Abstract
Normal female fertility relies on proper development of the oocyte. This growth culminates just prior to ovulation, when oocyte maturation occurs. Oocyte maturation refers to a release of meiotic arrest that allows oocytes to advance from prophase I to metaphase II of meiosis. This precisely regulated meiotic progression is essential for normal ovulation and subsequent fertilization, and involves changes in the delicate balance between factors promoting meiotic arrest and others that are stimulating maturation. Most of the inhibitory mechanisms appear to involve the upregulation of intracellular cyclic adenosine monophosphate levels. These processes may include direct transport of the nucleotide into oocytes via gap junctions, G protein-mediated stimulation of adenylyl cyclase, and inhibition of intracellular phosphodiesterases. In contrast, potential factors that play roles in triggering oocyte maturation include gonadotropins (e.g., follicle-stimulating factor and luteinizing hormone), growth factors (e.g., amphiregulin and epiregulin), sterols (e.g., follicular fluid-derived meiosis-activating sterol), and steroids (e.g., testosterone progesterone, and estradiol). Delineating the complex interactions between these positive and negative components is critical for determining the role that oocyte maturation plays in regulating follicle development and ovulation, and may lead to novel methods that can be used to modulate these processes in women with both normal and aberrant fertility.
Keywords: Oocyte, ovary, maturation, steroids, nongenomic
One of the final steps in the development of an oocyte is maturation. Oocyte maturation is defined as a re-entry into meiosis that occurs just prior to ovulation and subsequent fertilization.1,2 Oocytes within the ovary are arrested in prophase I of meiosis until the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), stimulate follicular growth and development, which then triggers the resumption of meiosis up to metaphase II. Oocytes are subsequently again held in meiotic arrest until fertilization, when meiosis is completed. The induction of oocyte maturation appears to involve a complex interaction of several important intracellular, paracrine, and structural factors that include sterols, steroids, growth factors, cyclic adenosine monophosphate (cAMP), and gap junctions. The following review highlights many of these components, focusing on the remarkable conservation between higher vertebrates such as mammals and the lower vertebrates, including frogs and fish.
OOGENESIS
During human fetal development, primordial germ cells originate in the dorsal endoderm of the yolk sac, close to the allantoic evagination. By weeks 4 to 5, these cells migrate through the hindgut before settling in the urogenital ridge.3 By week 25 of gestation, approximately 7 million oogonia have been formed by mitosis and developed into primary oocytes.4 At this stage, a single layer of flattened epithelial cells surrounds the primary oocyte to form a primordial follicle. The first steps of meiosis I occur in the primordial follicle, including the replication of DNA and arrest in the diplotene stage of prophase I. Controversy has existed for many decades regarding the status of the oocyte population within the mammalian ovary after birth. The more traditional conviction has been that mammals are born with their full contingent of oocytes that are held in meiotic arrest until puberty, when small populations in growing follicles are triggered to mature during each cycle.5 In contrast, recent evidence suggests that, at least in mice, oocytes might be steadily destroyed and then regenerated from a small population of germline stem cells throughout the early and reproductive years of mammalian life.6 Although still controversial with regard to mammals, this latter hypothesis is consistent with the ovarian biology of lower vertebrates, such as Xenopus laevis, in which evidence is relatively strong that new oocytes are constantly developing from oogonia throughout most of the lifespan of the female frog.7–9
MEIOTIC ARREST
Before discussing the external triggers and intracellular events that promote oocyte maturation, the signals that hold oocytes in meiotic arrest must be described. These inhibitory signals appear to originate from the surrounding ovary, given that removal of mammalian oocytes from the ovary results in spontaneous maturation within hours.10,11 The unprompted re-entry into meiosis that occurs in denuded mammalian oocytes contrasts with lower vertebrates such as frogs and fish, in which signals maintaining meiotic arrest appear to be endogenous to the oocytes themselves. For example, isolated amphibian oocytes remain locked in meiotic arrest indefinitely until triggered by exogenous addition of steroids and other compounds.12,13
