Abstract
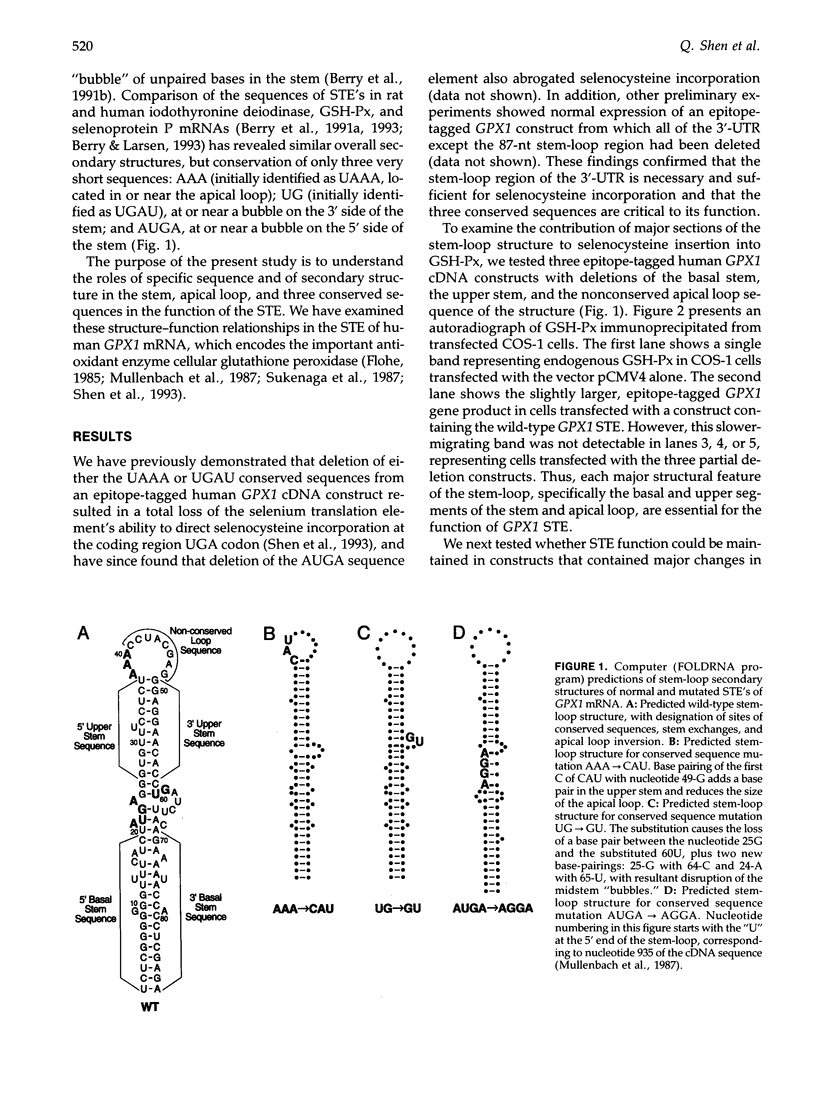
In eukaryotes, incorporation of selenocysteine into the polypeptide chain at a UGA codon requires a unique sequence motif, or "selenium translation element" (STE), located in the 3'-untranslated region of the mRNA. The present study examines structure-function relationships of conserved sequence elements and of the putative stem-loop secondary structure in the STE of human GPX1 mRNA, which encodes the important antioxidant enzyme cellular glutathione peroxidase (EC 1.11.1.9). Deletion of the basal stem, upper stem, or apical loop of the stem-loop structure eliminated the ability of the STE to direct selenocysteine incorporation at the UGA codon of an epitope-tagged GPX1 reporter construct transfected into COS1 cells. However, mutations that change the primary nucleotide sequence of nonconserved portions of the stem-loop, but preserve its overall secondary structure, by inversion of apical loop sequences or exchange of 5' and 3' sides of stem segments, had little or no effect on selenocysteine incorporation. Effects of single- and double-nucleotide substitutions in three short, highly conserved elements in the GPX1 STE depended in large part on their computer-predicted perturbation of the stem-loop and its midstem bulge. Only in the conserved "AAA" apical loop sequence did mutations show major effects on function without predicted changes in secondary structure. Our results demonstrate the critical role of the three short, highly conserved sequences. However, outside of these elements, the function of the human GPX1 STE appears to depend strongly on the stem-loop secondary structure.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Berry M. J., Banu L., Chen Y. Y., Mandel S. J., Kieffer J. D., Harney J. W., Larsen P. R. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3' untranslated region. Nature. 1991 Sep 19;353(6341):273–276. doi: 10.1038/353273a0. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Harney J. W., Larsen P. R. Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons. EMBO J. 1993 Aug;12(8):3315–3322. doi: 10.1002/j.1460-2075.1993.tb06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Larsen P. R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991 Jan 31;349(6308):438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Larsen P. R. Recognition of UGA as a selenocysteine codon in eukaryotes: a review of recent progress. Biochem Soc Trans. 1993 Nov;21(4):827–832. doi: 10.1042/bst0210827. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Hill K. E. Regulation of selenoproteins. Annu Rev Nutr. 1993;13:65–81. doi: 10.1146/annurev.nu.13.070193.000433. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Hill K. E. Selenoprotein P. A selenium-rich extracellular glycoprotein. J Nutr. 1994 Oct;124(10):1891–1897. doi: 10.1093/jn/124.10.1891. [DOI] [PubMed] [Google Scholar]
- Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA. EMBO J. 1986 Jun;5(6):1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Avissar N. Molecular and biochemical aspects of selenium metabolism and deficiency. Prog Clin Biol Res. 1993;380:191–202. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esworthy R. S., Chu F. F., Paxton R. J., Akman S., Doroshow J. H. Characterization and partial amino acid sequence of human plasma glutathione peroxidase. Arch Biochem Biophys. 1991 May 1;286(2):330–336. doi: 10.1016/0003-9861(91)90048-n. [DOI] [PubMed] [Google Scholar]
- Flohé L. The glutathione peroxidase reaction: molecular basis of the antioxidant function of selenium in mammals. Curr Top Cell Regul. 1985;27:473–478. doi: 10.1016/b978-0-12-152827-0.50047-5. [DOI] [PubMed] [Google Scholar]
- Forchhammer K., Leinfelder W., Böck A. Identification of a novel translation factor necessary for the incorporation of selenocysteine into protein. Nature. 1989 Nov 23;342(6248):453–456. doi: 10.1038/342453a0. [DOI] [PubMed] [Google Scholar]
- Hill K. E., Lloyd R. S., Yang J. G., Read R., Burk R. F. The cDNA for rat selenoprotein P contains 10 TGA codons in the open reading frame. J Biol Chem. 1991 Jun 5;266(16):10050–10053. [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Marino J. P., Gregorian R. S., Jr, Csankovszki G., Crothers D. M. Bent helix formation between RNA hairpins with complementary loops. Science. 1995 Jun 9;268(5216):1448–1454. doi: 10.1126/science.7539549. [DOI] [PubMed] [Google Scholar]
- Mullenbach G. T., Tabrizi A., Irvine B. D., Bell G. I., Hallewell R. A. Sequence of a cDNA coding for human glutathione peroxidase confirms TGA encodes active site selenocysteine. Nucleic Acids Res. 1987 Jul 10;15(13):5484–5484. doi: 10.1093/nar/15.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburger P. E., Skalnik D. G., Hopkins P. J., Eklund E. A., Curnutte J. T. Mutations in the promoter region of the gene for gp91-phox in X-linked chronic granulomatous disease with decreased expression of cytochrome b558. J Clin Invest. 1994 Sep;94(3):1205–1211. doi: 10.1172/JCI117437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read R., Bellew T., Yang J. G., Hill K. E., Palmer I. S., Burk R. F. Selenium and amino acid composition of selenoprotein P, the major selenoprotein in rat serum. J Biol Chem. 1990 Oct 15;265(29):17899–17905. [PubMed] [Google Scholar]
- Shen Q., Chu F. F., Newburger P. E. Sequences in the 3'-untranslated region of the human cellular glutathione peroxidase gene are necessary and sufficient for selenocysteine incorporation at the UGA codon. J Biol Chem. 1993 May 25;268(15):11463–11469. [PubMed] [Google Scholar]
- Sukenaga Y., Ishida K., Takeda T., Takagi K. cDNA sequence coding for human glutathione peroxidase. Nucleic Acids Res. 1987 Sep 11;15(17):7178–7178. doi: 10.1093/nar/15.17.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Cohen H. J. Selenium-dependent glutathione peroxidase protein and activity: immunological investigations on cellular and plasma enzymes. Blood. 1986 Sep;68(3):640–645. [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoni F., Heider J., Böck A. Features of the formate dehydrogenase mRNA necessary for decoding of the UGA codon as selenocysteine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4660–4664. doi: 10.1073/pnas.87.12.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]





