Palliation for osteosarcoma
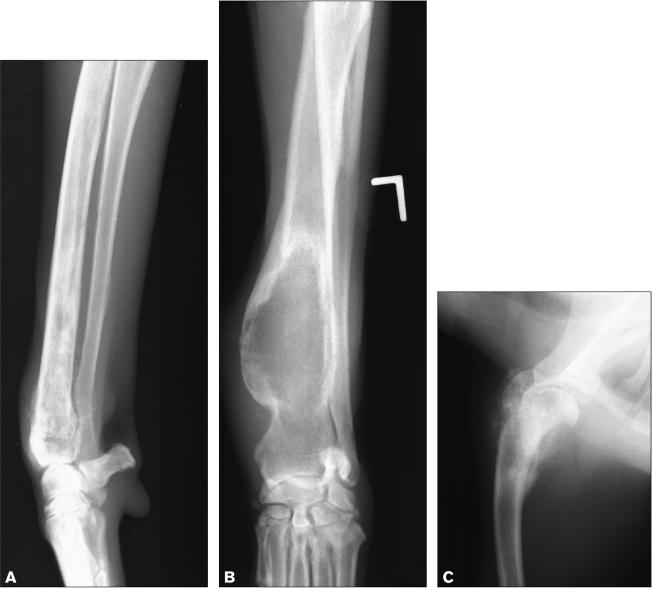
Osteosarcoma accounts for approximately 85% of primary bone cancers in the dog (1). It is a common cancer of large to giant breed dogs, and it occurs primarily in the appendicular skeleton (1). Osteosarcoma can appear as a lytic, productive or, most commonly, mixed lytic-productive lesion on radiographs (Figure 1) (2). Even with removal of the primary tumor before spread of the cancer is clinically detectable, metastases to lung, bone, or other sites eventually develop in almost all dogs. Palliative radiation therapy for bone pain is indicated in those dogs that do not undergo amputation or a limb-sparing surgery.
Figure 1.
Radiographs of 3 dogs with histologically confirmed osteosarcoma. The bone lesions show varying degrees of lysis and proliferation.
The goal of palliative therapy in cancer patients is to control pain from an incurable tumor and to support overall quality of life (3). Radiation protocols that have minimal risk of acute side effects are used, so that no discomfort results from treatment. The presence of pulmonary metastases is not a contraindication to palliative radiation treatment, as long as the patient is not showing clinical signs related to their metastatic disease.
Bone cancer pain
Two types of bone cancer pain are described in human patients, ongoing pain and breakthrough pain (4). Ongoing pain is described as a dull, constant, aching pain that increases in intensity with progression of the tumor. Breakthrough pain, intermittent episodes of extreme pain, occurs spontaneously or after movement or weight-bearing of the affected leg (4). Canine osteosarcoma has many clinical and biological similarities to human osteosarcoma, and affected dogs show signs of both ongoing and breakthrough pain (1).
Bone cancer pain results from the direct stimulation of afferent pain fibers by mechanical injury or chemical mediators (5). Specialized sensory neurons capable of detecting physical and chemical noxious stimuli, known as nociceptors, are present in periosteum, mineralized bone, and bone marrow (6). Nociceptors recognize physical stretch, as well as products released by cells during excessive mechanical stimulation. Potential mechanical stimuli associated with osteosarcoma include increased pressure within bone, stretching of the periosteum, microfractures, and compression of nerves resulting from bone collapse (5). Nociceptors also express receptors that are activated by chemical mediators released by tumor cells and by host cells in the tumor microenvironment (6).
In response to sustained stimulation, the threshold of nociceptor activation is lowered, resulting in peripheral sensitization characterized by hyperalgesia (an increased pain response to a stimulus that is normally painful) and allodynia (a pain response to a stimulus that normally does not cause pain) (6). Bone cancer pain may also result in central sensitization at the level of the spinal cord (7). Dogs being presented for bone cancer pain often exhibit hyperalgesia and allodynia (1).
Because bone cancer pain appears to be caused by multiple mechanisms, using therapies that target only 1 or 2 of these components may result in lack of effective pain control. For dogs not undergoing amputation for pain relief, palliative therapy may include a combination of radiation therapy, nonsteroidal anti-inflammatories, opioids, bisphosphonates, and drugs to treat neuropathic pain (8).
Palliative intent radiation therapy is an effective treatment for bone cancer pain, with 74% to 96% of canine patients experiencing some degree of pain relief (9–12). Irradiation may decrease bone cancer pain by a direct killing of tumor cells and inflammatory cells, and by reducing bone destruction by osteoclasts (5,13). Irradiation of lytic bone will often result in formation of mature organized bone in unfractured tumor sites (14).
