Abstract
Crossing the hands over, whether across the body midline or with respect to each other, leads to measurable changes in spatial compatibility, spatial attention, and frequently to a general decrement in discrimination performance for tactile stimuli. The majority of multisensory crossed hands effects, however, have been demonstrated with explicit or implicit spatial discrimination tasks, raising the question of whether non-spatial discrimination tasks also show spatial effects when the hands are crossed. We designed a novel, non-spatial tactile discrimination task to address this issue. Participants made speeded discriminations of single-versus double-pulse vibrotactile targets, while trying to ignore simultaneous visual distractor stimuli, in both hands uncrossed, and hands crossed postures. Tactile discrimination performance was significantly affected by the visual distractors (demonstrating a significant crossmodal congruency effect), and was affected most by visual distractors in the same external location as the tactile target (i.e., spatial modulation), regardless of the posture (uncrossed or crossed) of the hands (i.e., spatial ‘re-mapping’ of visual-tactile interactions). Finally, crossing the hands led to a general performance decrement with visual distractors, but not in a control task with unimodal visual or tactile judgements. These results demonstrate, for the first time, significant spatial and postural modulations of crossmodal congruency effects in a non-spatial discrimination task.
Keywords: Multisensory, Crossmodal congruency, Crossed hands, Somatosensation, Vision
1. Introduction
For the majority of functions performed with our hands, our left hand operates on the left side of the workspace, and the right hand operates on the right side. In this context, the ‘side’ of the workspace may be determined in a number of ways, for example, with respect to the anatomical sagittal midline (i.e., body-centred), with respect to the head (i.e., head-centred), or the direction of gaze (i.e., head and/or eye-centred). Regardless of the specific reference point used to determine what is left and what is right, it is still generally true that the left hand works on the left and the right hand on the right. There are exceptions to this general rule, however, and the brain needs to be able to adapt to situations where the hands are crossed. Skilful manual performance in a number of domains, for example in piano-playing and many racquet sports sometimes requires the left hand to operate on the right side of the right hand, and vice versa. How does the brain cope with such situations?
The effects of crossing the hands on speeded or unspeeded responses to a variety of sensory stimuli have been shown to depend upon the specific task requirements. If the participant’s task is simply to press a button as fast as possible in response to a single visual stimulus (in the case of a simple reaction time experiment), then the posture of the responding hand with respect to the non-responding hand does not affect reaction time (e.g., [16, Experiment 1]). If two hands are used to respond to two visual stimuli, one positioned to the left of the other, and the participant is required to press the left button for the left stimulus and the right button for the right stimulus, then crossing the hands significantly slows participants’ responses (e.g., by about 50-60 ms, [16, Experiments 2 & 3; see also 31]), regardless of the absolute spatial position of the two stimuli (i.e., to the left or right of the body midline). This general decrement in performance following the crossing of the hands has been called the ‘crossed hands effect’. Alongside this general impairment,‘spatial compatibility’ is also modulated when the hands are crossed. Spatial compatibility in an uncrossed posture refers to the fact that responses made by the left hand on the left side are faster in response to stimuli presented on the left, and slower for stimuli presented on the right (and vice versa). Crossing the hands reverses this spatial stimulus-response compatibility - when the left hand is on the right side of the right hand, it now responds fastest to stimuli presented on the right [18, 32, 33]. This reversal also occurs when the hands themselves remain in their respective anatomical hemispace, but the responses are emitted with two long sticks, crossing the midline and responding with the button on the opposite side of space [18] (see also [5]).
Crossing the hands has also been shown to impair performance on tactile temporal order judgement (TOJ) tasks, where participants are required to say which side (or modality) was presented first (or second) when two stimuli are presented at a range of temporal separations [3, 19, 23, 24, 31, 34, 35, 36, see also 2]. Further, crossing the hands results in a spatial ‘re-mapping’ of tactile and multisensory attentional orienting for both exogenous [8, 9] and endogenous forms of orienting [11, 28].
Finally, very similar effects were found using the visual-tactile crossmodal congruency task [29], in which participants made speeded elevation (‘upper’ vs. ‘lower’) discrimination responses to vibrotactile targets, while trying to ignore nearly-simultaneous visual distractor stimuli. In this task, crossmodal congruency effects (i.e., the difference in discrimination performance between congruent and incongruent trials, in which vibrotactile targets and visual distractors appear at the same or at different elevations, respectively) are typically strongest when both visual and vibrotactile stimuli are presented from the same side of space (i.e., to the same hemisphere of the brain when the hands are uncrossed). When the hands were crossed, however, congruency effects remained highest for stimuli presented in the same external location, regardless of the fact that different brain hemispheres now processed the initial sensory information [29]. This spatial crossmodal congruency task (i.e., judging ‘upper’ versus ‘lower’) therefore also shows a significant spatial modulation of performance with changes in hand-posture.
The studies mentioned above collectively highlight several consistent behavioural consequences of crossing the hands: 1) A reversal of spatial compatibility; 2) A spatial ‘remapping’ of multisensory attentional cueing and selective attention or ‘congruency’ effects (with stronger interactions occurring for bimodal stimuli presented in the same external location); and 3) A general decrement in performance for tactile discrimination tasks. The first of these three consequences, spatial compatibility, will not be discussed further here, and instead will be controlled for in the following experiments by using orthogonal stimulus-response mappings, and counterbalancing extraneous response factors across participants.
