Abstract
Background
The amyloid-β (Aβ) peptide has a central role in the neurodegeneration of Alzheimer disease (AD). Immunization of AD transgenic mice with Aβ1–42 (Aβ42) peptide reduces both the spatial memory impairments and AD-like neuropathologic changes in these mice. Therapeutic immunization with Aβ in patients with AD was shown to be effective in reducing Aβ deposition, but studies were discontinued owing to the development of an autoimmune, cell-mediated meningoencephalitis. We hypothesized that gene vaccination could be used to generate an immune response to Aβ42 that produced antibody response but avoided an adverse cell-mediated immune effect.
Objective
To develop an effective genetic immunization approach for treatment and prevention of AD without causing an autoimmune, cell-mediated meningoencephalitis.
Methods
Mice were vaccinated with a plasmid that encodes Aβ42, administered by gene gun. The immune response of the mice to Aβ42 was monitored by measurement of (1) antibody levels by enzyme-linked immunosorbent assay (ELISA) and Western blot and (2) Aβ42-specific T-cell response as measured by interferon-γ enzyme-linked immunospot (ELISPOT) assay.
Results
Gene-gun delivery of the mouse Aβ42 dimer gene induced significant humoral immune responses in BALB/c wild-type mice after 3 vaccinations in 10-day intervals. All 3 mice in the treated group showed significant humoral immune responses. The ELISPOT assay for interferon-γ release with mouse Aβ42 peptide and Aβ9–18 showed no evident cytotoxic T-lymphocyte response. We further tested the responses of wild-type BALB/c mice to the monomer Aβ42 gene vaccine. Western blot evaluation showed both human and mouse Aβ monomer gene vaccine elicited detectable humoral immune responses. We also introduced the human Aβ42 monomer gene vaccine into AD double transgenic mice APPswe/PSEN1(A246E). Mice were vaccinated with plasmids that encode Aβ1–42 and Aβ1–16, or with plasmid without the Aβgene. Treated mice showed significant humoral immune responses as demonstrated by ELISA and by Western blot. These mice also showed no significant cellular immune response as tested by ELISPOT. One of the treated mice was killed at 7 months of age for histological observations, and scattered amyloid plaques were noted in all layers of the cerebral cortex and in the hippocampus in both Aβ42- and control-vaccinated mice. No definite difference was discerned between the experimental and control animals.
Conclusions
Gene-gun–administered genetic immunization with the Aβ42 gene in wild-type BALB/c and AD transgenic mice can effectively elicit humoral immune responses without a significant T-cell–mediated immune response to the Aβ peptide. This immunotherapeutic approach could provide an alternative active immunization method for therapy and prevention of AD.
INTRODUCTION
Alzheimer disease (AD) is a progressive neurodegenerative disease defined pathologically by extracellular neuritic plaques and intraneuronal neurofibrillary tangles. The fibrillar neuritic plaques comprise deposits of amyloid-β (Aβ) protein and the tangles consist of helical filaments of hyperphosphorylated tau protein.1–2 A large body of data from autosomal dominant, early-onset AD research strongly support the pathogenetic basis of the disease as the amyloid cascade, which states that the neurodegeneration of AD is primarily initiated by the formation of neurotoxic Aβ-amyloid aggregates.3–7
Current treatments for AD are largely symptomatic. No therapies have been clinically proven to be able to slow or to prevent the progression of AD. The Aβ deposition and aggregation is an early event in AD neuropathology, suggesting the hypothesis that Aβ gene vaccination would be a promising solution to reverse and prevent progressive neuropathologic change by stimulating the host immune system to recognize and target Aβ, thereby clearing and/or preventing the deposition of Aβ plaques in brain. In fact, active immunization with synthetic Aβ (1–42) has been shown to be effective in a mouse model7–9 to reduce significantly brain Aβ burden and further was the impetus to proceed to clinical trials in patients with AD.
