Abstract
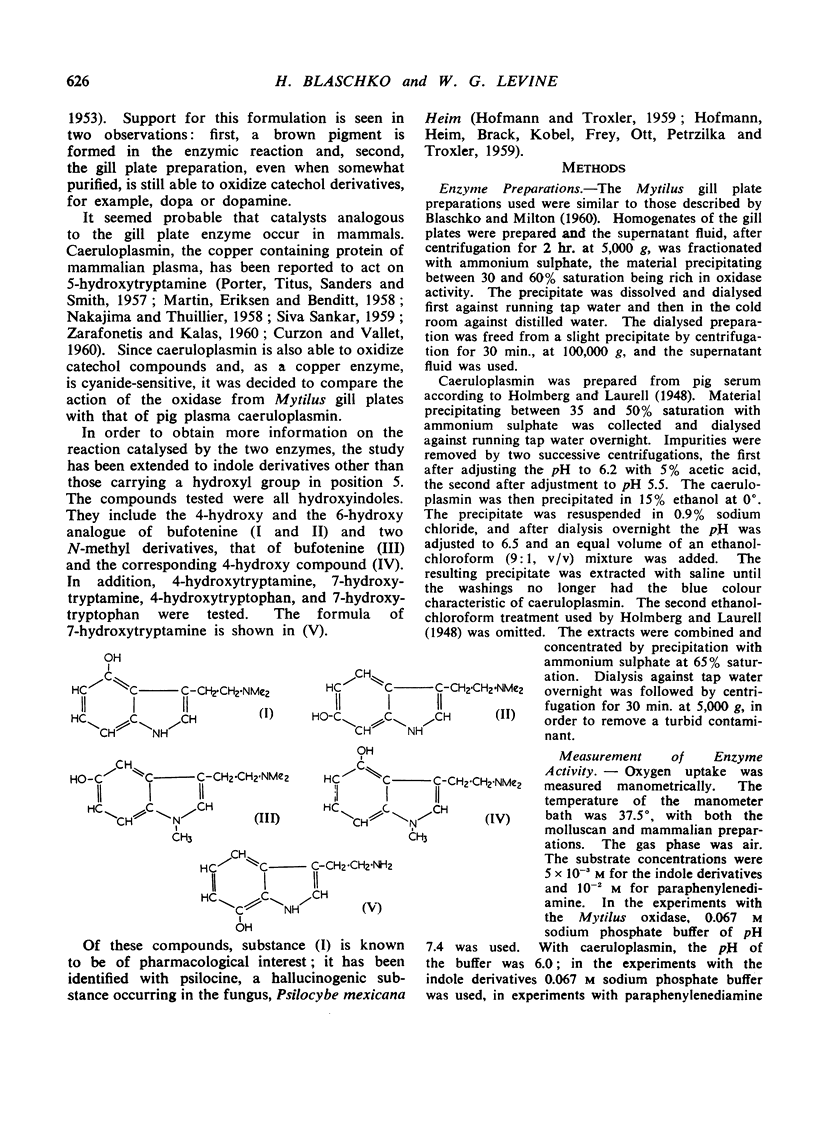
A comparative study has been carried out of the oxidation of 5-hydroxytryptamine and related compounds by the oxidase present in the gill plates of Mytilus edulis and of caeruloplasmin, the copper containing oxidase of mammalian plasma. Both preparations oxidized indole derivatives carrying a hydroxyl group in the 4-, 5-, 6-, or 7- position. The oxidation of bufoteni ne was compared with that of its 4- and 6-hydroxy analogues; the 4-hydroxy analogue is psil ocine, a naturally occurring hallucinogenic compound. Bufotenine and the 6-hydroxy analogue were oxidized by both preparations with the formation of brown pigments; psilocine was more rapidly oxidized with the appearance of a blue colour. 4-Hydroxytryptamine and 7-hydroxytryptamine were also oxidized, the former with the formation of a blue compound. The N-1-methyl derivatives of both bufotenine and psilocine were also oxidized. The Mytilus preparation acted also on 4-, 5-, and 7-hydroxytryptophan and on 5-hydroxyindole, none of which was oxidized by caeruloplasmin. The Mytilus enzyme also oxidized 5-hydroxyindoleacetic acid. Paraphenylenediamine, a very good substrate of caeruloplasmin, was much more slowly oxidized by the gill plate enzyme. The evidence suggests that the two enzymes catalyse the same reactions, but that the substrate specificity of the mammalian oxidase is somewhat more restricted. Both enzymes are “hydroxyindole oxidases,” not specific for 5-hydroxyindoles alone. Inhibitors of the Mytilus oxidase included inhibitors of copper enzymes but not edetate or carbon monoxide. The action of pig serum on 5-hydroxytryptamine was due to caeruloplasmin and not to amine oxidase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLASCHKO H., FRIEDMAN P. J., HAWES R., NILSSON K. The amine oxidases of mammalian plasma. J Physiol. 1959 Mar 3;145(2):384–404. doi: 10.1113/jphysiol.1959.sp006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