With regards to the effects of crossing the hands on spatial ‘remapping’ and tactile discrimination performance, however, one particular questions of interest is relevant to the present report. In all of the relevant studies mentioned above, the participants were required to make either an explicit spatial discrimination response (e.g., to respond ‘left’ or ‘right’ for TOJ tasks, and ‘upper’ or ‘lower’ for covert exogenous cueing or the visual-tactile congruency task), or to respond on a response button in a location compatible with that of the target stimulus (i.e., the left button for a left stimulus). Given that all these studies involved explicit spatial judgements, it is not yet known whether crossing the hands impairs performance on all types of tactile discrimination tasks, on the subset of tactile tasks performed in the presence of distractors in a second sensory modality, or only on explicitly or implicitly spatial tactile tasks. This question is important following the work of McDonald and Ward ([14]; see also [25], who show a similar pattern of results) showing that significant auditory spatial orienting effects only emerge when the task involves explicit or implicit spatial judgements, or when spatial information is informative about the target location (see also refs [4, 26, 27]). More importantly, those tasks that require participants to make explicit ‘left’ or ‘right’ judgements about stimuli (e.g., the majority of tactile TOJ studies) are susceptible to participants being confused about the response labels ‘left’ and ‘right.’ Judgements about left and right are inherently more difficult and more prone to error than judgements of other stimulus dimensions, even when compared to spatial judgements of ‘up’ versus ‘down’ (see especially ref [5] on this point).
The present study was designed to address the question: “Does crossing the hands lead to a performance deficit in a non-spatial visual-tactile discrimination task?” In order to answer this, we designed a novel crossmodal discrimination task based on a crossmodal congruency paradigm used in our laboratory (see [13, 29], for reviews). We asked participants to make non-spatial judgements concerning vibrotactile stimuli presented to either their left or right index fingers, which were positioned on the left and right side of space, with their hands held in either an uncrossed (the left hand on the left target stimulator, and the right hand on the right target), or a crossed posture. In order to rule out any effects of left-right confusion, the task did not involve explicit left-right judgements of the stimuli. Participants therefore discriminated single- from double-pulse vibrotactile stimuli, while trying to ignore random single- and double-pulse visual distractors. A similar task has recently been used in other unspeeded tasks with audio-visual stimuli [20, 21]. We were interested in the extent to which participants could ignore the random visual distractors on different sides of space, while selectively attending and responding only to vibrotactile target information.
2. Materials & Methods
2.1 Participants
Twenty-four healthy participants (16 female, aged 19 to 40 years, M ± SE age 24.8 ± 5.0 years) were recruited from the population of staff and students of, and visitors to, Oxford University. All reported being right-handed, having normal or corrected-to-normal vision, and being free from neurological impairment. The experiments were approved by the local ethics committee, were conducted in accordance with the Declaration of Helsinki, and participants gave their informed consent prior to the experiment, and were fully debriefed after the experiment was completed. Participants were rewarded with a £5 (UK Sterling) gift voucher in exchange of their participation. Each experiment took around 30 minutes to complete.
2.2 Apparatus and Stimuli
The experiment was performed in a dimly illuminated booth. The stimulus presentation and data collection were controlled by a standard personal computer, operating bespoke software programmed in the Turbo Pascal language. A custom-built parallel-port hardware interface device was used to control the visual and vibrotactile stimuli. The vibrotactile stimulators were two Oticon bone-conducting vibrators (Somerset, New Jersey, US; p/n: BC461-1, 100-ohm), driven by a broadband white-noise signal controlled by an amplifier. The vibrotactile stimulators were positioned 30 cm apart, 15 cm to either side of the middle of a table, and 30 cm from the front edge of the table (from the participant’s perspective). The visual stimuli were emitted from two 10 mm diameter yellow-orange LEDs, one positioned 1 cm directly above the vibrotactile stimulator on each side. A small (5 mm diameter) red LED was positioned centrally, 40 cm from the front of the table, and served as the visual fixation point.
2.3 Design
The experiment was a within-participants repeated measures design, with the independent variables of target stimulus (single vs. double vibration), target side (left vs. right), distractor stimulus (single vs. double flash), and distractor side (left vs. right), these conditions were fully factorised and repeated six times within each block of experimental trials (i.e., 2×2×2×2×6 repetitions = 96 trials per block). The participants performed four blocks of trials, two with their hands in an uncrossed posture and two while adopting the crossed posture. For the data analysis, the experimental variables were pooled into the following variables: Congruency (same number of visual and tactile stimuli vs. different number); relative side (visual and tactile stimuli presented from the same external location vs. different locations), and posture (hands uncrossed vs. hands crossed).
2.4 Procedure
The participants sat down behind the table, with their chest approximately 20 cm from the near edge of the table, and placed their arms out in front of them, with the index finger of one hand on the left vibrator, and the index finger of their other hand on the right vibrator. The general purposes of the experiment and instructions were given to the participants, who were also given the opportunity to ask questions.
The participants were required to discriminate between single (200 ms duration) and double (65 ms on, 70 ms off, 65 ms on) vibrotactile stimuli presented to either their left or right index finger, while trying to ignore near-simultaneous single or double visual distractor stimuli (a continuous 200 ms flash or two 65 ms flashes separated by 70 ms respectively, i.e., similar in duration to the vibrotactile target stimuli) presented immediately above the vibrotactile stimulators. Visual distractors were random, irrelevant, and non-predictive of either the side of the vibrotactile stimulus (left or right) or the type of vibrotactile stimulus (single or double): Participants were therefore instructed to ignore the visual stimuli as much as possible, and to concentrate on, and respond only to, the vibrotactile stimuli. Participants were encouraged to respond both as quickly and as accurately as possible.