Amyloid-β42 immunization in the AD transgenic (Tg) mouse elicited specific Aβ42 antibodies and these antibodies move across the blood-brain barrier resulting in the removal of amyloid plaques and the reduction of Aβ burden accompanied by improved cognitive performance.8 In clinical trials, about 300 patients with AD received multiple doses of the Aβ42 peptide with the adjuvant QS21. The program was discontinued after the signs of aseptic meningoencephalitis developed in about 6% of the treated patients. The follow-up in patients showed 60% produced antibodies against amyloid-containing plaques and patients with higher antibody titer (20%) had a slowing of the progression of cognitive loss.10–11
From preclinical Tg mouse studies and clinical trials of patients with AD, it has been concluded that active immunization with Aβ42 is effective in both reducing brain amyloid burden and in slowing cognitive loss.7, 10–11 However, an alternative vaccination method must be developed to avoid the development of autoimmune, cell-mediated meningoencephalitis. Therefore, we have developed a new vaccine strategy, gene vaccination, using a chemically synthesized gene for Aβ as the immunizing agent. We demonstrate that gene vaccination can efficiently elicit humoral immune responses without significantly activating cytotoxic T cells responsible for the autoimmune, cell-mediated meningoencephalitis that develops in patients with AD immunized with Aβ peptide.12
METHODS
PLASMID CONSTRUCTS AND PREPARATION
Both mouse and human Aβ1–42 genes were chemically synthesized with the codons optimized for expression in mammalian cells and cloned into an immunization vector system under the control of the synthetic promoter SP72.13 The Aβ42 was fused to an α-antitrypsin secretory signal upstream and a major histocompatibility complex II–targeting sequence downstream to elicit a better humoral immune response.14 The base plasmid and the Aβ42 genes are shown in Figure 1. The plasmid was amplified in DH5∂ cells and purified using a plasmid preparation kit (Gen Elute HP Plasmid Preparation Kit; Sigma-Aldrich Inc, St Louis, Mo). The presence of the Aβ genes was confirmed by sequencing.
Figure 1.
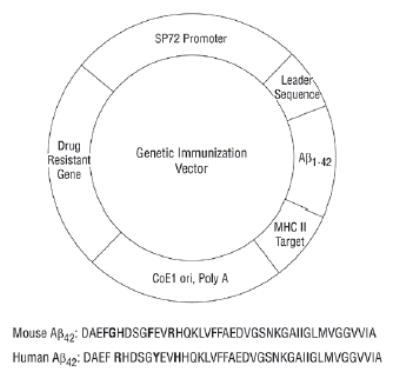
Gene immunization vector contains SP72, a synthetic mammalian expression promoter; an Aβ42 gene sequence fused between a human α-antitrypsin secretory signal and a major histocompatibility complex class (MHC) II–targeting peptide sequence, and the ampicillin-resistance gene, Aβ1–42 monomer or dimer gene, followed by an MHC II–targeting sequence.
Aβ GENE VACCINATION
The Aβ gene vaccination was delivered by the gene-gun ballistic bombardment method.15 Briefly, DNA-coated gold particles were prepared by combining 60 mg of 1- to 2-μm gold beads (Bio-Rad, Hercules, Calif) and 100 μg of DNA in 200-μL volume, and then 100 μL of 0.05M spermidine and 100 μL of 2.5M calcium chloride were added sequentially to the beads. The beads were then centrifuged, washed with alcohol, and finally suspended in 3 mL of alcohol. The solution was loaded into tubing and the alcohol was removed and the beads were evenly attached to the sides of the tubing. The tube was dried with nitrogen gas and the tube was cut into 1.3-cm sections. Each section contained 2 μg of plasmid DNA and 1.2 mg of gold beads. The DNA-coated gold particles (2 μg of DNA per shot) were delivered to both sides of the ears (4 shots) of mice using a helium-driven gene gun (Bio-Rad) at a discharge pressure of 400 lb/sq in. Mice vaccinated with no-Aβ-insert construct–coated gold particles were used as controls. The mice were boosted at 2-week intervals to a maximum of 6 times, blood was drawn from the tail vein every 2 weeks, and the serum samples were used for monitoring the humoral immune response.
