Abstract
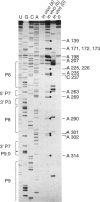
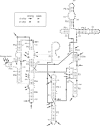
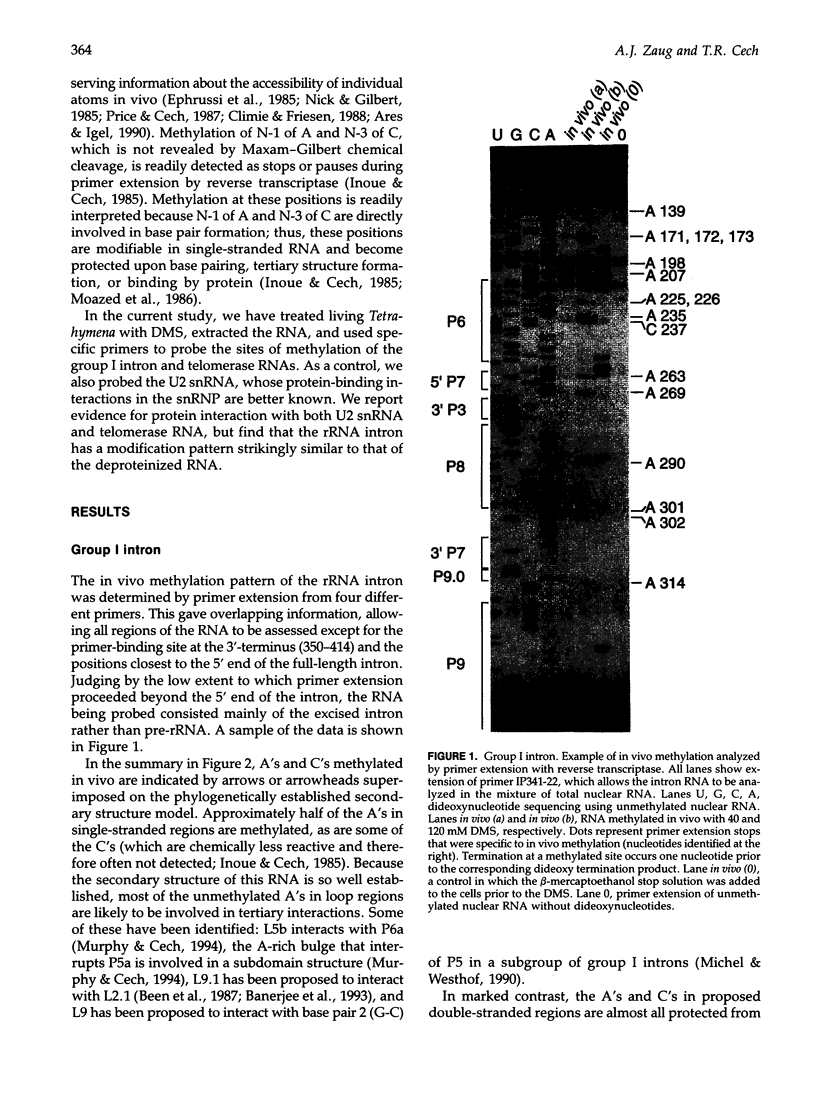
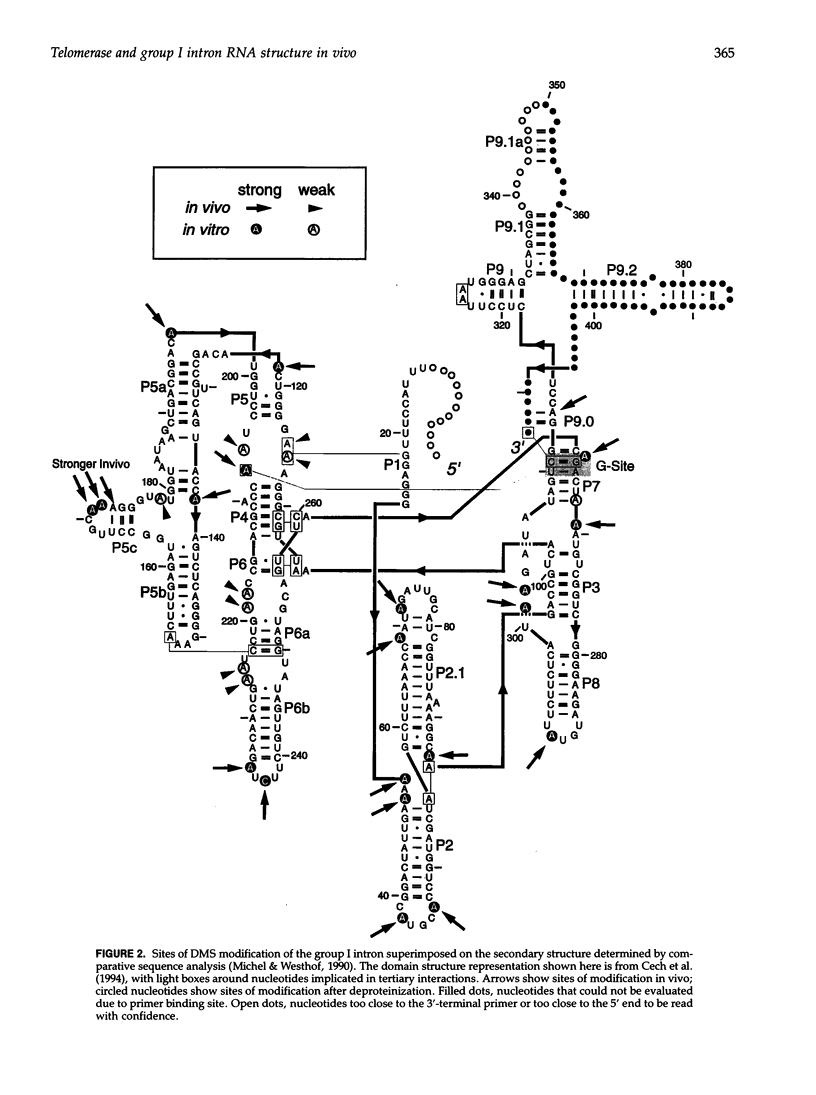
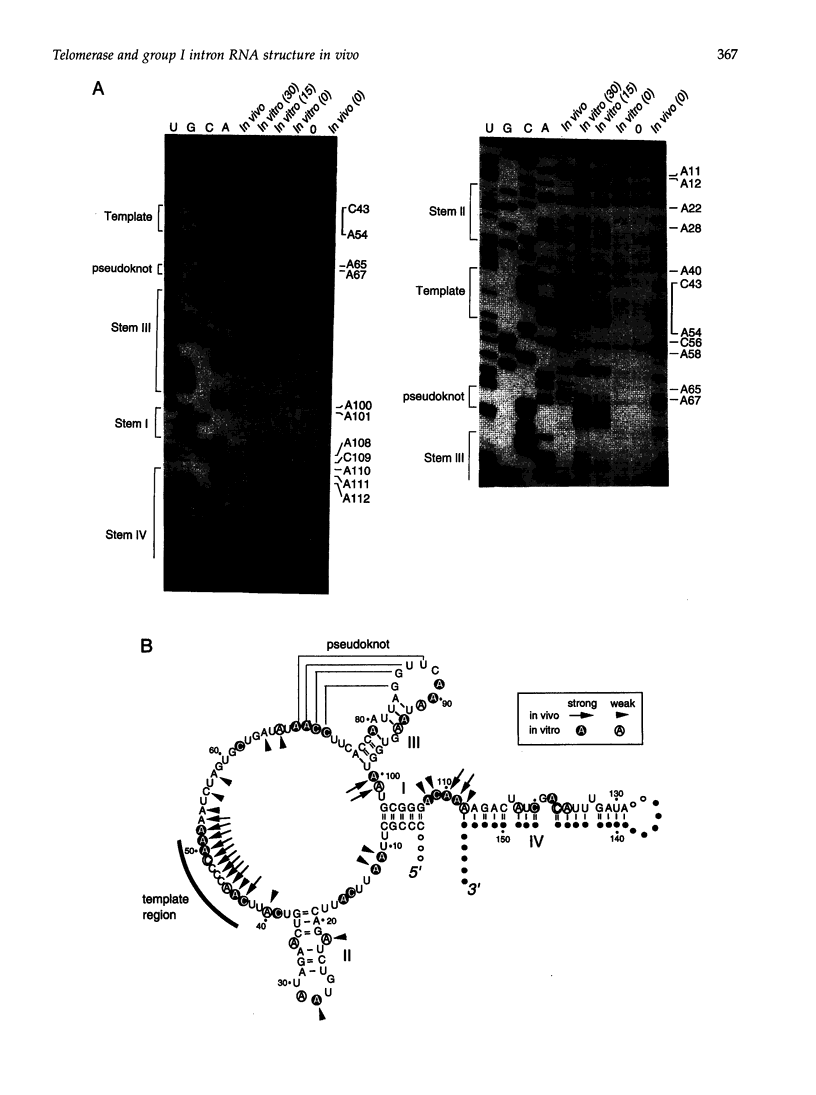
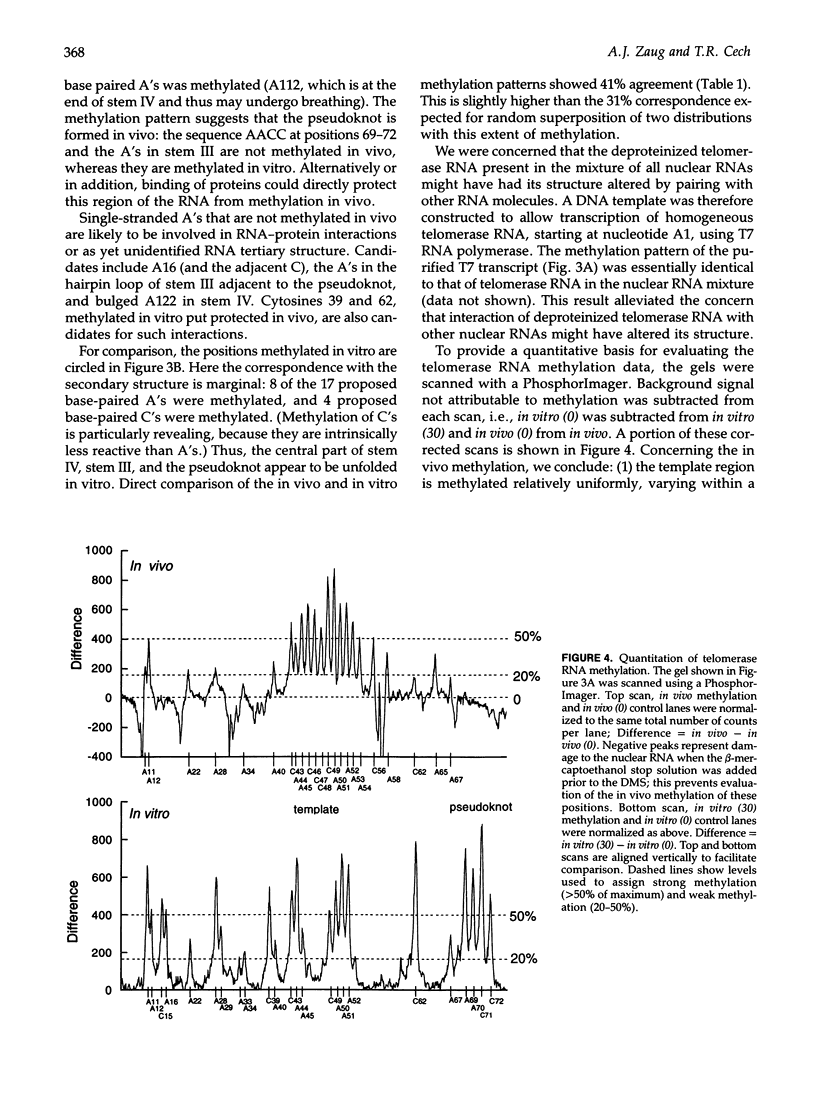
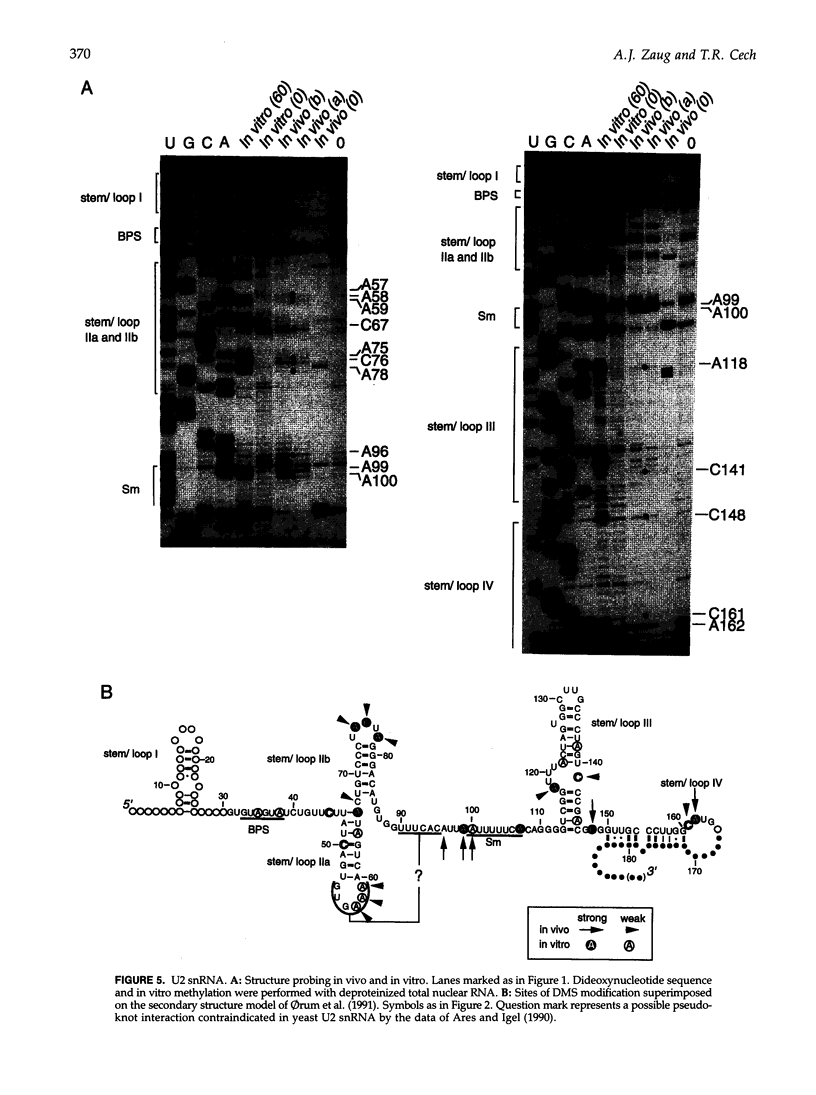
Dimethyl sulfate modification of RNA in living Tetrahymena thermophila allowed assessment of RNA secondary structure and protein association. The self-splicing rRNA intron had the same methylation pattern in vivo as in vitro, indicating that the structures are equivalent and suggesting that this RNA is not stably associated with protein in the nucleolus. Methylation was consistent with the current secondary structure model. Much of telomerase RNA was protected from methylation in vivo, but the A's and C's in the template region were very reactive. Thus, most telomerase is not base paired to telomeres in vivo. Protein-free telomerase RNA adopts a structure different from that in vivo, especially in the template and pseudoknot regions. The U2 snRNA showed methylation protection at the Sm protein-binding sequence and the mRNA branch site recognition sequence. For both telomerase RNA and U2 snRNA, the in vivo methylation pattern corresponded much better to the structure determined by comparative sequence analysis than did the in vitro methylation pattern. Thus, as expected, comparative analysis gives the structure of the RNA in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ares M., Jr, Igel A. H. Lethal and temperature-sensitive mutations and their suppressors identify an essential structural element in U2 small nuclear RNA. Genes Dev. 1990 Dec;4(12A):2132–2145. doi: 10.1101/gad.4.12a.2132. [DOI] [PubMed] [Google Scholar]