The participants responded using two foot pedals, one positioned under the toes and the other under the heel of one of the participant’s feet. The participants lifted one of the pedals to make their vibrotactile discrimination response. Half of the participants used their left foot to respond and the other half used their right foot. Half of each of these groups responded by lifting their toes off the front pedal in response to a single continuous vibration, and by lifting their heel off the rear pedal in response to a double-pulsed vibration. The other half of each subgroup responded in the opposite manner. Finally, half of each of these four subgroups performed the uncrossed hands condition in the first and third experimental blocks, and the crossed hands condition in the second and fourth blocks. The other half of the participants performed the experimental blocks in the opposite order. In short, all experimental conditions and stimulus-response mappings were fully counterbalanced across the 24 participants. In the crossed hands blocks, participants were provided with small foam pads to rest their uppermost arm upon to minimise any discomfort associated with maintaining the crossed hands posture. The left arm was uppermost in one of the two crossed-hand blocks, while the right was uppermost in the other crossed hands block. The arms rested upon each other in the crossed position, with no separation between them. Both of the participant’s hands and lower arms were visible throughout the experimental session.
The participants were instructed to fixate the central LED throughout the experiment and to refrain from making eye movements to the left or right. A closed-circuit television camera was installed directly in front of the participants, and the experimenter observed the participants’ eyes on a monitor outside the testing booth to ensure that visual fixation was maintained, giving prompts to fixate centrally when necessary. All of the participants apart from one were able to maintain correct central fixation throughout the experiment. The data from that participant are not included in the present report, and an additional participant was recruited. The participants wore headphones through which white noise was presented at approximately 70 dB to mask any sounds associated with the presentation of the experimental stimuli.
The participants were given short practice blocks of 32 trials prior to the main experimental session. In the first practice block, vibrotactile stimuli were presented alone in order to familiarise the participants with the target stimuli and the required responses. In the second short practice block, the visual stimuli were added, and participants were reminded that they should ignore the visual stimuli as much as possible, while keeping their eyes open and fixated on the central fixation spot. Typically, participants achieved around 85-95% correct on their first practice block. Several participants could not feel the stimulus clearly at the initial intensity setting, so the intensity was increased in subsequent practice blocks in the absence of visual distractor stimulation until their performance reached at least 75% correct. Once this initial criterion was reached, one additional practice block with concurrent visual stimuli was run, and the experiment proper then began. The intensity of vibrotactile stimulation then remained constant throughout the remainder of the experiment. The majority of participants required only two short practice blocks, while none required more than four to reach the 75% accuracy criterion in the absence of visual stimuli. The range of vibrotactile intensities used in the experiment, measured with an audiometer, was approximately 65-75 dB when measured immediately above the vibrator. This intensity attenuated to about 45 dB over a distance of 40cm, approximately the distance to the participants’ head. The background noise level was around 40 dB. The white noise played over headphones (∼70 dB) to the participants masked the sounds associated with vibrotactile stimulus presentation.
A typical trial proceeded as follows. When both of the pedals under the participant’s foot were fully depressed, the central fixation LED was illuminated for a random foreperiod of 750-1000 ms. On every experimental trial, one vibrotactile stimulus (either a single- or double-pulse vibration) and one visual stimulus (a single- or double-pulse flash) were presented nearly simultaneously: the onset of the vibrotactile stimulus occurred 30 ms after the onset of the visual stimulus. This visual-vibrotactile stimulus onset asynchrony (SOA) was chosen following previous work suggesting enhanced visual-vibrotactile congruency effects at this SOA as compared to when both stimuli were presented simultaneously [29]. Participants then responded by lifting either their toes or heel from one of the foot pedals, the fixation LED was turned off, and the next trial began after a delay of 1500 ms. Following correct responses, the LED was extinguished, and was not illuminated again until the start of the next trial. Following an incorrect response, the central LED flashed twice briefly (2 × 250 ms, separated by 250 ms) before the trial ended. Participants were encouraged to use the feedback to try to improve their performance as the experiment progressed. Trials in which one or both foot pedals were released before the stimuli were presented were aborted. Trials in which responses were made before 200 ms (‘anticipation errors’) or after 3000 ms (‘omission errors’) were removed from the data set and not analysed further. Neither aborted trials nor trials on which an anticipation or omission error occurred were replaced during the experiment.
2.5 Analysis
Median reaction time (RT) data were analysed after removing incorrect responses, anticipation errors, and omission errors. The RT and error data were analysed in an initial three-way repeated measures analysis of variance (ANOVA), with the variables of congruency (congruent = same number of tactile and visual stimuli, incongruent = different numbers of stimuli), relative side (tactile and visual stimuli on the same side vs. on different sides), and hand posture (uncrossed vs. crossed). To attempt to reduce any possible influence of speed-accuracy trade-offs in our data, we then combined the RT and error data to derive the Inverse Efficiency measure (median RT divided by the proportion of correct responses per condition,[30]).
Previous studies examining discrimination of the number of stimuli in one modality in the presence of distractors in another sensory modality have demonstrated asymmetrical effects of number (or of continuity vs. discontinuity of stimuli). For example, a single visual stimulus is more often perceived as two visual stimuli when accompanied by two auditory distractors, than with the opposite pairing of stimuli (i.e., two flashes are only rarely perceived as one flash when accompanied by a single auditory distractor [20, 21], but see ref [1]). For this reason, we also performed a secondary analysis to search for any asymmetries concerning the number of target and distractor stimuli. To simplify this analysis, the data were pooled across the relative side conditions - only the number of targets and the number of distractors were examined for their relative effects on reaction times and errors under different hand postures.