IMMUNOASSAYS
Enzyme-linked immunosorbent assay (ELISA) and Western blots were used to monitor the humoral immune responses.10, 15 In brief, mouse blood was drawn from tail vein and serum was used to detect Aβ peptide by ELISA with a 96-microwell plate coated with glutathione S-transferase–Aβ proteins. For Western blot, the glutathione S-transferase–Aβ proteins in bacteria extract were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, blotted on nitrocellulose incubated with mouse immune serum samples at a 1:2000 dilution. Antibodies against Aβ were detected using peroxidase-conjugated, affinity-purified rabbit antiserum against mouse IgG. The cell-mediated immune response was monitored by enzyme-linked immunospot (ELISPOT) assays for detection of peripheral blood T cells releasing interferon-γ during in vitro restimulation with Aβ peptide.16 Briefly, 96-well polyvinylidine difluoride microplates (Millipore, Bedford, Mass) were coated with antibody to interferon-γ. Cells were cultured at 2×105 per well in 0.2 mL of medium for restimulation with Aβ peptides. After incubation for 36 hours at 37°C, the microplates were washed, incubated with biotinylated anti-mouse interferon-γ, and with streptavidin–AP conjugate. After 3 washes, spots were developed with 1-step nitroblue tetrazolium/5-bromo-4-chloro-3-indolyphosphate reagent. Spots were counted using a stereomicroscope.
IMMUNIZATION IN WILD-TYPE MICE
The BALB/c mice were immunized with a plasmid DNA encoding the mouse or human Aβ42 gene delivered with a gene gun to the ear skin.15 Cellular and humoral immune responses were monitored with ELISA, Western blot, and ELISPOT. The mouse granulocyte-monocyte colony-stimulating factor gene was codelivered to further stimulate the immune system in 1 group of mice. The goal is to confirm that Aβ42 gene vaccination in mice is effective in breaking self-tolerance resulting in production of antibodies against both mouse and human Aβ42. All mice were immunized at 10- to 14-day intervals for 3 immunizations and then at 4-week intervals for up to 6 immunizations.
VACCINATION IN Tg AD MICE
Human amyloid precursor protein/presenilin 1 (APPswe/PSEN1[A246E]) double Tg mice17 were purchased from the Jackson Research Laboratory, Bar Harbor, Me. This Tg mouse strain expresses high concentrations of the mutant amyloid precursor protein, develops a significant amyloid plaque burden, and displays memory deficits. Based on the immunization data obtained using the wild-type mice, we focused on the preventive effects of Aβgene vaccination in the Tg AD mice. Experimental mice were vaccinated with human Aβ42 gene beginning at 2 months of age. Control mice received the vector lacking the Aβ42 insert. Immune responses were monitored and the mouse brain tissue was subjected to histological observation for the deposition of amyloid plaques.18
HISTOLOGICAL STAINING AND EXAMINATION
Five months after immunization, following induction of deep anesthesia with intraperitoneal injection of avertin, 1 genetically immunized and 1 control immunized mouse were perfused transcardially with a heparinized saline solution and then 4% paraformaldehyde in 0.1M Sorenson phosphate buffer (pH 7.4). Brains were removed and, after excision of frontal poles for freezing after cryoprotection in glycerol/dimethyl sulfoxide, fixed overnight in 4% paraformaldehyde, and embedded in paraffin. Sections were stained with hematoxylin-eosin and evaluated immunohistochemically for human Aβ (Signet Laboratories Inc, Dedham, Mass) and glial fibrillary acidic protein (DAKO, Copenhagen, Demark) immunohistochemistry.18–19
RESULTS
Aβ42 CLONING
Mouse and human Aβ42 dimer gene, monomer gene, and Aβ1–16-encoded DNA sequences were synthesized chemically with the optimal codons and successfully cloned into a genetic immunization vector. The Aβ gene was fused to the human α-antitrypsin secretory signal upstream and major histocompatibility complex II–targeting sequence downstream. This vector system was used in all of those mice that participated in the vaccination experiment. SP72, a synthetic mammalian expression promoter, was used to drive the Aβ gene expressions. The Aβ42 dimer, Aβ1–16, Aβ16–28, and Aβ28–42 were also cloned into a bacteria expression vector to express glutathione S-transferase–fused Aβ proteins. All constructs were sequenced and confirmed to be in the correct frame.