- Autexier C., Greider C. W. Functional reconstitution of wild-type and mutant Tetrahymena telomerase. Genes Dev. 1994 Mar 1;8(5):563–575. doi: 10.1101/gad.8.5.563. [DOI] [PubMed] [Google Scholar]
- Avilion A. A., Harrington L. A., Greider C. W. Tetrahymena telomerase RNA levels increase during macronuclear development. Dev Genet. 1992;13(1):80–86. doi: 10.1002/dvg.1020130113. [DOI] [PubMed] [Google Scholar]
- Banerjee A. R., Jaeger J. A., Turner D. H. Thermal unfolding of a group I ribozyme: the low-temperature transition is primarily disruption of tertiary structure. Biochemistry. 1993 Jan 12;32(1):153–163. doi: 10.1021/bi00052a021. [DOI] [PubMed] [Google Scholar]
- Been M. D., Barfod E. T., Burke J. M., Price J. V., Tanner N. K., Zaug A. J., Cech T. R. Structures involved in Tetrahymena rRNA self-splicing and RNA enzyme activity. Cold Spring Harb Symp Quant Biol. 1987;52:147–157. doi: 10.1101/sqb.1987.052.01.019. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A., Blackburn E. H. Architecture of telomerase RNA. EMBO J. 1994 Dec 1;13(23):5721–5731. doi: 10.1002/j.1460-2075.1994.tb06910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm S. L., Cech T. R. Fate of an intervening sequence ribonucleic acid: excision and cyclization of the Tetrahymena ribosomal ribonucleic acid intervening sequence in vivo. Biochemistry. 1983 May 10;22(10):2390–2397. doi: 10.1021/bi00279a014. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Esherick J. S., Burfeind W. R., King J. L. A 3' splice site-binding sequence in the catalytic core of a group I intron. Nature. 1990 Mar 1;344(6261):80–82. doi: 10.1038/344080a0. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Bass B. L. Biological catalysis by RNA. Annu Rev Biochem. 1986;55:599–629. doi: 10.1146/annurev.bi.55.070186.003123. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Damberger S. H., Gutell R. R. Representation of the secondary and tertiary structure of group I introns. Nat Struct Biol. 1994 May;1(5):273–280. doi: 10.1038/nsb0594-273. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Self-splicing of group I introns. Annu Rev Biochem. 1990;59:543–568. doi: 10.1146/annurev.bi.59.070190.002551. [DOI] [PubMed] [Google Scholar]
- Climie S. C., Friesen J. D. In vivo and in vitro structural analysis of the rplJ mRNA leader of Escherichia coli. Protection by bound L10-L7/L12. J Biol Chem. 1988 Oct 15;263(29):15166–15175. [PubMed] [Google Scholar]
- Coetzee T., Herschlag D., Belfort M. Escherichia coli proteins, including ribosomal protein S12, facilitate in vitro splicing of phage T4 introns by acting as RNA chaperones. Genes Dev. 1994 Jul 1;8(13):1575–1588. doi: 10.1101/gad.8.13.1575. [DOI] [PubMed] [Google Scholar]
- Conover R. K., Brunk C. F. Macronuclear DNA molecules of Tetrahymena thermophila. Mol Cell Biol. 1986 Mar;6(3):900–905. doi: 10.1128/mcb.6.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Gampel A., Nishikimi M., Tzagoloff A. CBP2 protein promotes in vitro excision of a yeast mitochondrial group I intron. Mol Cell Biol. 1989 Dec;9(12):5424–5433. doi: 10.1128/mcb.9.12.