One of the most important intracellular signaling molecules believed to be responsible for maintaining meiotic arrest is cAMP.14 In general, intracellular cAMP homeostasis is regulated by two important groups of enzymes: the adenylyl cyclases (ACs), which generate cAMP; and the phosphodiesterases (PDEs), which metabolize cAMP. Most of the well-characterized ACs are regulated by G proteins that either promote (Gαs) or inhibit (Gαi) their activity.15 In contrast, the mechanisms that control PDE activity are less well understood, but may involve short-term activation in response to protein kinase A (PKA)-mediated phosphorylation, as well as long-term regulation that entails changes in mRNA and protein expression.16 cAMP within oocytes appears to act as an inhibitor of oocyte maturation, perhaps through activation of PKA. Evidence supporting this claim includes the following: (1) Elevated intracellular cAMP levels in denuded mouse oocytes prevent spontaneous maturation. For example, isolated oocytes can be held in meiotic arrest by incubation with cAMP analogs such as dibutyryl cAMP, or PDE antagonists such as 3-isobutyl-1-methylxanthine or milrinone;14,17 (2) meiotic arrest of mouse oocytes within follicles can be released by injecting antibodies targeted against Gαs into oocytes18; (3) oocyte maturation can be blocked in vivo by feeding mice PDE inhibitors prior to and during ovulation19; and (4) intracellular cAMP appears to decrease rapidly under some conditions at the start of mammalian oocyte maturation.14
Interestingly, similar studies whereby cAMP levels in frog oocytes were artificially elevated resulted in inhibition of steroid-induced maturation, and a likewise drop in cAMP upon activation of meiosis with progesterone has been observed.20–22 In addition, detailed signaling studies using frog oocytes have produced a release of inhibition model whereby constitutive Gβγ and/or Gαs signaling within the oocyte appears to hold it in meiotic arrest, perhaps by stimulating AC to produce cAMP.23–25 Triggers of maturation, such as steroids, appear to overcome this inhibitory signal, releasing meiotic arrest and allowing maturation to occur.12,13,26,27a Perhaps these inhibitory G protein-mediated signals have evolved from being constitutively active within the oocytes of lower, egg-laying vertebrates to being activated by factors outside the oocyte in mammalian follicles. For example, a Gαs-coupled receptor termed GPR3 has recently been shown to play a potentially important role in mediating meiotic arrest in mouse oocytes, although its ligand has yet to be identified.27b Notably, however, although increased intracellular cAMP clearly inhibits maturation, whether a decrease in intracellular cAMP is either necessary or sufficient to promote oocyte maturation remains unknown.28–30
Finally, one of the earliest candidates to be considered an inhibitor of meiosis in mammalian oocytes was the purine hypoxanthine. Hypoxanthine appears to be produced in the follicle, and inhibits in vitro meiosis of oocytes that are either denuded or encased in follicles.31 Hypoxanthine functions as a PDE inhibitor that might prevent metabolism of cAMP, thus maintaining meiotic-arresting levels of cAMP within the oocyte32; however, the physiologic importance of hypoxanthine still remains uncertain.33
OVARIAN ANATOMY AND THE GAP JUNCTION
Appreciation of the structural relationships between oocytes and their surrounding follicle cells is important for understanding the mechanisms regulating meiosis in oocytes (Fig. 1A). The mammalian follicle consists of several outer layers of theca cells that play a prominent role in ovarian steroidogenesis. These theca cells surround layers of outer mural granulosa cells, which in turn encompass layers of inner cumulus granulosa cells. These cumulus granulosa cells then form close contacts with the oocytes via gap junctions. Gap junctions are composed of proteins from the connexin family; connexin 43 (Cx43) is the primary member in the ovary. Cx43-containing gap junctions appear to be necessary for expansion of granulosa cells during follicular growth,34 as well as for oocyte development. In lower vertebrates, gap junctions play important roles in supplying nutrition to the oocytes, as they regulate the transport of yolk proteins from the blood to the oocyte.35 Likewise, the role of gap junctions in transferring nutrients and metabolic precursors to mammalian oocytes is well established.36
Figure 1.
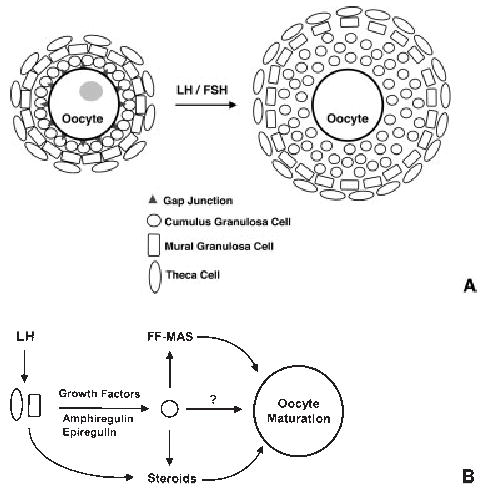
(A) Schematic model of follicle structure and gonadotropin-mediated cumulus cell expansion. Oocytes are directly surrounded by cumulus granulosa cells. These in turn are surrounded by mural granulosa cells, followed by layers of theca cells. Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) stimulate expansion of cumulus granulosa cells, loss of gap junctions, and oocyte maturation (depicted by loss of gray nucleus). (B) Model for gonadotropin-induced oocyte maturation. LH stimulates both steroid and growth factor production by theca and mural granulosa cells. These growth factors, including amphiregulin and epiregulin, then act in a paracrine fashion on cumulus granulosa cells, possibly to produce meiosis-activating sterol (MAS), steroids, or other unknown factors that can promote oocyte maturation. FF-MAS, follicular fluid-derived meiosis-activating sterol.
Interestingly, gap junctions may also be important for maintaining meiotic arrest. Oocytes surrounded by primarily cumulus granulosa cells remain in meiotic arrest when cultured in vitro, whereas disruption of oocyte-granulosa cell contacts during cumulus cell expansion (Fig. 1A) coincides with oocyte maturation.37 These observations suggest that gap junctions may therefore be serving as conduits to transport meiotic inhibitory factors such as cAMP from granulosa cells to oocytes. However, whether disruption of gap junctions actually precedes the initiation of oocyte maturation, or whether disturbance of gap junction integrity is necessary for maturation, remains uncertain; thus, this hypothesis has yet to be verified.