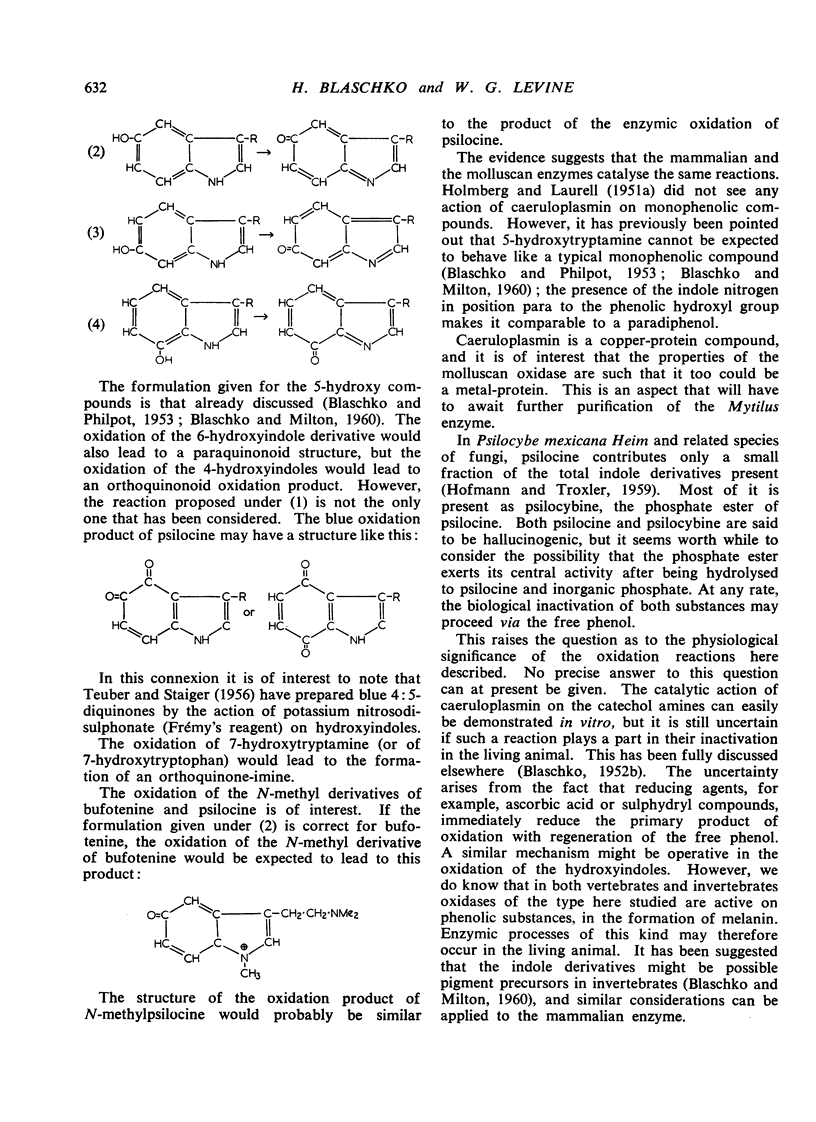
- BLASCHKO H., PHILPOT F. J. Enzymic oxidation of tryptamine derivatives. J Physiol. 1953 Nov 28;122(2):403–408. doi: 10.1113/jphysiol.1953.sp005010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko H. Amine oxidase in Sepia officinalis. J Physiol. 1941 Mar 25;99(3):364–369. doi: 10.1113/jphysiol.1941.sp003908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko H., Richter D., Schlossmann H. The oxidation of adrenaline and other amines. Biochem J. 1937 Dec;31(12):2187–2196. doi: 10.1042/bj0312187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURZON G. The excretion of indoles in argentaffinoma. Arch Biochem Biophys. 1957 Feb;66(2):497–499. doi: 10.1016/s0003-9861(57)80027-7. [DOI] [PubMed] [Google Scholar]
- CURZON G., VALLET L. The purification of human caeruloplasmin. Biochem J. 1960 Feb;74:279–287. doi: 10.1042/bj0740279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELLER E., EIDUSON S., YUWILER A. Oxidation of p-phenylenediamine and adrenaline in enzymic and copper-catalysed reactions. J Neurochem. 1959 Dec;5:73–79. doi: 10.1111/j.1471-4159.1959.tb13335.x. [DOI] [PubMed] [Google Scholar]
- McISAAC W. M., PAGE I. H. The metabolism of serotonin (5-hydroxytryptamine). J Biol Chem. 1959 Apr;234(4):858–864. [PubMed] [Google Scholar]
- NAKJIMA H., THUILLIER J. Inactivation de la sérotonine par la céruloplasmine. C R Seances Soc Biol Fil. 1958;152(2):270–272. [PubMed] [Google Scholar]
- PORTER C. C., TITUS D. C., SANDERS B. E., SMITH E. V. Oxidation of serotonin in the presence of ceruloplasmin. Science. 1957 Nov 15;126(3281):1014–1015. doi: 10.1126/science.126.3281.1014. [DOI] [PubMed] [Google Scholar]
- ZARAFONETIS C. J., KALAS J. P. Serotonin degradation by ceruloplasmin and its inhibition by isoniazid and iproniazid. Am J Med Sci. 1960 Feb;239:203–206. doi: 10.1097/00000441-196002000-00014. [DOI] [PubMed] [Google Scholar]



