women treated with other anticonvulsant monotherapies, B) untreated epileptic women, and C) healthy control subjects.
RR—relative risk (random).
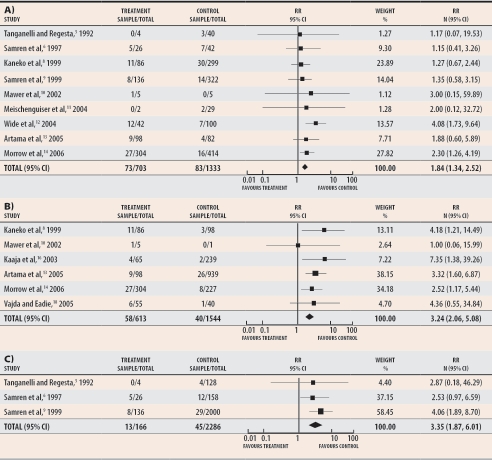
When valproic acid was administered as part of anticonvulsant polytherapy, the number of exposed babies was lower than those exposed to valproic acid monotherapy, yet the relative risk also increased significantly over that of other anticonvulsant drugs (1.84 [95% CI 1.34 to 2.52]), untreated epilepsy (3.24 [95% CI 2.06 to 5.08]), and healthy control subjects (3.35 [95% CI 1.87 to 6.01]) (Figure 2).5,6,8-14,18 Valproic acid polytherapy regimens varied greatly, and combinations with all commercially available treatments were reported.
Figure 2. Meta-analysis of major congenital malformations among women treated during pregnancy with valproic acid polytherapy.
Rates are compared with those of A) women treated with other polytherapies, B) untreated epileptic women, and C) healthy control subjects.
RR—relative risk (random).
Dose dependence
Several studies6-10,13,14,18-21 performed subanalyses evaluating dose effects. Most suggested that the risk for major congenital malformations was dose dependent, with risks increasing statistically at 600 mg/d.9,14 The largest attributable risks, however, were seen when doses exceeded 1000 mg/d.
Neurobehavioural risk
Several studies have compared child development and cognitive deficits among children exposed in utero to valproic acid with those among children exposed to other anticonvulsant drugs.17,22-25 Although variation in study designs, outcomes, and cognitive tests precluded synthesis of these data into meta-analyses, all researchers reported developmental delays and cognitive deficits associated with valproic acid use in pregnancy. The most prominent effect was on verbal intelligence quotient (IQ). Two of the studies, however, noted that mothers taking valproic acid had had lower levels of education,23,24 which could predict lower levels of cognitive functioning among their children. Another study17 found that frequent tonic-clonic seizures in pregnancy were significantly associated with a lower verbal IQ (P = .007). More neurodevelopment studies are needed to control for confounders affecting child development, such as maternal IQ and socioeconomic class.
Conclusion
A 3-fold increase in major congenital malformations above the general population is associated with use of valproic acid in early pregnancy. Consequently, practitioners should inform women of increased risk of malformation and of potentially higher risk of cognitive deficits when valproic acid is used in early pregnancy, especially at high doses. A combination of high-resolution ultrasonography, measuring maternal serum levels of alpha-fetoprotein, and (if necessary) fetal echocardiography between weeks 16 and 18 of pregnancy sometimes helps to evaluate major malformations.
Motherisk questions are prepared by the Motherisk Team at the Hospital for Sick Children in Toronto, Ont. Dr Koren is Director; Dr Nava-Ocampo, Ms Moretti, and Dr Nulman are members; and Mr Sussman is a student in the Motherisk Program. Dr Koren holds the Ivey Chair in Molecular Toxicology at the University of Western Ontario in London and is supported by the Research Leadership for Better Pharmacotherapy during Pregnancy and Lactation and, in part, by a grant from the Canadian Institutes of Health Research.