3. Results
Aborted trials, anticipation errors and omission errors were removed from the analysis and accounted for a total of 0.94 ± 0.30 % (mean ± SE) of responses. Reaction times were calculated using correct responses only. Pooling the data across all experimental conditions for each participant individually revealed that reaction times for responses made with the toes (796 ± 28 ms) were not significantly different from those made with the heels (794 ± 21 ms, t(23) = 0.19, n.s.), suggesting that there were no systematic differences between the two responses across participants. The results of the ANOVA for the primary experimental variables of interest are presented in Table 1, and the corresponding data in Figure 1. In the following, ‘same side’ and ‘different sides’ refer in all cases to the external spatial locations where the stimuli were presented, rather than to the anatomical side of the hand that was stimulated.
TABLE 1.
ANOVA statistics for showing degrees of freedom (d.f.), the F-statistic and p-values for all main effects and interactions. Side refers to the external spatial location, rather than the hand to which the stimuli were presented. IE - Inverse Efficiency (median RT/proportion correct responses, [30]). Significant effects are highlighted in bold.
| Reaction times |
Errors |
Inverse efficiency |
|||||
|---|---|---|---|---|---|---|---|
| Effects | d.f. | F | P | F | p | F | p |
| Congruency (single vs. double) | 1, 23 | 57.22 | <.001 | 71.05 | <.001 | 33.6 | <.001 |
| Posture (uncrossed vs. crossed) | 1, 23 | 3.98 | .06 | 5.06 | <.05 | 6.88 | <.05 |
| Side (same-side vs. different sides) | 1, 23 | 0.87 | .36 | 9.96 | <.01 | 3.34 | .08 |
| Congruency X Posture | 1, 23 | 0.16 | .69 | 3.22 | .09 | 4.88 | <.05 |
| Congruency X Side | 1, 23 | 0.1 | .76 | 7.87 | <.01 | 3.91 | .06 |
| Posture X Side | 1, 23 | 1.00 | .33 | 2.24 | .15 | 0.55 | .47 |
| Congruency X Posture X Side | 1, 23 | 0.02 | .88 | <0.01 | .98 | 0.10 | .76 |
Figure 1.
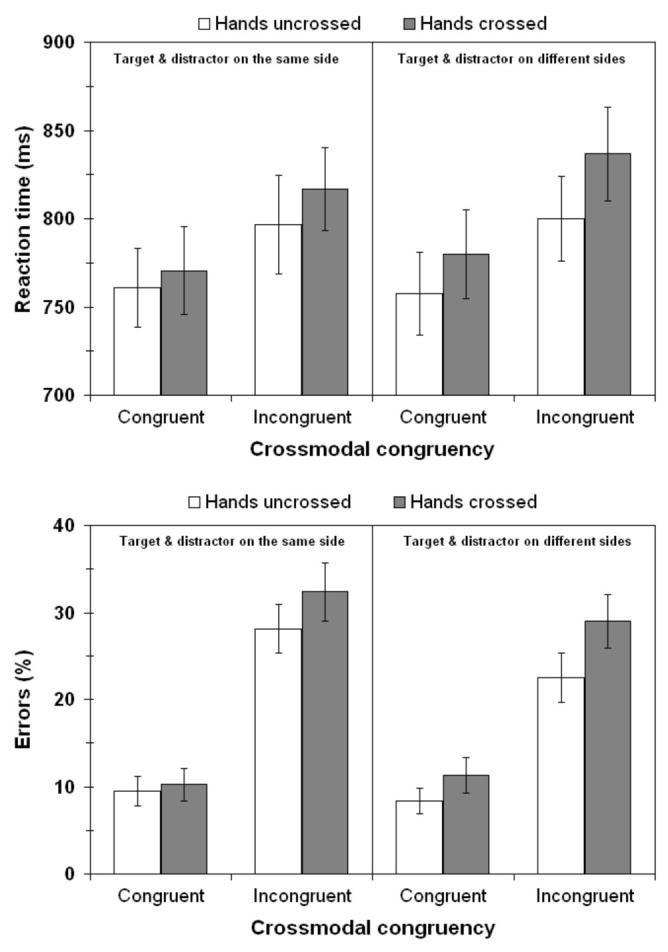
Results of Experiment 1: Vibrotactile judgements with visual distractors. Columns show the mean of median reaction time (RT) data (upper panel), and percentage error (lower panel). Open columns- hands uncrossed; Filled columns-hands crossed. Errors bars - standard errors of the means across participants. The left half of each chart shows responses for vibrotactile and visual stimuli presented from the same side of space. The right half of each chart shows responses for vibrotactile and visual stimuli presented from different sides of space. Congruent - vibrotactile and visual stimuli of the same type (single vs. double); Incongruent - vibrotactile and visual stimuli of different types.
3.1 Reaction times
Participants were significantly faster at discriminating the vibrotactile stimuli when the number of visual distractors presented was congruent with that of the vibrotactile target (M ± SE = 771 ± 23 ms) compared to when it was incongruent (820 ± 24 ms). Additionally, participants responded more rapidly when their hands were held in an uncrossed posture (787 ± 24 ms) than when their hands were crossed (804 ± 24 ms), as has been reported in other speeded tasks [16, 34]. This difference of 17 ± 9 ms across participants just failed to reach statistical significance (p = .058). There were no other significant main effects or interactions. The analyses were repeated using the mean RTs rather than the medians. The same pattern of results emerged, except that the main effect of posture now reached significance, F(1, 23) = 6.18, p < .05, with an overall crossed hands deficit of 21 ms as compared to the uncrossed hands posture.