GROWTH FACTORS
Although exposure of ovarian follicles to LH leads to oocyte maturation, neither oocytes nor surrounding cumulus granulosa cells contain LH receptors. Instead, the theca and mural granulosa cells express LH receptors, indicating that some paracrine factor(s) must be released by these outer cells in response to LH to promote expansion of cumulus cells and oocyte maturation.38 The identity of at least some of these paracrine markers has been elucidated recently, and they appear to be members of the epidermal growth factor (EGF) family. Although the ability of growth factors to promote cumulus cell expansion and subsequent oocyte maturation in follicle cultures has been long established,39 their physiologic role in regulating meiosis and ovulation had been uncertain. This recent work has now confirmed that the growth factors amphiregulin and epiregulin, and perhaps others, appear to stimulate oocyte maturation and ovulation via the following model (Fig. 1B)40: First, in addition to promoting steroid production, LH triggers the release of growth factors from the theca and/or mural granulosa cells. These growth factors then act in a paracrine fashion to stimulate inner cumulus granulosa cell expansion, which is followed by disruption of oocyte-granulosa cell contacts, oocyte maturation, and eventual ovulation. Although still not proven, growth factors may also stimulate cumulus granulosa cells to produce steroids, follicular fluid-derived meiosis-activating sterol (FF-MAS), and other factors that may play a role in promoting oocyte maturation.
In addition to promoting cumulus cell expansion, EGF-mediated signaling in cumulus granulosa cells may play a direct role in promoting the breakdown of cumulus cell-oocyte gap junctions. EGF treatment of granulosa cells leads to activation of mitogen-activated protein kinase (MAPK), which in turn phosphorylates Cx43.41 Phosphorylation of Cx43 appears to destabilize gap junctions, which disrupts intercellular junctional communication42 and may therefore prevent the aforementioned transport of meiotic inhibitory factors to the oocyte.
MEIOSIS-ACTIVATING STEROL
As mentioned, one of the factors that might be released from cumulus granulosa cells to promote oocyte maturation is the meiosis-activating C29 sterol, FF-MAS (Fig. 2). FF-MAS is an intermediate along the biosynthetic pathway from lanosterol to cholesterol that has been shown to promote maturation of either isolated oocytes held in meiotic arrest or oocytes in cumulus cell cultures.43,44 FF-MAS is found in ovarian follicular fluid, and may be secreted by granulosa cells. T-MAS—testes-derived meiosis-activating sterol—is a metabolite of FF-MAS that has been isolated from bull testis and also appears to promote oocyte maturation in vitro. Although FF-MAS clearly triggers maturation in vitro, some controversy still exists regarding its physiologic importance. Evidence supporting a physiologic role for FF-MAS in regulating meiosis is mainly based on studies demonstrating that inhibition of FF-MAS production or metabolism reduces or enhances, respectively, maturation in cumulus oocyte complexes in vitro.45–47 In addition, FF-MAS production has been shown to increase in response to gonadotropins. For example, injection of female rats with pregnant mare’s serum gonadotropin stimulates a threefold increase in the activity of P450 14α-demthylase, the rate-limiting enzyme in the conversion of lanosterol to FF-MAS (Fig. 2), after 48 hours.48 Furthermore, ovarian FF-MAS levels appear to increase after LH stimulation in rabbit follicles,49 which would be consistent with the timing of maturation and ovulation.
Figure 2.
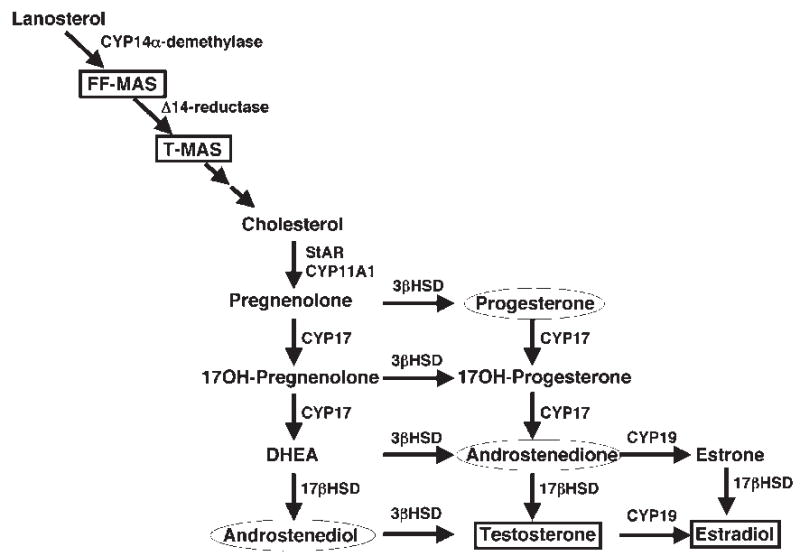
Steroidogenic pathway from the cholesterol precursor lanosterol to the sex steroids testosterone and estradiol. Sterols and steroids that have been shown to promote mouse oocyte maturation in vitro are enclosed in a box, whereas steroids known to trigger oocyte maturation in frogs (in addition to testosterone) are enclosed in a circle. FF-MAS, follicular fluid-derived meiosis-activating sterol; CYP, cytochrome P450 enzyme; DHEA, dehydroepiandrosterone; HSD, hydroxysteroid dehydrogenase.