IMMUNE RESPONSE TO Aβ42 DIMER GENE IN WILD-TYPE BALB/c MICE
Initially, we designed and chemically synthesized a mouse Aβ42 dimer gene because aggregated Aβ peptide is thought to be the major neurotoxic form.1 Gene-gun delivery of the mouse Aβ42 dimer gene induced significant humoral immune responses in BALB/c wild-type mice after 3 vaccinations in 10-day intervals. All 3 mice in the treated group showed significant humoral immune responses as measured by ELISA and by Western blot. The antibody titers specific for Aβ42 were from 1:4000 to 1:10 000. The ELISPOT assay for interferon-γ release with mouse Aβ42 peptide and Aβ9–18 mixture showed no evident cytotoxic T-lymphocyte response (Figure 2).
Figure 2.
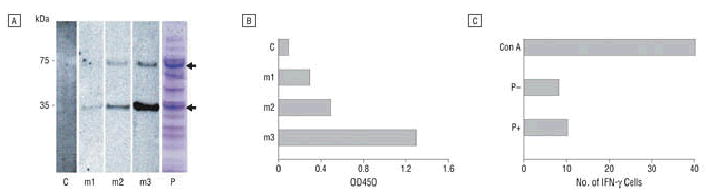
Amyloid-β42 (Aβ42)–specific immune responses in BALB/c wild-type mice immunized with mouse Aβ42 dimer gene vaccine. A, Western blot shows that all 3 mice in the vaccinated group produced specific anti-Aβ42 antibodies that detected mouse recombinant glutathione S-transferase–Aβ42 (GST-Aβ42) dimers (lower arrow in lane P) and tetramers (upper arrow in lane P) (lanes m1, m2, m3). Mice in the control group (vector lacking Aβ gene) were negative (lane C). Recombinant GST-Aβ42 protein in Escherichia coli extracts was shown in lane P stained with Coomassie blue. Serum samples were obtained 14 days after a third vaccination at 10-day intervals with dilution of 1:2000. kDa indicates kilodalton. B, Same serum samples as in A were tested by enzyme-linked immunosorbent assay for Aβ42 peptide and show that all 3 mice (m1, m2, m3) vaccinated with Aβ42 dimer gene showed specific antibody against human Aβ42 peptide with 1:2000 dilution. C, Enzyme-linked immunospot assay demonstrated that no detectable cellular immune response was observed in Aβ42 dimer gene–vaccinated mice. Concanavalin A (Con A) was added as a positive control. P–indicates no peptide added in peripheral blood T-cell culture; P+, a mixture of Aβ9–18 and Aβ1–42 peptide was added to the culture for 36 hours for specific antigen stimulation for T cells to release interferon-γ (INF-γ).
We further tested the responses of wild-type BALB/c mice to the monomer gene of Aβ42 by gene-gun vaccination. Western blot showed both human and mouse Aβ monomer gene vaccine elicited detectable humoral immune responses at a dilution of 1:2000 after 3 separate immunizations although a weaker response was seen for mouse Aβ gene vaccine in a short vaccine schedule. A longer time schedule of mouse Aβ42 gene immunization with a gene gun with 6 vaccinations did break tolerance and resulted in similar antibody production for both human and mouse Aβ42. Coshooting with granulocyte-macrophage colony-stimulating factor aided in breaking tolerance to mouse Aβ42 in BALB/c wild-type mice (Figure 3).
Figure 3.