3.2 Errors
The participants made significantly fewer errors when the visual and vibrotactile stimuli were congruent in number (9.8 ± 1.6 %) as compared to when they were incongruent (28.0 ± 2.6 %). Additionally, participants made significantly fewer errors when they held their hands in an uncrossed posture (17.1 ± 1.8 %), than when they held their hands in a crossed posture (20.7 ± 2.3 %). Finally, participants made significantly more errors when the vibrotactile and visual stimuli were presented from the same side (20.1 ± 2.0 %) than when the vibrotactile and visual stimuli were presented from different sides (17.8 ± 1.9 %).
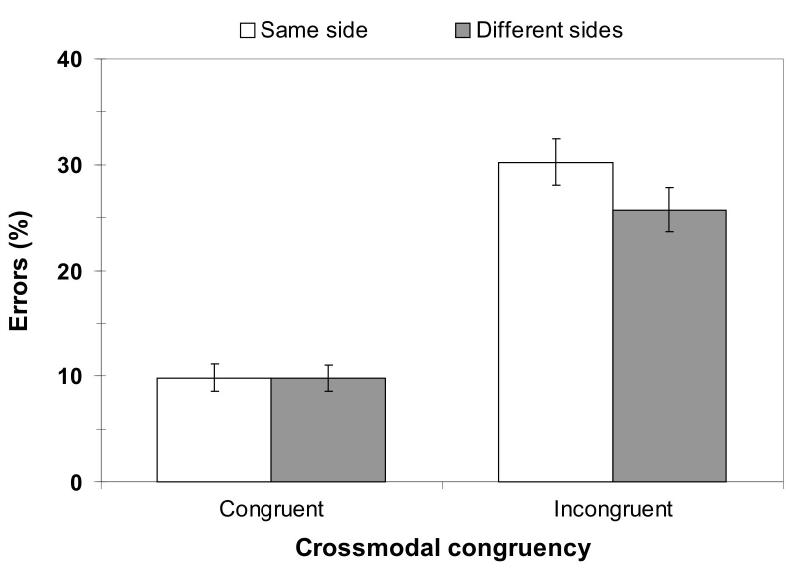
In addition to the main effects of congruency, posture, and side, the interaction between congruency and side was also significant. Inspection of the data for this interaction revealed that while congruent vibrotactile and visual stimulation resulted in the same number of errors for same-side (9.9 ± 1.7 %) and for different-sides trials (9.8 ± 1.6 %), when the vibrotactile and visual stimuli were incongruent with each other participants made more errors when they were both on the same side of space (30.3 ± 2.8 %) than when they were presented from different sides of space (25.7 ± 2.6 %, see Figure 2). This result is similar to the findings of Spence and colleagues ([29], Appendix), in which an unspeeded version of the elevation discrimination crossmodal congruency task produced a greater number of errors for incongruent trials when the targets and distractors were presented from the same side of space (5.7%) as compared to on different sides (1.1%). In the present analysis, there was also a trend towards a significant interaction between posture and congruency (p = .086), with the effect of congruency being slightly larger in the crossed hands posture.
Figure 2.
The effect of relative location of visual and vibrotactile stimuli on the target-distractor congruency effects (percentage errors). The left half of the chart shows percentage of errors made on congruent trials, the right half shows performance on incongruent trials. Open columns - target and distractor stimuli on the same side of space. Filled columns - target and distractor on different sides of space. The interaction between congruency and side was significant (p = .01).
3.3 Asymmetrical effects of target and distractor number
The analysis of the RT data revealed a significant main effect of the number of distractor stimuli, F(1, 23) = 35.97, p < .001, but no other main effects. RTs to discriminate vibrotactile stimuli were shorter when they were accompanied by a single long visual distractor (771 ± 24 ms), than when accompanied by two short visual distractors (822 ± 25 ms). In addition, there was a significant interaction between the number of target stimuli and the number of distractor stimuli (i.e., the effect of congruency), F(1, 23) = 14.07, p < .001, and a marginal interaction between posture, target number, and distractor number, F(1, 23) = 4.09, p = .055.
The analysis of the error data revealed a significant main effect of distractor number, F(1, 23) = 46.2, p < .001. Again, performance was better when targets were accompanied by a single visual distractor (10.3 ± 1.7 % errors), than when there were two visual distractors (27.5 ± 2.7 %). Overall, the effect of congruency between the number of target and distractor stimuli was higher when there was a single long vibrotactile target (congruency effect of 75 ms and 20.4 % errors), compared to when two short targets were presented (28 ms and 14.0 % errors). This asymmetry: a large influence of two short (discontinuous) distractors on responses to one long (continuous) target, but small influence of one long distractor on responses to two short targets, is consistent with other recent findings from the literature on audiovisual interactions in information processing ([20, 21], though see [1]).
4. Discussion
The primary aims of this experiment were: 1) To demonstrate a non-spatial congruency effect for visual distractors and vibrotactile targets; 2) To assess whether the spatial location of the visual distractors modulated the congruency effects; and 3) To assess whether crossing the hands impaired performance on this non-spatial task, and/or reversed any spatial relationship between responses to visual and tactile stimuli on the same versus on different sides.