In contrast, other studies using similar inhibitors of FF-MAS production do not support its physiologic role in regulating maturation. Furthermore, some laboratories have demonstrated that the time course of FF-MAS-mediated maturation and activation of MAPK, as well as the conditions whereby FF-MAS-induced maturation occur, differ from spontaneous or gonadotropin-induced maturation, suggesting that the sterol is unlikely to be the primary mediator of oocyte maturation in vivo.50–55 Finally, micromolar amounts of FF-MAS are required to promote maturation, raising concerns that such high sterol concentrations may be having nonspecific effects on cellular membranes.56
Although the physiologic importance of FF-MAS-induced oocyte maturation remains controversial, recent work has demonstrated that, in addition to enhancing meiotic progression from metaphase I to metaphase II, the sterol may play a role in stabilizing oocytes in metaphase II arrest. In fact, treatment of oocytes with FF-MAS markedly improve their ability to be successfully fertilized in vitro,57,58 suggesting that FF-MAS may eventually prove useful as an adjunct during in vitro fertilization.
STEROIDS
Steroids have been known to promote oocyte maturation in lower vertebrates such as fish and frogs for many decades. Interestingly, this steroid-mediated maturation occurs independent of transcription, and may be regulated by steroid receptors located at the plasma membrane of cells.12,13,59 One of the best-studied models for oocyte maturation comes from the frog Xenopus laevis. Several steroids are known to promote Xenopus oocyte maturation, including progestins, glucocorticoids, and androgens, with the latter being the most potent.13,23,60 Recent evidence has suggested that the membrane receptors regulating progestin- and androgen-triggered maturation might in fact be the classical receptors that are traditionally thought to be located in the nucleus or cytoplasm.23,61–63 For example, elimination of the classical Xenopus androgen receptor (AR) by RNA interference, or inhibition studies using AR antagonists, significantly and specifically reduced androgen-mediated maturation, as well as androgen-induced activation of accompanying MAPK and cyclin-dependent kinasel (CDC1) signals. Furthermore, blockade of androgen production in intact ovaries reduced human chorionic gonadotropin-induced oocyte maturation, suggesting that androgens play an important physiologic role in the regulation of gonadotropin-induced oocyte maturation in X. laevis.23,61 Finally, selective androgen receptor modulators (SARMs) have been characterized that specifically regulate genomic versus nongenomic AR-mediated signaling, and therefore may prove useful for specifically modulating oocyte maturation in vivo.61
Experiments aimed at determining the effects of steroids on mammalian oocyte maturation have been difficult to interpret. Several studies have attempted to examine the role of steroidogenesis in regulating meiosis in cultured oocytes by using ketoconazole or other compounds to block sterol production. The results of these studies were variable,46,51,53,56,64 perhaps due to the nonspecific nature of the inhibitor’s effects. Ketoconazole blocks the activity of nearly all cytochrome P450 enzymes (designated CYP in Fig. 2), and is therefore a potent inhibitor of steroid, FF-MAS, and cholesterol production. As such, ketoconazole can have profound effects on cholesterol content and transport within cells. Because recent evidence indicates that intracellular cholesterol may play a critical role in maturation of frog oocytes,65 ketoconazole may not be the most appropriate inhibitor of steroidogenesis to use when studying oocyte maturation in any species.
In addition to blocking steroidogenesis, several studies have directly examined steroid effects on the spontaneous maturation of isolated mammalian oocytes, finding that micromolar amounts of some steroids slowed maturation.66–69 However, these studies were hampered in that they examined steroid-mediated changes in oocyte maturation using oocytes that were already spontaneously maturing rather than studying steroid-triggered maturation of oocytes arrested in prophase I. Furthermore, the high concentrations of steroids may have been toxic to the oocytes, thus complicating interpretation of the results.
Recent work examining steroid-induced maturation of mouse oocytes held in meiotic arrest with a PDE inhibitor demonstrated the following:70 (1) mouse oocytes in meiotic arrest were triggered to mature by nanomolar amounts of testosterone or estradiol independent of transcription; (2) maturation was accompanied by activation of MAPK and CDK1; (3) androgen-and estrogen-mediated signals were inhibited by AR and ER antagonists respectively; (4) AR was expressed in oocytes; and (5) the pharmacology of androgen signaling using AR antagonists and SARMs was the same in mouse and frog oocytes. This study indicated that steroids were capable of triggering oocyte maturation in a fashion very similar to that seen in the well-characterized frog model, and that they may play an important, although likely not exclusive, role in regulating mammalian oocyte maturation in vivo. For example, in addition to growth factors, steroids might serve as second messengers secreted by theca and mural granulosa cells to promote cumulus cell expansion and oocyte maturation. Alternatively, steroids may be tertiary messengers that, along with FF-MAS and perhaps other substances, are secreted by cumulus granulosa cells in response to growth factors to promote maturation (Fig. 1B). Further studies using follicle cultures and in vivo models will be necessary to confirm these results; however, injection of progestins and androgens has recently been shown to enhance oocyte maturation in monkey models,71 suggesting that, as in lower vertebrates, steroids may indeed play a physiologic role in the regulation of meiosis in mammalian oocytes.