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987 Dec 24;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Higashinakagawa T., Tashiro F., Mita T. DNA-dependent RNA polymerase from a protozoan, Tetrahymena pyriformis. Extraction and partial characterization. J Biochem. 1975 Apr;77(4):783–793. doi: 10.1093/oxfordjournals.jbchem.a130783. [DOI] [PubMed] [Google Scholar]
- Inoue T., Cech T. R. Secondary structure of the circular form of the Tetrahymena rRNA intervening sequence: a technique for RNA structure analysis using chemical probes and reverse transcriptase. Proc Natl Acad Sci U S A. 1985 Feb;82(3):648–652. doi: 10.1073/pnas.82.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J. A., Zuker M., Turner D. H. Melting and chemical modification of a cyclized self-splicing group I intron: similarity of structures in 1 M Na+, in 10 mM Mg2+, and in the presence of substrate. Biochemistry. 1990 Nov 6;29(44):10147–10158. doi: 10.1021/bi00496a002. [DOI] [PubMed] [Google Scholar]
- Jaeger L., Westhof E., Michel F. Monitoring of the cooperative unfolding of the sunY group I intron of bacteriophage T4. The active form of the sunY ribozyme is stabilized by multiple interactions with 3' terminal intron components. J Mol Biol. 1993 Nov 20;234(2):331–346. doi: 10.1006/jmbi.1993.1590. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Perlman P. S. Involvement of aminoacyl-tRNA synthetases and other proteins in group I and group II intron splicing. Trends Biochem Sci. 1990 Nov;15(11):440–444. doi: 10.1016/0968-0004(90)90283-h. [DOI] [PubMed] [Google Scholar]