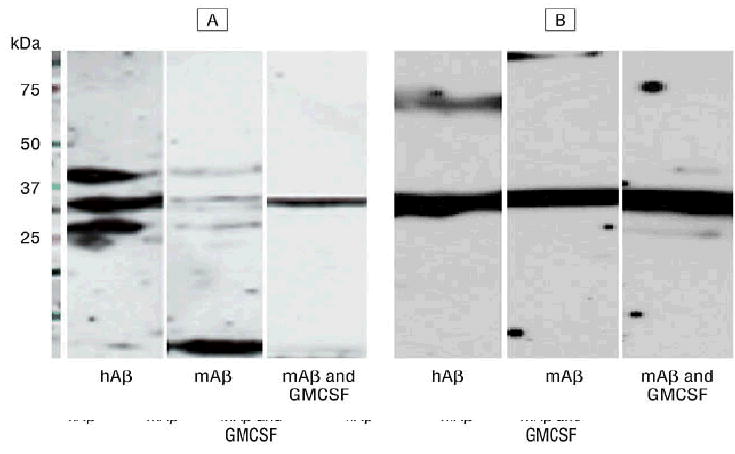
Western blot to show humoral immune response of BALB/c wild-type mice against monomer Aβ42. Mice were immunized with monomer human (amyloid-β [hAβ] protein) and mouse (mAβ) Aβ1–42 gene vaccine by gene gun. Human Aβ induced a higher immune response compared with mAβ in these mice in short schedule (3 immunizations in a 10-day interval and blood was drawn 2 weeks after the third shot) immunizations (panel A), but responses were similar with a long-schedule vaccination (additional 3 shots in a 1-month interval and blood was drawn 2 weeks after the last shot) (panel B). Administration of granulocyte-monocyte colony-stimulating factor (GMSF) (performed with mAβ42 immunizations) seemed to enhance antibody production. This result demonstrated that genetic vaccination can break the self-tolerance in mice for mAβ42 protein and also elicit strong immune responses to hAβ42.
IMMUNE RESPONSE IN APPswe/PSEN1(A246E) Tg MICE
On the basis of data obtained in wild-type BALB/c mice, we introduced the human Aβ42 monomer gene vaccine into double Tg mice APPswe/PSEN1(A246E). These mice begin to develop amyloid plaques at about 6 months of age and reached significant levels of accumulation by 10 to 11 months. We purchased 10 Tg mice and divided them into treated and control groups. Two treated mice died and 1 control mouse died at about 4 months of age. Therefore, 3 treated mice were codelivered plasmids encoding the human Aβ1–42 gene and Aβ1–16 gene constructs and 4 control mice received DNA without the Aβ gene. As shown in Figure 4, among 3 treated mice, 2 showed significant humoral immune responses as demonstrated by ELISA and by Western blot. The antibody titer against Aβ1–16 in one treated mouse was estimated at 1:10 000, and 1:5000 for Aβ17–28 and Aβ29–42 peptides and in the second treated mouse at 1:4000 against Aβ1–6 and 1:2000 against Aβ17–28 and Aβ29–42. The result of ELISA and Western blots showed similar conclusions. The third treated mouse was killed at 7 months of age for histological observations. It showed no detectable immune response against Aβ42. These mice also showed no significant cellular immune responses as tested by ELISPOT stimulated with Aβ1–42 and Aβ9–18 synthetic peptide although a slightly higher number of interferon-positive T cells was observed. No specific immune responses were seen in the control mice against Aβ1–42.
Figure 4.
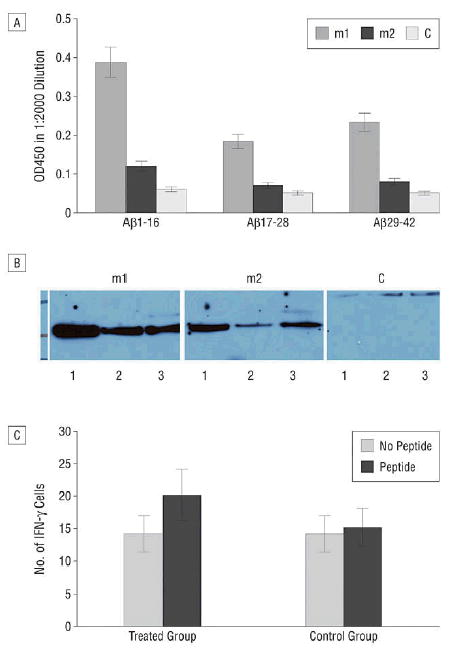
Human amyloid-β 42 (Aβ 42)–specific immune responses in transgenic (Tg) mice immunized with human Aβ 42 gene vaccine. A, Anti-Aβ peptide antibody titer assayed by enzyme-linked immunosorbent assay in Tg mice immunized with both Aβ 1–42 and Aβ 1–16 gene construct for 4 times in 2-week intervals. The serum sample was obtained 2 weeks after the last immunization and titers were tested against Aβ peptide 1–16, 17–28, 29–42 fused to glutathione S-transferase (GST) protein produced in Escherichia coli. A higher response against Aβ 1–16 was seen. B, The same serum sample tested with Western blot shows a similar result; both mouse 1 (m1) and 2 (m2) in the treated group show a higher response against Aβ peptide. A higher titer is achieved for the m1 than for the m2. Control mice are negative for antibodies. Lane 1; Aβ 1–16, lane 2; Aβ 17–28, lane 3; Aβ 29–42 fused to GST was loaded and probed with serum in a 1:2000 dilution. The third treated mouse showed no detectable humoral response (data not shown). C, Enzyme-linked immunospot assay shows that no significant cellular immune response was observed in human Aβ 42 gene–vaccinated Tg mice. Peripheral blood T cells were pooled from the vaccinated and control groups of mice and the cells were cultured in quadruplicate (2 × 105 cells per well in a 96-well microplate format) in the presence of peptide or absence of peptide using a mixture of Aβ 1–42 and Aβ 9–18 peptide at 10 μg/mL for 36 hours and further processed for detection of released interferon-γ.