OOCYTE MATURATION, FERTILITY, AND OVARIAN PATHOLOGY
Whether or not androgens are physiologic mediators of oocyte maturation, their ability to trigger promeiotic signals in oocytes brings into question the consequences of excess androgens on oocyte development in diseases such as polycystic ovarian syndrome (PCOS). Although PCOS is complicated by a high incidence of insulin resistance and metabolic syndrome,72–74 anovulation and abnormal follicle growth occur under any conditions in which androgens are in excess, including in women taking exogenous androgens,75 women with congenital adrenal hyperplasia,76 and women with aromatase deficiency.77 In addition, fertility can be improved in PCOS patients by treatment with AR antagonists.78–81 Together, these data suggest that excess androgen signaling in the ovary might be a major contributor to the unregulated folliculogenesis and infertility in diseases of androgen excess. Could these androgens also be altering normal oocyte maturation? Although the ovaries of PCOS patients contain degenerated follicles with abnormal oocytes, the maturation status of these oocytes is not certain. Furthermore, PCOS patients often have increased sensitivity to gonadotropins during in vitro fertilization protocols,82–84 suggesting that follicles and/or oocytes might be primed by the excess androgens. If the high androgen levels are indeed stimulating meiosis, then perhaps the aforementioned SARMs can be used specifically to regulate oocyte maturation and improve fertility in patients with androgen excess. In addition, the use of FF-MAS during the capture, maturation, and fertilization of human oocytes might improve success rates of in vitro fertilization protocols. Thus, understanding the mechanisms regulating oocyte development and maturation is crucial in future efforts to regulate and improve fertility in women.
Acknowledgments
S.R.H. is a W.W. Caruth, Jr., Endowed Scholar in Biomedical Research. M.J. is funded in part by the National Institutes of Health (NIH) training grant T32 GM07062-29. This work was also supported by funding from the NIH (DK59913) and the Welch Foundation (I-1506).
Footnotes
New Frontiers in Gamete Biology; Editor in Chief, Bruce R. Carr, M.D.; Guest Editors, Jerome F. Strauss III, M.D., Ph.D., Carmen J. Williams, M.D., Ph.D. Seminars in Reproductive Medicine, Volume 23, Number 3, 2005.
References
- 1.Albertini DF, Carabatsos MJ. Comparative aspects of meiotic cell cycle control in mammals. J Mol Med. 1998;76:795–799. doi: 10.1007/s001090050283. [DOI] [PubMed] [Google Scholar]
- 2.Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 3.diZerega GS, Hodgen GD. Folliculogenesis in the primate ovarian cycle. Endocr Rev. 1981;2:27–49. doi: 10.1210/edrv-2-1-27. [DOI] [PubMed] [Google Scholar]
- 4.Griffin JE, Ojeda SR. Textbook of Endocrine Physiology, 4th ed. New York: Oxford University Press; 2000
- 5.Mandl AM, Zuckerman S. The relation of age to numbers of oocytes. J Endocrinol. 1951;7:190–193. doi: 10.1677/joe.0.0070190. [DOI] [PubMed] [Google Scholar]
- 6.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 7.Hausen P.The Early Development of Xenopus laevis: An Atlas of the Histology New York: Springer-Verlag; 1991
- 8.Keem K, Smith LD, Wallace RA, Wolf D. Growth rate of oocytes in laboratory maintained Xenopus laevis. Gamete Res. 1979;2:125–135. [Google Scholar]
- 9.Smith CL. Reproduction in female Amphibia. Mem Soc Endocrinol. 1955;4:39–56. [Google Scholar]
- 10.Edwards RG. Maturation in vitro of human ovarian oocytes. Lancet. 1965;2:926–929. doi: 10.1016/s0140-6736(65)92903-x. [DOI] [PubMed] [Google Scholar]
- 11.Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–351. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- 12.Maller JL, Krebs EG. Regulation of oocyte maturation. Curr Top Cell Regul. 1980;16:271–311. doi: 10.1016/b978-0-12-152816-4.50012-1. [DOI] [PubMed] [Google Scholar]
- 13.Smith LD, Ecker RE. The interaction of steroids with Rana pipiens oocytes in the induction of maturation. Dev Biol. 1971;25:232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- 14.Conti M, Andersen CB, Richard F, et al. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187:153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 15.Freissmuth M, Casey PJ, Gilman AG. G proteins control diverse pathways of transmembrane signaling. FASEB J. 1989;3:2125–2131. [PubMed] [Google Scholar]
- 16.Mehats C, Andersen CB, Filopanti M, Jin SL, Conti M. Cyclic nucleotide phosphodiesterases and their role in endocrine cell signaling. Trends Endocrinol Metab. 2002;13:29–35. doi: 10.1016/s1043-2760(01)00523-9. [DOI] [PubMed] [Google Scholar]
- 17.Conti M, Andersen CB, Richard FJ, Shitsukawa K, Tsafriri A. Role of cyclic nucleotide phosphodiesterases in resumption of meiosis. Mol Cell Endocrinol. 1998;145:9–14. doi: 10.1016/s0303-7207(98)00187-7. [DOI] [PubMed] [Google Scholar]