References
- 1.Robert E, Guibaud P. Maternal valproic acid and congenital neural tube defects. Lancet. 1982;2:937. doi: 10.1016/s0140-6736(82)90908-4. [DOI] [PubMed] [Google Scholar]
- 2.Lammer EJ, Sever LE, Oakley GP., Jr Teratogen update: valproic acid. Teratology. 1987;35:465–473. doi: 10.1002/tera.1420350319. [DOI] [PubMed] [Google Scholar]
- 3.Nau H, Zierer R, Spielmann H, Neubert D, Gansau C. A new model for embryotoxicity testing: teratogenicity and pharmacokinetics of valproic acid following constant-rate administration in the mouse using human therapeutic drug and metabolite concentrations. Life Sci. 1981;29:2803–2814. doi: 10.1016/0024-3205(81)90541-5. [DOI] [PubMed] [Google Scholar]
- 4.Koren G, Kennedy D. Safe use of valproic acid during pregnancy [Motherisk Update]. Can Fam Physician. 1999;45:1451–1453. [PMC free article] [PubMed] [Google Scholar]
- 5.Tanganelli P, Regesta G. Epilepsy, pregnancy, and major birth anomalies: an Italian prospective, controlled study. Neurology. 1992;42(4 Suppl 5):89–93. [PubMed] [Google Scholar]
- 6.Samren EB, van Duijn CM, Koch S, Hiilesmaa VK, Klepel H, Bardy AH, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–990. doi: 10.1111/j.1528-1157.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 7.Canger R, Battino D, Canevini MP, Fumarola C, Guidolin L, Vignoli A, et al. Malformations in offspring of women with epilepsy: a prospective study. Epilepsia. 1999;40:1231–1236. doi: 10.1111/j.1528-1157.1999.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko S, Battino D, Andermann E, Wada K, Kan R, Takeda A, et al. Congenital malformations due to antiepileptic drugs. Epilepsy Res. 1999;33:145–158. doi: 10.1016/s0920-1211(98)00084-9. [DOI] [PubMed] [Google Scholar]
- 9.Samren EB, van Duijn CM, Christiaens GC, Hofman A, Lindhout D. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann Neurol. 1999;46:739–746. [PubMed] [Google Scholar]
- 10.Mawer G, Clayton-Smith J, Coyle H, Kini U. Outcome of pregnancy in women attending an outpatient epilepsy clinic: adverse features associated with higher doses of sodium valproate. Seizure. 2002;11:512–518. doi: 10.1016/s1059-1311(02)00135-8. [DOI] [PubMed] [Google Scholar]
- 11.Meischenguiser R, D’Giano CH, Ferraro SM. Oxcarbazepine in pregnancy: clinical experience in Argentina. Epilepsy Behav. 2004;5:163–167. doi: 10.1016/j.yebeh.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Wide K, Winbladh B, Kallen B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatr. 2004;93:174–176. doi: 10.1080/08035250310021118. [DOI] [PubMed] [Google Scholar]
- 13.Artama M, Auvinen A, Raudaskoski T, Isojarvi I, Isojarvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64:1874–1878. doi: 10.1212/01.WNL.0000163771.96962.1F. [DOI] [PubMed] [Google Scholar]
- 14.Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–198. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyszynski DF, Nambisan M, Surve T, Alsdorf RM, Smith CR, Holmes LB. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64:961–965. doi: 10.1212/01.WNL.0000154516.43630.C5. [DOI] [PubMed] [Google Scholar]
- 16.Kaaja E, Kaaja R, Hiilesmaa V. Major malformations in offspring of women with epilepsy. Neurology. 2003;60:575–579. doi: 10.1212/01.wnl.0000044157.28073.dc. [DOI] [PubMed] [Google Scholar]
- 17.Adab N, Kini U, Vinten J, Ayres J, Baker G, Clayton-Smith J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–1583. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vajda FJ, Eadie MJ. Maternal valproate dosage and foetal malformations. Acta Neurol Scand. 2005;112:137–143. doi: 10.1111/j.1600-0404.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 19.Vajda FJ, O’brien TJ, Hitchcock A, Graham J, Cook M, Lander C, et al. Critical relationship between sodium valproate dose and human teratogenicity: results of the Australian register of anti-epileptic drugs in pregnancy. J Clin Neurosci. 2004;11:854–858. doi: 10.1016/j.jocn.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Omtzigt JG, Los FJ, Grobbee DE, Pijpers L, Jahoda MG, Brandenburg H, et al. The risk of spina bifida aperta after first-trimester exposure to valproate in a prenatal cohort. Neurology. 1992;42(4 Suppl 5):119–125. [PubMed] [Google Scholar]
- 21.Lindhout D, Meinardi H, Meijer JW, Nau H. Antiepileptic drugs and teratogenesis in two consecutive cohorts: changes in prescription policy paralleled by changes in pattern of malformations. Neurology. 1992;42(4 Suppl 5):94–110. [PubMed] [Google Scholar]
- 22.Vinten J, Adab N, Kini U, Gorry J, Gregg J, Baker GA. Neuropsychological effects of exposure to anticonvulsant medication in utero. Neurology. 2005;64:949–954. doi: 10.1212/01.WNL.0000154514.82948.69. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson K, Viinikainen K, Monkkonen A, Aikia M, Nieminen P, Heinonen S, et al. Children exposed to valproate in utero—population based evaluation of risks and confounding factors for long-term neurocognitive development. Epilepsy Res. 2005;65:189–200. doi: 10.1016/j.eplepsyres.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Gaily E, Kantola-Sorsa E, Hiilesmaa V, Isoaho M, Matila R, Kotila M, et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology. 2004;62:28–32. doi: 10.1212/wnl.62.1.28. [DOI] [PubMed] [Google Scholar]
- 25.Adab N, Jacoby A, Smith D, Chadwick D. Additional educational needs in children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2001;70:15–21. doi: 10.1136/jnnp.70.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]