- Lingner J., Hendrick L. L., Cech T. R. Telomerase RNAs of different ciliates have a common secondary structure and a permuted template. Genes Dev. 1994 Aug 15;8(16):1984–1998. doi: 10.1101/gad.8.16.1984. [DOI] [PubMed] [Google Scholar]
- McCormick-Graham M., Romero D. P. Ciliate telomerase RNA structural features. Nucleic Acids Res. 1995 Apr 11;23(7):1091–1097. doi: 10.1093/nar/23.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Netter P., Xu M. Q., Shub D. A. Mechanism of 3' splice site selection by the catalytic core of the sunY intron of bacteriophage T4: the role of a novel base-pairing interaction in group I introns. Genes Dev. 1990 May;4(5):777–788. doi: 10.1101/gad.4.5.777. [DOI] [PubMed] [Google Scholar]
- Michel F., Westhof E. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol. 1990 Dec 5;216(3):585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Murphy F. L., Cech T. R. GAAA tetraloop and conserved bulge stabilize tertiary structure of a group I intron domain. J Mol Biol. 1994 Feb 11;236(1):49–63. doi: 10.1006/jmbi.1994.1117. [DOI] [PubMed] [Google Scholar]
- Nick H., Gilbert W. Detection in vivo of protein-DNA interactions within the lac operon of Escherichia coli. 1985 Feb 28-Mar 6Nature. 313(6005):795–798. doi: 10.1038/313795a0. [DOI] [PubMed] [Google Scholar]