HISTOLOGICAL AND IMMUNOSTAINING OF BRAIN TISSUES
Five months after immunization, scattered amyloid plaques were noted in all layers of the cerebral cortex and in the hippocampus in both Aβ 42- (Figure 5A and B) and control- (not shown) vaccinated mice as evaluated by both hematoxylineosin stain and amyloid-β immunoperoxidase preparations. No definite difference in the number of plaques was discerned between the experimental and control animals. A brisk astrocytic response was noted in association with plaques in all mice, identified by glial fibrillary acid protein immunohistochemistry (Figure 5C). By hematoxylineosin assessment, no evidence of lymphocytic inflammation or evidence of prominent macrophage response was noted in either experimental or control mice.
Figure 5.
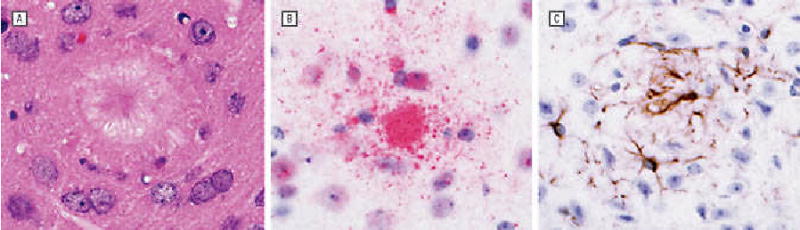
Preliminary examination of brains of transgenic (Tg) mice 5 months after immunization. Mice were killed 5 months after immunization. Brains were fixed in paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylineosin (A) and evaluated by immunohistochemistry for amyloid- β (Aβ ) (B) and glial fibrillary acid protein (C). Scattered amyloid plaques were noted diffusely in the brains in both the control- and Aβ gene vaccine–immunized mice without evidence of lymphocytic inflammation. A brisk astrocytic response to plaque deposition was identified. Analysis of older animals is necessary to assess for differences between the DNA-vaccinated and control vector–vaccinated mice for amyloid deposition and other parameters.
COMMENT
As the major brain amyloid plaque component is Aβ 1–40 and Aβ 1–42, targeting this peptide by gene vaccination is intended to reduce the amyloid burden in the AD-affected brain. Based on striking effectiveness in mouse studies,7 a clinical trail using Aβ 42 peptide vaccination was conducted in a large number of patients with AD but had to be terminated because of the occurrence of an autoimmune, cell-mediated response that resulted in meningoencephalitis in 6% of the immunized patients.10–11 Gene immunization has proven to be effective in treating or preventing several infectious as well as noninfectious diseases20–23 and our intent has been to test the potential of gene vaccination as a new therapeutic approach in AD while also minimizing the risk of encephalitis.12
The gene-gun delivery of a gene vaccine has the advantage over peptide vaccination of higher efficiency in breaking self-tolerance and for inducing beneficial Th2-based immune responses24–25 to reduce the possible adverse effects related to Th1 adverse responses seen with Aβ 42 peptide vaccine. We demonstrate that immunization with the mouse Aβ 42 gene was effective in breaking self-tolerance of mouse to mouse Aβ 42 peptide and also in Tg mice with the human Aβ 42 gene in breaking tolerance to human Aβ 42. The breaking of self-tolerance of mouse to mouse Aβ 42 corresponds to the required human therapeutic response, as vaccination with human Aβ 42 in patients must also result in breaking self-tolerance. Overcoming self-tolerance may be the key step in the treatment of the patient with AD using the Aβ 42 gene vaccine, as this peptide is produced in most cells and is also present in high concentration in plasma.