Prostaglandins produced by cancer cells and tumor-associated macrophages can directly stimulate nociceptors as well as promote inflammation (6). Seventy-seven percent of canine appendicular osteosarcomas were positive for cyclooxygenase-2 (COX2) expression (15). Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce pain through COX2 inhibition at the peripheral tumor level, as well as at the level of the spinal cord neurons (16). The COX2 inhibition may also directly retard tumor growth and have an antiangiogenic effect (6).
Opioids can be used at a range of doses to achieve optimal pain relief for an individual animal with a minimum of side effects (8). Tramadol is a weak opioid that has been used to treat cancer-related pain in dogs (17). In addition to a moderate mu opioid receptor affinity, tramadol also activates both the descending serotoninergic and noradrenergic pain inhibitory pathways by inhibiting serotonin and norepinephrine reuptake (18).
Bisphosphonates are a family of drugs that inhibit bone resorption through an inhibitory effect on osteoclast activity (19). Bisphosphonates have also been proposed to inhibit proliferation of tumor cells, inhibit angiogenesis, and induce apoptosis of tumor cells. A recent study evaluating the safety of pamidronate in 33 dogs with primary or metastatic bone cancer concluded that pamidronate was well tolerated and may help to relieve pain related to bone tumors (20).
Destruction of sensory nerve fibers by tumors has been demonstrated in animal models of bone cancer, resulting in a neuropathic component to pain (6). Drugs effective against neuropathic pain, such as gabapentin, may increase the level of pain relief. Gabapentin may inhibit pain signal transmission at the level of the dorsal horn of the spinal cord through antagonism of NMDA (N-methyl d-aspartate) receptors and inhibition of calcium channels (21). Gabapentin has been shown to be efficacious on hyperalgesia and allodynia in a rat model of bone cancer pain, and in human patients with cancer-related pain (22,23).
The benefit of chemotherapy in combination with palliative radiation treatment is not well defined at this time. In one study of 95 dogs treated palliatively for appendicular osteosarcoma, dogs receiving platinum chemotherapy were more likely to exhibit a decrease in pain or an improvement in lameness after radiation therapy than dogs that did not receive chemotherapy (10). The duration of decreased pain or improved lameness was also significantly longer in dogs receiving chemotherapy in this study. However, other studies did not report an increase in response rate or in duration of response when chemotherapy was added to palliative radiation protocols (11,12).
Palliative external beam radiation therapy
Palliative radiation protocols vary and the optimal dose and timing of treatment is not known. A recent prospective clinical trial in human patients with bone metastases showed equivalent pain relief when a single larger dose of radiation and multiple smaller doses were used over a 3-wk period (24). In most published veterinary palliative protocols, a larger dose is given approximately once a week for 2 to 4 treatments (9–12). Median time to improvement in limb function after radiation treatment has been reported at 11 to 15 d (9–12). The median duration of partial or complete pain relief ranges from 1.8 to 4.3 mo. Median survival in dogs with osteosarcoma after palliative radiation therapy ranges from 4.1 to 10.4 mo (9–12). Cause of death is most often euthanasia due to a decreased quality of life caused by local tumor progression or metastatic disease.
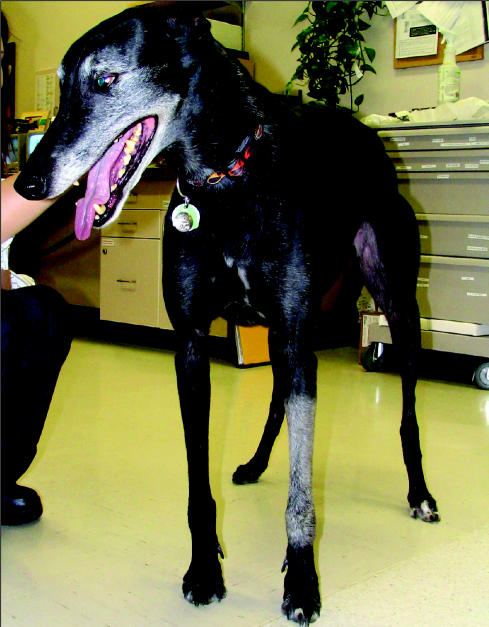
The current protocol at the Western College of Veterinary Medicine (WCVM) for palliation of extremity osteosarcoma is 2 fractions: 800 cGy (1 cGy = 1 rad) followed by 800 cGy 24 h later for a total of 1600 cGy. If a presumptive diagnosis of osteosarcoma is supported by history, signalment, and radiographic appearance of the lesion, a patient may be treated palliatively at the WCVM without a cytological or histopathological diagnosis. Radiation therapy is generally not recommended if a pathological fracture is present. Acute skin side effects of radiation therapy, such as erythema and desquamation, are generally absent to minimal with this protocol. Most patients develop a change in hair color at the treated site several months after completing radiation treatment (Figure 2). If relief of pain is achieved in a patient after the 1st radiation treatment, additional treatments can be administered following a similar protocol when pain progresses.
Figure 2.
A dog treated 3 mo previously with palliative intent radiation for osteosarcoma. Leukotrichia is present in the treated area over the left radius.