- 18.Mehlmann LM, Jones TL, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343–1345. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- 19.Wiersma A, Hirsch B, Tsafriri A, et al. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J Clin Invest. 1998;102:532–537. doi: 10.1172/JCI2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrill GA, Schatz F, Kostellow AB, Poupko JM. Changes in cyclic AMP levels in the amphibian ovarian follicle following progesterone induction of meiotic maturation. Effect of phosphodiesterase inhibitors and exogenous calcium on germinal vesicle breakdown. Differentiation. 1977;8:97–104. doi: 10.1111/j.1432-0436.1977.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 21.Sadler SE, Maller JL. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J Biol Chem. 1981;256:6368–6373. [PubMed] [Google Scholar]
- 22.Sadler SE, Maller JL. Inhibition of Xenopus oocyte adenylate cyclase by progesterone: a novel mechanism of action. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:179–194. [PubMed] [Google Scholar]
- 23.Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR. Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc Natl Acad Sci USA. 2001;98:13728–13733. doi: 10.1073/pnas.241471598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng Y, Tiberi M, Booth RA, Ma C, Liu XJ. Regulation of Xenopus oocyte meiosis arrest by G protein betagamma subunits. Curr Biol. 2001;11:405–416. doi: 10.1016/s0960-9822(01)00123-3. [DOI] [PubMed] [Google Scholar]
- 25.Gallo CJ, Hand AR, Jones TL, Jaffe LA. Stimulation of Xenopus oocyte maturation by inhibition of the G-protein alpha S subunit, a component of the plasma membrane and yolk platelet membranes. J Cell Biol. 1995;130:275–284. doi: 10.1083/jcb.130.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammes SR. The further redefining of steroid-mediated signaling. Proc Natl Acad Sci USA. 2003;100:2168–2170. doi: 10.1073/pnas.0530224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Hammes SR. Steroids and oocyte maturation—a new look at an old story. Mol Endocrinol. 2004;18:769–775. doi: 10.1210/me.2003-0317. [DOI] [PubMed] [Google Scholar]
- 27b.Mehlmann LM, Saeki Y, Tanaka S, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 28.Gelerstein S, Shapira H, Dascal N, Yekuel R, Oron Y. Is a decrease in cyclic AMP a necessary and sufficient signal for maturation of amphibian oocytes? Dev Biol. 1988;127:25–32. doi: 10.1016/0012-1606(88)90185-6. [DOI] [PubMed] [Google Scholar]
- 29.Faure S, Morin N, Doree M. Inactivation of protein kinase A is not required for c-mos translation during meiotic maturation of Xenopus oocytes. Oncogene. 1998;17:1215–1221. doi: 10.1038/sj.onc.1202056. [DOI] [PubMed] [Google Scholar]
- 30.Eppig JJ, Downs SM. Gonadotropin-induced murine oocyte maturation in vivo is not associated with decreased cyclic adenosine monophosphate in the oocyte-cumulus cell complex. Gamete Res. 1988;20:125–131. doi: 10.1002/mrd.1120200203. [DOI] [PubMed] [Google Scholar]
- 31.Shim C, Lee DK, Lee CC, Cho WK, Kim K. Inhibitory effect of purines in meiotic maturation of denuded mouse oocytes. Mol Reprod Dev. 1992;31:280–286. doi: 10.1002/mrd.1080310409. [DOI] [PubMed] [Google Scholar]
- 32.Downs SM, Daniel SA, Bornslaeger EA, Hoppe PC, Eppig JJ. Maintenance of meiotic arrest in mouse oocytes by purines: modulation of cAMP levels and cAMP phosphodiesterase activity. Gamete Res. 1989;23:323–334. doi: 10.1002/mrd.1120230309. [DOI] [PubMed] [Google Scholar]
- 33.Eppig JJ, Downs SM. The effect of hypoxanthine on mouse oocyte growth and development in vitro: maintenance of meiotic arrest and gonadotropin-induced oocyte maturation. Dev Biol. 1987;119:313–321. doi: 10.1016/0012-1606(87)90037-6. [DOI] [PubMed] [Google Scholar]
- 34.Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol. 2001;233:258–270. doi: 10.1006/dbio.2001.0216. [DOI] [PubMed] [Google Scholar]
- 35.Browne CL, Wiley HS, Dumont JN. Oocyte-follicle cell gap junctions in Xenopus laevis and the effects of gonadotropin on their permeability. Science. 1979;203:182–183. doi: 10.1126/science.569364. [DOI] [PubMed] [Google Scholar]
- 36.Herlands RL, Schultz RM. Regulation of mouse oocyte growth: probable nutritional role for intercellular communication between follicle cells and oocytes in oocyte growth. J Exp Zool. 1984;229:317–325. doi: 10.1002/jez.1402290217. [DOI] [PubMed] [Google Scholar]
- 37.Eppig JJ. Further reflections on culture systems for the growth of oocytes in vitro. Hum Reprod. 1994;9:974–976. doi: 10.1093/oxfordjournals.humrep.a138669. [DOI] [PubMed] [Google Scholar]
- 38.Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207. doi: 10.1210/endo-129-6-3200. [DOI] [PubMed] [Google Scholar]
- 39.Downs SM, Daniel SA, Eppig JJ. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool. 1988;245:86–96. doi: 10.1002/jez.1402450113. [DOI] [PubMed] [Google Scholar]