First, the results confirmed that there was indeed a large and significant effect of the congruency between the type (single vs. double) of visual distractor and vibrotactile target stimuli (for related multisensory effects, see [20, 21, 29]). This result demonstrates a failure of tactile selective attention, where participants’ responses to vibrotactile stimuli are significantly influenced by random and irrelevant visual distractors. The congruency effects in this non-spatial task were large - incongruent visual distractors slowed responses by around 50 ms and increased the percentage of errors by around 19% with respect to the congruent conditions. Second, this basic congruency effect was modulated by the spatial position of the visual distractor with respect to the vibrotactile target, with distractors on the same side as the target resulting in larger crossmodal congruency effects than distractors on the opposite side. This latter effect was seen only in the analysis of the error data, with a difference between same-side and opposite-sides stimuli of 2.3%.
Third, crossing the hands had two important additional effects: 1) With the hands crossed, RTs were on average 17 ms slower, and participants made 3.6% more errors than when the hands were uncrossed; 2) Crossing the hands did not lead to any change in the main effect of relative sides (i.e., there was no interaction between posture and side). The largest crossmodal congruency effects were always observed for visual and tactile stimuli presented on the same side of space (i.e., in external or allocentric coordinates). This effect can be seen most clearly in the lower panel of Figure 1 as the greater difference between congruent and incongruent conditions for the ‘same-side’ data compared to the ‘different-sides’ data. This result indicates both a spatial and a postural modulation of the multisensory congruency effect even though the task itself did not itself require any kind of explicit or implicit spatial or postural judgement - the largest multisensory interactions ‘followed’ the hands as they crossed the midline.
Following the results of the experiment, we considered possible reasons why crossing the hands impaired performance in the vibrotactile discrimination task. First, we hypothesised that crossing the hands might impair performance in any behavioural task, perhaps because it is less comfortable to maintain a crossed hands posture than a straight-hands posture while at the same time performing demanding speeded discrimination tasks. If this ‘general impairment’ hypothesis were true, then crossing the hands should impair both visual and vibrotactile discrimination performance equally, for example when tested under unimodal discrimination conditions (though see [16], for contradictory evidence). Alternatively, one might hypothesise that, since crossing the hands affects the spatial position of the somatosensory receptors, but not those of the visual receptors, then somatosensory discrimination should be impaired to a greater extent than visual discrimination [23]. This is the ‘somatosensory impairment’ hypothesis. Third, it is at least logically possible (if not very likely) that crossing the hands may not impair somatosensory processing directly, but rather enhance visual processing, thus leading to a greater distracting effect of the irrelevant visual stimuli on vibrotactile performance (the ‘visual enhancement’ hypothesis).
These three possible alternative hypotheses led us to examine the effects of hand-crossing in the same task, but under conditions of unimodal stimulus presentation. In this additional control experiment, 24 new right-handed participants (13 female, 11 male, aged 18-35 years) discriminated both single from double vibrotactile stimuli, and single from double visual stimuli, but with the different sensory modalities presented in different ‘unimodal’ blocks of trials (i.e., no distractors were presented). The participants performed two blocks of trials for each sensory modality, one with the arms uncrossed, and one with arms held crossed over the midline. All between-participant factors were fully counterbalanced, as in the experiment reported above. We found no significant effects of crossing the hands on either vibrotactile (uncrossed, RT = 686 ms, 6.3% errors; crossed, 677 ms, 6.9%) or visual judgements (uncrossed, RT = 668 ms, 7.0% errors; crossed, 678 ms, 5.5%), and no overall RT or error differences between responses to vibrotactile and visual targets (see also Table 2). The only effects of interest related to the type of target (single vs. double) presented. Double targets in both touch (RT = 668 ms, 4.0% errors), and vision (672 ms, 5.0%) were discriminated more efficiently than single targets (touch, 694 ms, 9.2%; vision, 674 ms, 7.5%). This difference resulted in a main effect of target type, and an interaction between target type and modality, since the effects of target type were stronger for tactile targets (both analyses were significant only for the error data). This additional control experiment showed that neither the ‘general’ ‘somatosensory’ or ‘visual’ impairment hypotheses could account for the effects of crossing the hands in the main experiment. The remaining possible explanations for the results are discussed further below.
Table 2.
Mean (± SE) of median RT and percent error data for unimodal tactile and visual judgements.
| Posture | Uncrossed | Crossed | ||||||
|---|---|---|---|---|---|---|---|---|
| Modality | Tactile | Visual | Tactile | Visual | ||||
| Targets | Single | Double | Single | Double | Single | Double | Single | Double |
| Reaction times (ms) | 701(35) | 670(31) | 696(26) | 700(23) | 687(37) | 666(34) | 712(25) | 705(20) |
| Errors (%) | 8.8(1.4) | 3.9(0.7) | 8.1(1.7) | 6.0(1.0) | 9.6(1.6) | 4.2(0.8) | 6.9(1.3) | 4.1(0.7) |
To our knowledge, the results of the present study provide the first empirical evidence that visual-tactile space (or perhaps just the position of the individual vibrotactile stimuli) is updated (or ‘remapped’) during a non-spatial tactile discrimination task performed with the hands crossed over the midline in the presence of visual distractors. The magnitude of the spatial effects reported here are smaller than those typically found in spatial tasks. For example, in the elevation discrimination version of the crossmodal congruency task, bimodal stimuli presented on the same side typically result in congruency effects approximately 50 ms larger than for visual and tactile stimuli presented on opposite sides [6, 12, 29]. The present results therefore differ from these previous data (and from those concerning multisensory spatial cueing, e.g., [8, 9]) by examining spatial modulations of a non-spatial tactile discrimination response with simultaneous visual distractors, and show that smaller, but nevertheless still significant, spatial modulations occur.