In AD Tg mice, by coshooting the Aβ 1–16 gene with the Aβ 1–42 gene, a higher titer of antibodies is induced against the Aβ 1–16 peptide. The Aβ 1–16 is expected to be a better vaccine target than the other subunits, as the N-terminal region of the Aβ is a key position in protein conformation and the cell-mediated adverse effects may be associated with the C-terminal portion of the Aβ peptide.26 Further studies are in progress to determine if the Aβ 1–16 vaccine can reduce the Aβ burden in brain.
We also demonstrate by ELISPOT that the Aβ 1–42 gene vaccine did not induce significant cell-mediated responses specifically against Aβ 1–42 or Aβ 9–18 peptides. We have shown that Aβ 9–18 does not induce significant stimulating effects of T cells by releasing interferon-γ in the ELISPOT assay. Thus, gene-gun vaccination with Aβ demonstrated herein could prevent inducing autoimmune, cell-mediated meningoencephalitis in immunized patients with AD encountered with peptide immunization.
Studies in progress to assess the effectiveness of gene vaccine therapy in Tg mice include behavioral analysis, survival time, and evaluation of histological and histochemical changes in the brain in immunized and control vector–vaccinated mice. While we have demonstrated that genetically vaccinated AD Tg mice make antibody against Aβ , a critical question is whether the antibody response is effective in limiting the extent of amyloid accumulation in these double AD Tg mice. The histological findings to date in gene-vaccinated Aβ 42 and control vector–vaccinated mice 5 months after immunization are limited and the number of animals examined is too few to draw conclusions. Analysis of older animals is ongoing to assess for differences with respect to amyloid burden, glial response, inflammatory cell response, and importantly, behavioral improvement or slowing of cognitive loss. Additional studies are planned to increase antibody titers by coshooting with Th2 cytokines including interleukin 4 and interleukin 5 and specifically targeting B cells.
CONCLUSIONS
We have demonstrated that gene-gun–mediated genetic immunization with Aβ 42 gene can efficiently elicit humoral immune responses against mouse Aβ 1–42 peptide in wild-type BALB/c mice as well as against human Aβ 1–42 in Tg mice. Further, induction of the humoral immune response did not induce a significant cellular immune response, potentially circumventing an autoimmune, cell-mediated meningoencephalitis in patients. These studies demonstrate that in principle self-tolerance can be broken to produce a humoral response to the Aβ 1–42 peptide with minimal cellular response.
Acknowledgments
The University of Texas Southwestern Pathology and Immunohistochemistry Laboratory, Dallas, performed the histological processing and immunohistochemical stains.
Footnotes
Financial Disclosure: Dr Rosenberg has received educational grants from Pfizer Inc, Novartis, Janssen Pharmaceutica, and Forest Laboratories. He has been a consultant for Elan Pharmaceuticals Inc.
Author Contributions: Study concept and design: Qu, Rosenberg, and Johnston. Acquisition of data: Qu, Rosenberg, and Johnston. Analysis and interpretation of data: Qu, Rosenberg, Li, Boyer, and Johnston. Drafting of the manuscript: Qu, Rosenberg, Li, and Johnston. Critical revision of the manuscript for important intellectual content: Rosenberg, Boyer, and Johnston. Statistical analysis: Li. Obtained funding: Qu and Johnston. Administrative, technical, and material support: Qu, Li, and Johnston. Study supervision: Qu, Rosenberg, Boyer, and Johnston.
Funding/Support: This study was supported by grants P30AG12300 the Alzheimer’s Disease Center grant from the National Institutes of Health–National Institute on Aging, Bethesda, Md; U01 AG16976 the National Alzheimer’s Coordinating Center grant, Seattle, Wash; and The Rudman Foundation, Dallas; and unrestricted funds of Center for Biomedical Inventions. The Winspear Family Special Center for Research on the Neuropathology of Alzheimer Disease, Dallas, provided support for this research.