References
- 1.Dernell WS, Straw RC, Withrow SJ. Tumors of the skeletal system. In: Withrow SJ, MacEwen EG, eds. Small Animal Clinical Oncology. Philadelphia, WB Saunders, 2001:378–417.
- 2.Thrall DE. Bone tumors versus bone infections. In: Thrall DE, ed. Textbook of Veterinary Diagnostic Radiology. Philadelphia, WB Saunders, 2002:179–186.
- 3.Thrall DE, LaRue SM. Palliative radiation therapy. Semin Vet Med Surg (Small Anim) 1995;10:205–208. [PubMed] [Google Scholar]
- 4.Peters CM, Ghilardi JR, Keyser CP, et al. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp Neurol. 2005;193:85–100. doi: 10.1016/j.expneurol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Vakaet LA, Boterberg T. Pain control by ionizing radiation of bone metastasis. Int J Dev Biol. 2004;48:599–606. doi: 10.1387/ijdb.041817lv. [DOI] [PubMed] [Google Scholar]
- 6.Sabino MA, Mantyh PW. Pathophysiology of bone cancer pain. J Support Oncol. 2005;3:15–24. [PubMed] [Google Scholar]
- 7.Schwei MJ, Honore P, Rogers SD, et al. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999;19:10886–10897. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lorimier L, Fan TM. Treating cancer pain in dogs and cats. Vet Med. 2005;100:364–379. [Google Scholar]
- 9.McEntee MC, Page RL, Novotney CA, Thrall DE. Palliative radiotherapy for canine appendicular osteosarcoma. Vet Radiol Ultrasound. 1993;34:367–370. [Google Scholar]
- 10.Ramirez O, III, Dodge RK, Page RL, et al. Palliative radiotherapy of appendicular osteosarcoma in 95 dogs. Vet Radiol Ultrasound. 1999;40:517–522. doi: 10.1111/j.1740-8261.1999.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 11.Green EM, Adams WM, Forrest LJ. Four fraction palliative radiotherapy for osteosarcoma in 24 dogs. J Am Anim Hosp Assoc. 2002;38:445–451. doi: 10.5326/0380445. [DOI] [PubMed] [Google Scholar]
- 12.Mueller F, Poirier V, Melzer K, Nitzl D, Roos M, Kaser-Hotz B. Palliative radiotherapy with electrons of appendicular osteosarcoma in 54 dogs. In Vivo. 2005;19:713–716. [PubMed] [Google Scholar]
- 13.Goblirsch MJ, Zwolak P, Clohisy DR. Advances in understanding bone cancer pain. J Cell Biochem. 2005;96:682–688. doi: 10.1002/jcb.20589. [DOI] [PubMed] [Google Scholar]
- 14.Perez CA, Brady LW, Halperin EC, Schmidt-Ullrich RK. Principles and Practice of Radiation Oncology. Philadelphia: Lippincott Williams & Wilkins, 2004.
- 15.Mullins MN, Lana SE, Dernell WS, Ogilvie GK, Withrow SJ, Ehrhart EJ. Cyclooxygenase-2 expression in canine appendicular osteosarcomas. J Vet Intern Med. 2004;18:859–865. doi: 10.1892/0891-6640(2004)18<859:ceicao>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Sabino MA, Ghilardi JR, Jongen JL, et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. 2002;62:7343–7349. [PubMed] [Google Scholar]
- 17.Parker R. Tramadol. Compend Contin Educ Pract Vet. 2004;26:800–802. [Google Scholar]
- 18.Leppert W, Luczak J. The role of tramadol in cancer pain treatment — a review. Support Care Cancer. 2005;13:5–17. doi: 10.1007/s00520-004-0720-4. [DOI] [PubMed] [Google Scholar]
- 19.Milner RJ, Farese J, Henry CJ, Selting K, Fan TM, de Lorimier LP. Bisphosphonates and cancer. J Vet Intern Med. 2004;18:597–604. doi: 10.1892/0891-6640(2004)18<597:bac>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Fan TM, de Lorimier LP, Charney SC, Hintermeister JG. Evaluation of intravenous pamidronate administration in 33 cancer-bearing dogs with primary or secondary bone involvement. J Vet Intern Med. 2005;19:74–80. doi: 10.1892/0891-6640(2005)19<74:eoipai>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Rose MA, Kam PC. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57:451–462. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 22.Caraceni A, Zecca E, Bonezzi C, et al. Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. J Clin Oncol. 2004;22:2909–2917. doi: 10.1200/JCO.2004.08.141. [DOI] [PubMed] [Google Scholar]
- 23.Donovan-Rodriguez T, Dickenson AH, Urch CE. Gabapentin normalizes spinal neuronal responses that correlate with behavior in a rat model of cancer-induced bone pain. Anesthesiology. 2005;102:132–140. doi: 10.1097/00000542-200501000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short-versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]