The absence of any detrimental effects of crossing the hands in the unimodal control task suggests either: 1) That only when visual and tactile stimuli are presented together do any crossed hands deficits emerge, or; 2) That crossed hands effects only arise with difficult tactile discrimination tasks. With regard to the latter possibility, note that the error rate in the multisensory experiment was much higher in the incongruent conditions (22-32%), compared both to the congruent conditions in that experiment (6-12%), and to all conditions in the unimodal control experiment (4-10%). Indeed, the crossed hands effects themselves seem to be due almost entirely to changes in the performance on the incongruent, rather than in the congruent conditions (see Figure 1).
The possibility that crossed hands effects in the tactile modality only occur in the presence of incongruent visual distractors is an interesting one, and is worthy of further research (see also the discussion of this point in ref [24]). Perhaps it is only in the presence of additional visual stimulation in close temporal sequence that tactile crossed hands effects may arise for non-spatial discrimination tasks, suggesting that crossed hands impairments may be due to the conflict between external, allocentric, and internal, somatotopic coordinates. This conflict would only arise in the presence of near-simultaneous stimulation in both sensory modalities, thus forcing the brain to use both allocentric and somatotopic reference frames to code the relative locations of the stimuli - even if the locations themselves were not relevant to the task (see also ref [2]). Such an automatic mechanism comparing visual and tactile spatial locations might be useful for visually locating sudden, transient events on the body surface (such as, for example, mosquito bites!). In addition, the present results are in part compatible with the idea that, in order to identify (or become conscious of) a tactile stimulus, it must first be processed spatially [8, 37], suggesting that “what” and “where” tactile processing streams, rather than being independent, may interact substantially (for recent neuroimaging data, see [15, 17]). However, this ‘space-first’ view can only be supported for explicitly spatial tactile judgements, or for tactile judgements performed in the presence of visual distractors. The present results show that crossing the hands did not impair non-spatial tactile numerosity (single vs. double) judgments. In the case of non-spatial judgments, processing the location of the tactile targets in an external frame of reference either did not occur, or occurred but had very little effect on the performance of the tactile discrimination task.
In summary, the present experiment has provided the first empirical evidence that spatial modulations of crossmodal congruency effects occur in a non-spatial tactile discrimination task in the presence of visual distractors, and that these spatial modulations are unaffected by the posture of the hands. We conclude that the largest multisensory interactions occur in external (allocentric) rather than internal (somatotopic) coordinates, and follow the hands as they cross the midline.
References
- [1].Andersen TS, Tiippana K, Sams M. Factors influencing audiovisual fission and fusion illusions, Cogn. Brain Res. 2004;21:301–308. doi: 10.1016/j.cogbrainres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [2].Axelrod S, Thompson LW, Cohen LD. Effects of senescence on the temporal resolution of somesthetic stimuli presented to one hand or both. J. Gerontol. 1968;23:191–195. doi: 10.1093/geronj/23.2.191. [DOI] [PubMed] [Google Scholar]
- [3].Drew F. Attention: Experimental and critical. Am. J. Psychol. 1896;7:533–573. [Google Scholar]
- [4].Driver J, Grossenbacher PG. Multimodal spatial constraints on tactile selective attention. In: Inui T, McClelland JL, editors. Attention and Performance, XVI. MIT Press; Cambridge, MA: 1996. pp. 209–235. [Google Scholar]
- [5].Farrell WSJ. Coding left and right. J. Exp. Psychol. Human Percept. Perform. 1979;5:42–51. doi: 10.1037//0096-1523.5.1.42. [DOI] [PubMed] [Google Scholar]
- [6].Holmes NP, Calvert GA, Spence C. Extending or projecting peripersonal space with tools? Multisensory interactions highlight only the distal and proximal ends of tools. Neurosci. Lett. 2004;372:62–67. doi: 10.1016/j.neulet.2004.09.024. [DOI] [PubMed] [Google Scholar]
- [7].Hommel B. Inverting the Simon effect by intention: Determinants of direction and extent of effects of irrelevant spatial information. Psychol. Res. 1993;55:270–279. [Google Scholar]
- [8].Kennett S, Eimer M, Spence C, Driver J. Tactile-visual links in exogenous spatial attention under different postures: Convergent evidence from psychophysics and ERPs. J. Cogn. Neurosci. 2001;13:462–478. doi: 10.1162/08989290152001899. [DOI] [PubMed] [Google Scholar]
- [9].Kennett S, Spence C, Driver J. Visuo-tactile links in covert exogenous spatial attention remap across changes in unseen hand posture. Percept. Psychophys. 2002;64:1083–1094. doi: 10.3758/bf03194758. [DOI] [PubMed] [Google Scholar]
- [10].Kitazawa S. Where conscious sensation takes place. Consciousness Cognition. 2002;11:475–477. doi: 10.1016/s1053-8100(02)00031-4. [DOI] [PubMed] [Google Scholar]