- 40.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 41.Kanemitsu MY, Lau AF. Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12–0-tetrade-canoylphorbol 13-acetate-sensitive protein kinase C: the possible involvement of mitogen-activated protein kinase. Mol Biol Cell. 1993;4:837–848. doi: 10.1091/mbc.4.8.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia G, Byskov AG, Andersen CY. Cumulus cells secrete a meiosis-inducing substance by stimulation with forskolin and dibutyric cyclic adenosine monophosphate. Mol Reprod Dev. 1994;39:17–24. doi: 10.1002/mrd.1080390104. [DOI] [PubMed] [Google Scholar]
- 44.Byskov AG, Yding Andersen C, Hossaini A, Guoliang X. Cumulus cells of oocyte-cumulus complexes secrete a meiosis-activating substance when stimulated with FSH. Mol Reprod Dev. 1997;46:296–305. doi: 10.1002/(SICI)1098-2795(199703)46:3<296::AID-MRD8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 45.Byskov AG, Andersen CY, Nordholm L, et al. Chemical structure of sterols that activate oocyte meiosis. Nature. 1995;374:559–562. doi: 10.1038/374559a0. [DOI] [PubMed] [Google Scholar]
- 46.Lu Z, Xia G, Byskov AG, Andersen CY. Effects of amphotericin B and ketoconazole on mouse oocyte maturation: implications on the role of meiosis-activating sterol. Mol Cell Endocrinol. 2000;164:191–196. doi: 10.1016/s0303-7207(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 47.Xie H, Xia G, Byskov AG, Andersen CY, Bo S, Tao Y. Roles of gonadotropins and meiosis-activating sterols in meiotic resumption of cultured follicle-enclosed mouse oocytes. Mol Cell Endocrinol. 2004;218:155–163. doi: 10.1016/j.mce.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida Y, Yamashita C, Noshiro M, Fukuda M, Aoyama Y. Sterol 14-demethylase P450 activity expressed in rat gonads: contribution to the formation of mammalian meiosis-activating sterol. Biochem Biophys Res Commun. 1996;223:534–538. doi: 10.1006/bbrc.1996.0929. [DOI] [PubMed] [Google Scholar]
- 49.Grondahl C, Breinholt J, Wahl P, et al. Physiology of meiosis-activating sterol: endogenous formation and mode of action. Hum Reprod. 2003;18:122–129. doi: 10.1093/humrep/deg028. [DOI] [PubMed] [Google Scholar]
- 50.Gross MD, Gosnell M, Tsarbopoulos A, Hunziker W. A functional and degenerate pair of EF hands contains the very high affinity calcium-binding site of calbindin-D28K. J Biol Chem. 1993;268:20917–20922. [PubMed] [Google Scholar]
- 51.Tsafriri A, Popliker M, Nahum R, Beyth Y. Effects of ketoconazole on ovulatory changes in the rat: implications on the role of a meiosis-activating sterol. Mol Hum Reprod. 1998;4:483–489. doi: 10.1093/molehr/4.5.483. [DOI] [PubMed] [Google Scholar]
- 52.Tsafriri A, Cao X, Vaknin KM, Popliker M. Is meiosis activating sterol (MAS) an obligatory mediator of meiotic resumption in mammals. Mol Cell Endocrinol. 2002;187:197–204. doi: 10.1016/s0303-7207(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 53.Downs SM, Ruan B, Schroepfer GJ., Jr Meiosis-activating sterol and the maturation of isolated mouse oocytes. Biol Reprod. 2001;64:80–89. doi: 10.1095/biolreprod64.1.80. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita M, Kajiura H, Tanaka T, Onoe S, Nagahama Y. Molecular mechanisms of the activation of maturation-promoting factor during goldfish oocyte maturation. Dev Biol. 1995;168:62–75. doi: 10.1006/dbio.1995.1061. [DOI] [PubMed] [Google Scholar]
- 55.Faerge I, Terry B, Kalous J, et al. Resumption of meiosis induced by meiosis-activating sterol has a different signal transduction pathway than spontaneous resumption of meiosis in denuded mouse oocytes cultured in vitro. Biol Reprod. 2001;65:1751–1758. doi: 10.1095/biolreprod65.6.1751. [DOI] [PubMed] [Google Scholar]
- 56.Vaknin KM, Lazar S, Popliker M, Tsafriri A. Role of meiosis-activating sterols in rat oocyte maturation: effects of specific inhibitors and changes in the expression of lanosterol 14alpha-demethylase during the preovulatory period. Biol Reprod. 2001;64:299–309. doi: 10.1095/biolreprod64.1.299. [DOI] [PubMed] [Google Scholar]
- 57.Cavilla JL, Kennedy CR, Baltsen M, Klentzeris LD, Byskov AG, Hartshorne GM. The effects of meiosis activating sterol on in-vitro maturation and fertilization of human oocytes from stimulated and unstimulated ovaries. Hum Reprod. 2001;16:547–555. doi: 10.1093/humrep/16.3.547. [DOI] [PubMed] [Google Scholar]
- 58.Marin Bivens CL, Grondahl C, Murray A, Blume T, Su YQ, Eppig JJ. Meiosis-activating sterol promotes the metaphase I to metaphase II transition and preimplantation developmental competence of mouse oocytes maturing in vitro. Biol Reprod. 2004;70:1458–1464. doi: 10.1095/biolreprod.103.026351. [DOI] [PubMed] [Google Scholar]
- 59.Smith LD, Ecker RE, Subtelny S. In vitro induction of physiological maturation in Rana pipiens oocytes removed from their ovarian follicles. Dev Biol. 1968;17:627–643. doi: 10.1016/0012-1606(68)90010-9. [DOI] [PubMed] [Google Scholar]
- 60.Le Goascogne C, Sananes N, Gouezou M, Baulieu EE. Testosterone-induced meiotic maturation of Xenopus laevis oocytes: evidence for an early effect in the synergistic action of insulin. Dev Biol. 1985;109:9–14. doi: 10.1016/0012-1606(85)90340-9. [DOI] [PubMed] [Google Scholar]
