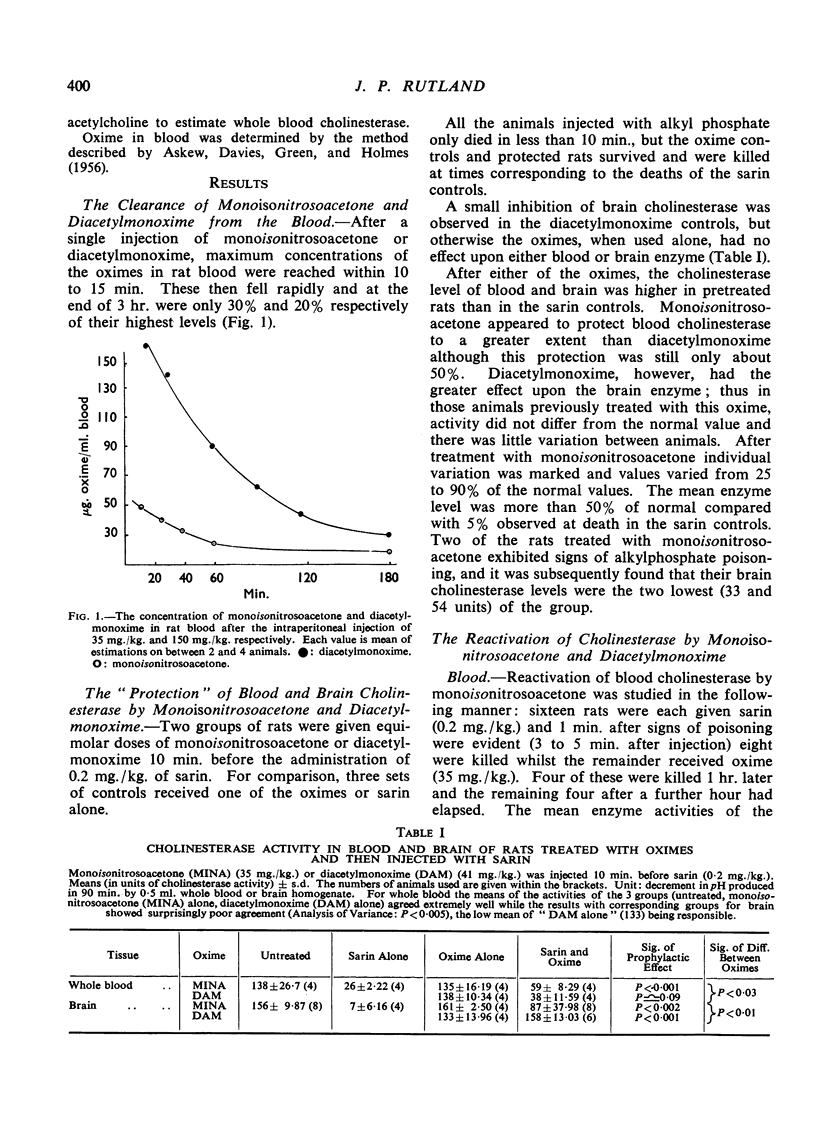
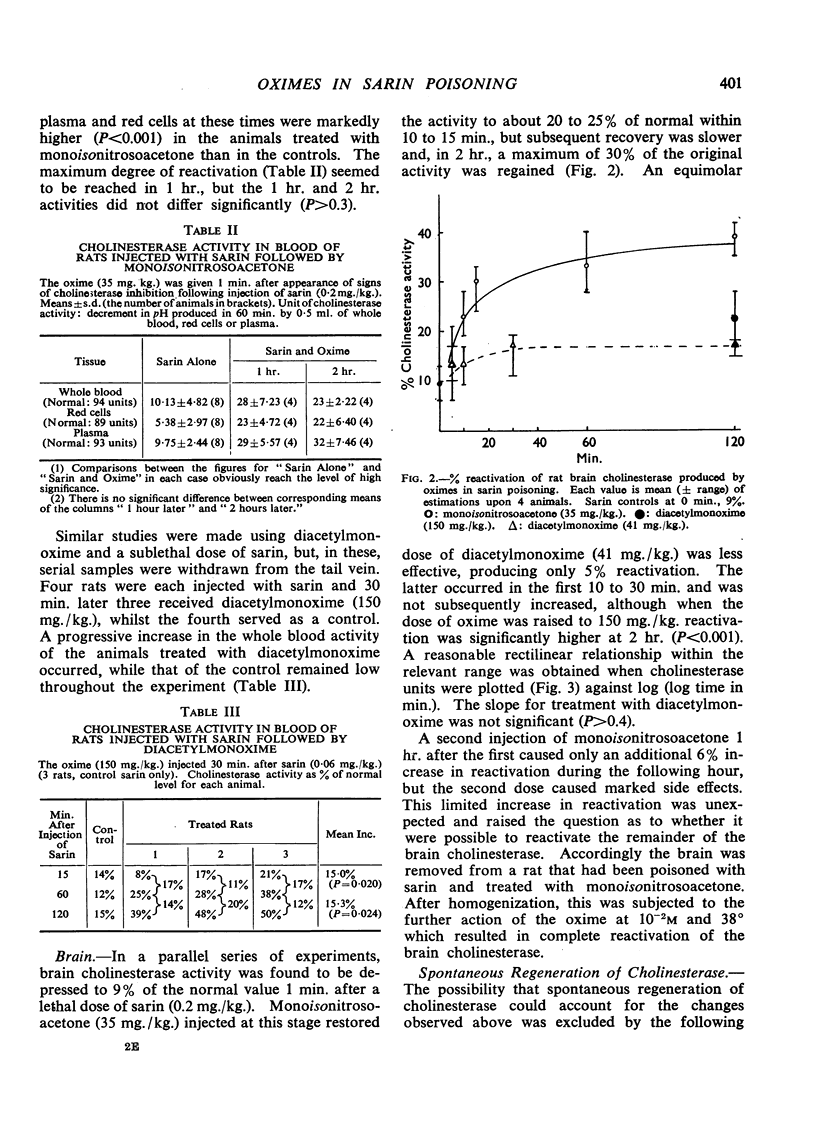
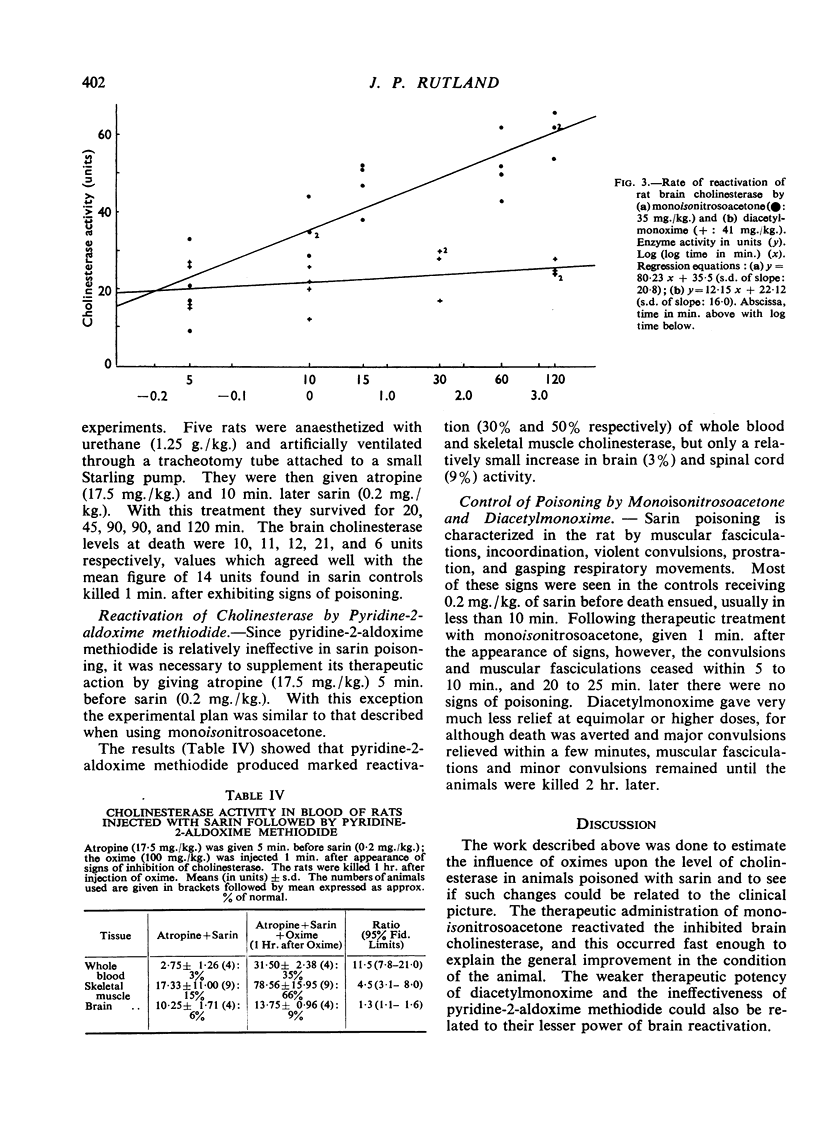
Abstract
The effects of monoisonitrosoacetone (MINA), diacetylmonoxime (DAM) and pyridine-2-aldoxime methiodide (P2AM) upon the cholinesterase of sarin poisoned rats have been studied. Monoisonitrosoacetone and diacetylmonoxime given before sarin protected blood and brain cholinesterase from inhibition. Monoisonitrosoacetone given after the appearance of signs of poisoning caused a rapid reactivation of brain cholinesterase. Diacetylmonoxime, at an equimolar dose, produced only a slight increase in enzyme activity, and pyridine-2-aldoxime methiodide, the best reactivator in vitro, reactivated blood but not brain cholinesterase. There is a relationship between protection and reactivation of brain cholinesterase and prevention and alleviation of signs of poisoning.