References
- 1.Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease: hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid β-protein. Ann N Y Acad Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J. Amyloid, the preseniline and Alzheimer’s disease. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399(6738 suppl):A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 5.Monsonego A, Weiner HL. Immunotherapeutic approaches to Alzheimer’s disease. Science. 2003;302:834–838. doi: 10.1126/science.1088469. [DOI] [PubMed] [Google Scholar]
- 6.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat Med. 2004;10(suppl):S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 7.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 8.Kotilinek LA, Bacskai B, Westerman M, et al. Reversible memory loss in a mouse transgenic model of Alzheimer's disease. J Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid-beta peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 10.Hock C, Konietzko U, Papassotiropoulos A, et al. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer's disease. Nat Med. 2002;8:1270–1275. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- 11.Hock C, Konietzko U, Streffer JR, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 12.Qu B, Rosenberg RN, Johnston SA. Genetic Immunization approach for therapy of Alzheimer disease [abstract] Neurobiol Aging. 2004;25(suppl):S568–S569. [Google Scholar]
- 13.Chambers RS, Johnston SA. High-level generation of polyclonal antibodies by genetic immunization. Nat Biotechnol. 2003;21:1088–1092. doi: 10.1038/nbt858. [DOI] [PubMed] [Google Scholar]
- 14.de Arruda LB, Chikhlikar PR, August JT, Marques ET. DNA vaccine encoding human immunodeficiency virus-1 Gag, targeted to the major histocompatibility complex II compartment by lysosomal-associated membrane protein, elicits enhanced long-term memory response. Immunology. 2004;112:126–133. doi: 10.1111/j.1365-2567.2004.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Weiss W, Tine JA, Hoffman SL, Rogers WO. ELISPOT assay for detection of peptide specific interferon-gamma secreting cells in rhesus macaques. J Immunol Methods. 2001;247:49–60. doi: 10.1016/s0022-1759(00)00310-0. [DOI] [PubMed] [Google Scholar]
- 17.Borchelt DR, Ratovitski T, van Lare J, et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 18.Lehman EJ, Kulnane LS, Gao Y, et al. Genetic background regulates β-amyloid precursor protein processing and β-amyloid deposition in the mouse. Hum Mol Genet. 2003;12:2949–2956. doi: 10.1093/hmg/ddg322. [DOI] [PubMed] [Google Scholar]
- 19.Guo-Ross SX, Yang EY, Walsh TJ, Bondy SC. Decrease of glial fibrillary acidic protein in rat frontal cortex following aluminum treatment. J Neurochem. 1999;73:1609–1614. doi: 10.1046/j.1471-4159.1999.0731609.x. [DOI] [PubMed] [Google Scholar]
- 20.Barry MA, Lai WC, Johnston SA. Protection against mycoplasma infection using expression-library immunization. Nature. 1995;377:632–635. doi: 10.1038/377632a0. [DOI] [PubMed] [Google Scholar]
- 21.Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 22.Ji H, Wang TL, Chen CH, et al. Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-exprssing tumors. Hum Gene Ther. 1999;10:2727–2740. doi: 10.1089/10430349950016474. [DOI] [PubMed] [Google Scholar]
- 23.Schultz JG, Salzer U, Mohajeri MH, et al. Antibodies from a DNA peptide vaccination decrease the brain amyloid burden in a mouse model of Alzheimer’s disease. J Mol Med [online series]. Available at: http://www.2004;0570-z [DOI] [PubMed]
- 24.Feltquate DM, Heaney S, Webster RG, Robinson HL. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 25.Weiss R, Scheiblhofer S, Freund J, et al. Gene gun bombardment with gold particles displays a particular Th2-promoting signal that over-rules the Th1-inducing effect of immunostimulatory CpG motifs in DNA. Vaccine. 2002;20:3148–3154. doi: 10.1016/s0264-410x(02)00250-5. [DOI] [PubMed] [Google Scholar]
- 26.Solomon B. Alzheimer’s disease and immunotherapy. Curr Alzheimer Res. 2004;1:149–163. doi: 10.2174/1567205043332126. [DOI] [PubMed] [Google Scholar]