- 61.Lutz LB, Jamnongjit M, Yang WH, Jahani D, Gill A, Hammes SR. Selective modulation of genomic and non-genomic androgen responses by androgen receptor ligands. Mol Endocrinol. 2003;17:1106–1116. doi: 10.1210/me.2003-0032. [DOI] [PubMed] [Google Scholar]
- 62.Bayaa M, Booth RA, Sheng Y, Liu XJ. The classical progesterone receptor mediates Xenopus oocyte maturation through a nongenomic mechanism. Proc Natl Acad Sci USA. 2000;97:12607–12612. doi: 10.1073/pnas.220302597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian J, Kim S, Heilig E, Ruderman JV. Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation. Proc Natl Acad Sci USA. 2000;97:14358–14363. doi: 10.1073/pnas.250492197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamashita Y, Shimada M, Okazaki T, Maeda T, Terada T. Production of progesterone from de novo-synthesized cholesterol in cumulus cells and its physiological role during meiotic resumption of porcine oocytes. Biol Reprod. 2003;68:1193–1198. doi: 10.1095/biolreprod.102.010934. [DOI] [PubMed] [Google Scholar]
- 65.Sadler SE, Jacobs ND. Stimulation of Xenopus laevis oocyte maturation by methyl-beta-cyclodextrin. Biol Reprod. 2004;70:1685–1692. doi: 10.1095/biolreprod.103.026161. [DOI] [PubMed] [Google Scholar]
- 66.Eppig JJ, Freter RR, Ward-Bailey PF, Schultz RM. Inhibition of oocyte maturation in the mouse: participation of cAMP, steroid hormones, and a putative maturation-inhibitory factor. Dev Biol. 1983;100:39–49. doi: 10.1016/0012-1606(83)90198-7. [DOI] [PubMed] [Google Scholar]
- 67.Rice C, McGaughey RW. Effect of testosterone and dibutyryl cAMP on the spontaneous maturation of pig oocytes. J Reprod Fertil. 1981;62:245–256. doi: 10.1530/jrf.0.0620245. [DOI] [PubMed] [Google Scholar]
- 68.Smith DM, Tenney DY. Effects of steroids on mouse oocyte maturation in vitro. J Reprod Fertil. 1980;60:331–338. doi: 10.1530/jrf.0.0600331. [DOI] [PubMed] [Google Scholar]
- 69.Schultz RM, Montgomery RR, Ward-Bailey PF, Eppig JJ. Regulation of oocyte maturation in the mouse: possible roles of intercellular communication, cAMP, and testosterone. Dev Biol. 1983;95:294–304. doi: 10.1016/0012-1606(83)90030-1. [DOI] [PubMed] [Google Scholar]
- 70.Gill A, Jamnongjit M, Hammes SR. Androgens promote maturation and signaling in mouse oocytes independent of transcription: a release of inhibition model for mammalian oocyte meiosis. Mol Endocrinol. 2004;18:97–104. doi: 10.1210/me.2003-0326. [DOI] [PubMed] [Google Scholar]
- 71.Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, Zelinski-Wooten MB. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod. 2004;71:366–373. doi: 10.1095/biolreprod.103.023390. [DOI] [PubMed] [Google Scholar]
- 72.Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;96:520–527. doi: 10.1172/JCI118064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 74.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 75.Pache TD, Chadha S, Gooren LJ, et al. Ovarian morphology in long-term androgen-treated female to male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopathology. 1991;19:445–452. doi: 10.1111/j.1365-2559.1991.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 76.Speiser PW. Congenital adrenal hyperplasia: transition from childhood to adulthood. J Endocrinol Invest. 2001;24:681–691. doi: 10.1007/BF03343913. [DOI] [PubMed] [Google Scholar]
- 77.Ito Y, Fisher CR, Conte FA, Grumbach MM, Simpson ER. Molecular basis of aromatase deficiency in an adult female with sexual infantilism and polycystic ovaries. Proc Natl Acad Sci USA. 1993;90:11673–11677. doi: 10.1073/pnas.90.24.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gambineri A, Pelusi C, Genghini S, et al. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60:241–249. doi: 10.1111/j.1365-2265.2004.01973.x. [DOI] [PubMed] [Google Scholar]
- 79.Rittmaster RS. Antiandrogen treatment of polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:409–421. doi: 10.1016/s0889-8529(05)70077-3. [DOI] [PubMed] [Google Scholar]
- 80.Eagleson CA, Gingrich MB, Pastor CL, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–4052. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 81.De Leo V, Lanzetta D, D’Antona D, la Marca A, Morgante G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:99–102. doi: 10.1210/jcem.83.1.4500. [DOI] [PubMed] [Google Scholar]
- 82.Koivunen RM, Morin-Papunen LC, Ruokonen A, Tapanainen JS, Martikainen HK. Ovarian steroidogenic response to human chorionic gonadotrophin in obese women with polycystic ovary syndrome: effect of metformin. Hum Reprod. 2001;16:2546–2551. doi: 10.1093/humrep/16.12.2546. [DOI] [PubMed] [Google Scholar]
- 83.Balen A. Ovulation induction for polycystic ovary syndrome. Hum Fertil (Camb) 2000;3:106–111. doi: 10.1080/1464727002000198791. [DOI] [PubMed] [Google Scholar]
- 84.Coffler MS, Patel K, Dahan MH, et al. Evidence for abnormal granulosa cell responsiveness to follicle-stimulating hormone in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:1742–1747. doi: 10.1210/jc.2002-021280. [DOI] [PubMed] [Google Scholar]