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKEW B. M., DAVIES D. R., GREEN A. L., HOLMES R. The nature of the toxicity of 2-oxo-oximes. Br J Pharmacol Chemother. 1956 Dec;11(4):424–427. doi: 10.1111/j.1476-5381.1956.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASKEW B. M. Oximes and hydroxamic acids as antidotes in anticholinesterase poisoning. Br J Pharmacol Chemother. 1956 Dec;11(4):417–423. doi: 10.1111/j.1476-5381.1956.tb00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A. S. V., CHIPMAN L. M. The location of cholinesterase in the central nervous system. Q J Exp Physiol Cogn Med Sci. 1952;37(2):61–74. doi: 10.1113/expphysiol.1952.sp000983. [DOI] [PubMed] [Google Scholar]
- CHILDS A. F., DAVIES D. R., GREEN A. L., RUTLAND J. P. The reactivation by oximes and hydroxamic acids of cholinesterase inhibited by organo-phosphorus compounds. Br J Pharmacol Chemother. 1955 Dec;10(4):462–465. doi: 10.1111/j.1476-5381.1955.tb00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVISON A. N. Return of cholinesterase activity in the rat after inhibition by organophosphorus compounds. I. Diethyl p-nitro-phenyl phosphate (E 600, paraoxon). Biochem J. 1953 Jul;54(4):583–590. doi: 10.1042/bj0540583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOELLE G. B., STEINER E. C. The cerebral distributions of a tertiary and a quaternary anticholinesterase agent following intravenous and intraventricular injection. J Pharmacol Exp Ther. 1956 Dec;118(4):420–434. [PubMed] [Google Scholar]