- [11].Lloyd DM, Merat N, McGlone FP, Spence C. Crossmodal links between audition and touch in covert endogenous spatial attention. Percept. Psychophys. 2003;65:901–924. doi: 10.3758/bf03194823. [DOI] [PubMed] [Google Scholar]
- [12].Maravita A, Spence C, Kennett S, Driver J. Tool-use changes multimodal spatial interactions between vision and touch in normal humans. Cognition. 2002;83:25–34. doi: 10.1016/s0010-0277(02)00003-3. [DOI] [PubMed] [Google Scholar]
- [13].Marks LE. Cross-modal interactions in speeded classification. In: Calvert G, Spence C, Stein BE, editors. The handbook of multisensory processes. MIT Press; Cambridge, MA: 2004. pp. 85–105. [Google Scholar]
- [14].McDonald JJ, Ward LM. Spatial relevance determines facilitatory and inhibitory effects of auditory covert spatial orienting. J. Exp. Psychol. Human Percept. Perform. 1999;25:1234–1252. [Google Scholar]
- [15].Newell FN, Hansen PC, Steven MS, Bülthoff HH, Calvert GA. An fMRI investigation of visual, tactile and visuo-tactile “what” and “where” dissociations. International Multisensory Research Forum; Sitges, Spain. June 5-8, 2004; Paper presented at the. [Google Scholar]
- [16].Nicoletti R, Umiltà C, Làdavas E. Compatibility due to the coding of the relative position of the effectors. Acta Psychologica. 1984;57:133–143. doi: 10.1016/0001-6918(84)90039-8. [DOI] [PubMed] [Google Scholar]
- [17].Reed CL, Klatzky RL, Halgren E. What vs. where in touch. An fMRI study; NeuroImage in press. [DOI] [PubMed] [Google Scholar]
- [18].Riggio L, Gawryszewski L, Umiltà C. What is crossed in crossed-hand effects. Acta Psychologica. 1986;62:89–100. [Google Scholar]
- [19].Röder B, Rösler F, Spence C. Early vision impairs tactile perception in the blind. Curr. Biol. 2004;14:121–124. [PubMed] [Google Scholar]
- [20].Shams L, Kamitani Y, Shimojo S. Illusions: What you see is what you hear. Nature. 2000;408:788. doi: 10.1038/35048669. [DOI] [PubMed] [Google Scholar]
- [21].Shams L, Kamitani Y, Shimojo S. Visual illusion induced by sound. Cogn. Brain Res. 2002;14:147–152. doi: 10.1016/s0926-6410(02)00069-1. [DOI] [PubMed] [Google Scholar]
- [22].Shore DI, Barnes, Spence C. The temporal evolution of crossmodal congruency effects
- [23].Shore DI, Spry E, Spence C. Confusing the mind by crossing the hands. Cogn. Brain Res. 2002;14:153–163. doi: 10.1016/s0926-6410(02)00070-8. [DOI] [PubMed] [Google Scholar]
- [24].Spence C, Baddeley R, Zampini M, James R, Shore DI. Multisensory temporal order judgments: When two locations are better than one. Percept. Psychophys. 2003;65:318–328. doi: 10.3758/bf03194803. [DOI] [PubMed] [Google Scholar]
- [25].Spence C, Driver J. Covert spatial orienting in audition: Exogenous and endogenous mechanisms facilitate sound localization. J. Exp. Psychol. Human Percept. Perform. 1994;20:555–574. [Google Scholar]
- [26].Spence C, McDonald JJ, Driver J. Exogenous spatial-cuing studies of human cross-modal attention and multisensory integration. In: Spence C, Driver J, editors. Crossmodal space and crossmodal attention. Oxford University Press; Oxford: 2004. pp. 277–320. [Google Scholar]
- [27].Spence C, McGlone FP. Reflexive spatial orienting of tactile attention. Exp. Brain Res. 2001;141:324–330. doi: 10.1007/s002210100883. [DOI] [PubMed] [Google Scholar]
- [28].Spence C, Pavani F, Driver J. Crossmodal links between vision and touch in covert endogenous spatial attention. J. Exp. Psychol. Human Percept. Perform. 2000;26:1298–1319. doi: 10.1037//0096-1523.26.4.1298. [DOI] [PubMed] [Google Scholar]
- [29].Spence C, Pavani F, Driver J. Spatial constraints on visual-tactile cross-modal distractor congruency effects. Cogn. Affective Behav. Neurosci. 2004;4:148–169. doi: 10.3758/cabn.4.2.148. [DOI] [PubMed] [Google Scholar]
- [30].Townsend JT, Ashby FG. Stochastic Modelling of Elementary Psychological Processes. Cambridge University Press; New York: 1983. [Google Scholar]
- [31].Wada M, Yamamoto S, Kitazawa S. Effects of handedness on tactile temporal order judgment. Neuropsychologia. 2004;42:1887–1895. doi: 10.1016/j.neuropsychologia.2004.05.009. [DOI] [PubMed] [Google Scholar]
- [32].Wallace RJ. S-R compatibility and the idea of a response code. J. Exp. Psychol. 1971;88:354–360. doi: 10.1037/h0030892. [DOI] [PubMed] [Google Scholar]
- [33].Wallace RJ. Spatial S-R compatibility effects involving kinaesthetic cues. J. Exp. Psychol. 1972;93:163–168. doi: 10.1037/h0032462. [DOI] [PubMed] [Google Scholar]
- [34].Yamamoto S, Kitazawa S. Reversal of subjective temporal order due to arm crossing. Nat. Neurosci. 2001;4:759–765. doi: 10.1038/89559. [DOI] [PubMed] [Google Scholar]
- [35].Yamamoto S, Kitazawa S. Sensation at the tips of invisible tools. Nat. Neurosci. 2001;4:979–980. doi: 10.1038/nn721. [DOI] [PubMed] [Google Scholar]
- [36].Yamamoto S, Moizumi S, Kitazawa S. Referral of tactile sensation to the tips of L-shaped sticks. J. Neurophysiol. doi: 10.1152/jn.01015.2004. In press. [DOI] [PubMed] [Google Scholar]
- [37].Zampini M, Spence C. The effect of posture change on tactile perception: Impaired direction discrimination performance with interleaved fingers. Exp. Brain Res. doi: 10.1007/s00221-005-2390-y. (in press) [DOI] [PubMed] [Google Scholar]