- Parker R., Siliciano P. G., Guthrie C. Recognition of the TACTAAC box during mRNA splicing in yeast involves base pairing to the U2-like snRNA. Cell. 1987 Apr 24;49(2):229–239. doi: 10.1016/0092-8674(87)90564-2. [DOI] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. M., Cech T. R. Telomeric DNA-protein interactions of Oxytricha macronuclear DNA. Genes Dev. 1987 Oct;1(8):783–793. doi: 10.1101/gad.1.8.783. [DOI] [PubMed] [Google Scholar]
- Romero D. P., Blackburn E. H. A conserved secondary structure for telomerase RNA. Cell. 1991 Oct 18;67(2):343–353. doi: 10.1016/0092-8674(91)90186-3. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. Keeping RNA happy. RNA. 1995 Mar;1(1):4–6. [PMC free article] [PubMed] [Google Scholar]
- Wang J. F., Downs W. D., Cech T. R. Movement of the guide sequence during RNA catalysis by a group I ribozyme. Science. 1993 Apr 23;260(5107):504–508. doi: 10.1126/science.7682726. [DOI] [PubMed] [Google Scholar]
- Weeks K. M., Cech T. R. Efficient protein-facilitated splicing of the yeast mitochondrial bI5 intron. Biochemistry. 1995 Jun 13;34(23):7728–7738. doi: 10.1021/bi00023a020. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Bradley J. D., Attardi L. D., Blackburn E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990 Mar 8;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- Zhang F., Ramsay E. S., Woodson S. A. In vivo facilitation of Tetrahymena group I intron splicing in Escherichia coli pre-ribosomal RNA. RNA. 1995 May;1(3):284–292. [PMC free article] [PubMed] [Google Scholar]
- ten Dam E., van Belkum A., Pleij K. A conserved pseudoknot in telomerase RNA. Nucleic Acids Res. 1991 Dec 25;19(24):6951–6951. doi: 10.1093/nar/19.24.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]