Abstract
A young, male miniature poodle was presented with severe neurological problems. Laboratory tests and ultrasonograph examination were consistent with extrahepatic portosystemic shunts, resulting in hepatic encephalopathy. When surgical correction proved not to be a viable option, the dog was euthanized. Postmortem examination revealed multiple shunts likely acquired after severe hepatitis.
Résumé
Shunts portosystémiques extrahépatiques acquis chez un jeune chien. Un jeune caniche miniature mâle a été présenté pour de graves problèmes neurologiques. Les tests de laboratoires et l’examen échographique étaient compatibles avec des shunts portosystémiques extrahépatiques ayant pour résultat une encéphalopathie hépatique. Lorsque la correction chirurgicale s’est avérée être une option non viable, le chien a été euthanasié. L’examen post-mortem a révélé de multiples shunts vraisemblablement acquis à la suite d’une grave hépatite.
(Traduit par Docteur André Blouin)
A seemingly well-cared for male poodle, approximately 2- to 4-y-old, was admitted to the Small Animal Clinic at the Western College of Veterinary Medicine (WCVM) after being found almost comatose in a park. On initial examination, the dog had harsh, labored breathing and pale mucous membranes, and was completely nonresponsive to external stimuli. Although his temperature, heart rate, and respiratory rate were all within normal limits, the dog’s limbs were cold to the touch. His front limbs were rigidly extended and there were decreased reflexes in his hind limbs. An emergency biochemical and hematological panel (packed cell volume 0.46 L/L, normal range, 0.37 to 0.55 L/L; total protein 58 g/L, normal range, 55 to 75 g/L; blood glucose 5.1 mmol/L, normal range 3.4 to 6.0 mmol/L; blood urea nitrogen 5 to 10 mmol/L, normal range, 3.1 to 9.2 mmol/L) and an electrocardiogram (ECG) revealed no abnormalities. Abdominal radiographs showed increased opacity in the caudal part of the abdomen and an impacted colon. No fluid was obtained on abdominal aspiration. The dog was placed on IV lactated Ringer’s solution, supplemented with 20 mEq potassium chloride, at 30 mL/h and was closely observed overnight.
The following day, the dog was able to stand, although at first he was extremely ataxic with severe proprioceptive deficits (knuckling on all 4 limbs). Other signs of altered central nervous system (CNS) function included a bilateral lack of menace reflexes; constricted pupils; a head tilt; tremors; poor anal tone; and incessant, frantic barking. His temperature was slightly elevated (39.6°C).
A complete blood (cell) count (CBC) revealed acute, severe inflammation (white blood cells 21.3 × 109/L, normal range, 6 to 17 × 109/L; bands 5.112 × 109/L, normal range 0 to 0.3 × 109/L 1+ toxic change; lymphocytes 0.213 × 109/L, normal range, 1 to 4.8 × 109/L; and monocytes 2.130 × 109/L, normal range, 0.15 to 1.35 × 109/L). A biochemical panel showed extremely elevated hepatic enzymes, indicative of both hepatocellular damage (alanine aminotransferase 903 U/L, normal range, 8.2 to 57 U/L; sorbitol dehydrogenase 8.0 U/L, normal range, 3.1 to 7.6 U/L; glutamate dehydrogenase 22 U/L, normal range, 24 to 219 U/L) and cholestasis (alkaline phosphatase 267 U/L, normal range, 10.6 to 101 U/L; gamma glutamyl transferase 17 U/L, normal range, 1.0 to 9.7 U/L). There was increased total plasma bilirubin (13 μmol/L, normal range, 0.9 to 10.6 μmol/L) and low cholesterol (1.80 mmol/L, normal range, 3.0 to 6.6 mmol/L). The urea and albumin levels were low normal (3.8 mmol/L, normal range, 3.1 to 9.2 mmol/L, and 28 g/L normal range, 26 to 40 g/L, respectively). Urinalysis revealed the presence of ammonium urate crystals, which, when combined with the biochemical results, strongly supported a diagnosis of hepatic insufficiency. It was considered most likely that a hepatic encephalopathy was responsible for the dog’s neurological deficits.
Under normal circumstances, a more aggressive diagnostic and therapeutic plan would have been instituted immediately. However, this dog arrived at the clinic as a stray and, due to financial and ethical restrictions, therapy was initiated slowly while the local Society for the Prevention of Cruelty to Animals was contacted and attempts were made to find an owner.
Over the next few days, a variety of medical therapies were introduced in an effort to decrease the dog’s neurological signs. The dog was given a single dose of mannitol (Mannitol 25%; Abbott Laboratories, Montreal, Quebec), 0.5 g/kg bodyweight (BW), IV. As ammonia generated from endogenous and exogenous protein catabolism is considered one of the most important cerebral toxins implicated in hepatic encephalopathy, efforts were made to reduce the amount of ammonia present in the dog’s blood. A prescription diet (Hill’s k/d; Hill’s Pet Nutrition, Topeka, Kansas, USA) was fed in order to reduce the dietary nitrogen available to ammonia producing colonic bacteria. This diet is highly digestible, uses carbohydrates as a primary source of calories, contains high levels of branched-chain amino acids and arginine, and, most importantly, contains only 3.1 g of protein per 100 kcal fed. Lactulose (Lactulose; Pharmascience, Montreal, Quebec), 0.5 mL/kg BW, PO, q8h, was administered to decrease fecal transit time and acidify colonic contents. The dog was given amoxicillin (Amoxil; Pfizer Canada, Kirkland, Quebec), 20 mg/kg BW, PO, and warm water enemas q8h to reduce the ammonia producing bacterial populations in the colon. Gradually, the dog’s neurological signs dissipated and 1 wk after initial presentation, both his mental and physical status were outwardly normal.
An abdominal ultrasonograph revealed an unusually small liver and an abnormal vascular configuration at the caudal pole of the right kidney. The anomalous vessel was consistent with a large prehepatic portal-caval shunt. The dog was diagnosed presumptively as having a single congenital portosystemic shunt (PSS) whose injurious effects could most likely be mitigated with surgical attenuation or ligation. Funds were raised, and 11 d after the initial presentation, an attempt was made to ligate the anomalous vessel.
An anesthesic premedication of hydromorphone (WCVM Teaching Hospital), 0.1 mg/kg BW, SC, was administered. Induction and maintenance of anesthesia were achieved with inhaled isoflurane and nitrous oxide gas. A 12-cm, midline incision was made through the dog’s abdominal wall. On initial exploration of the peritoneal cavity, multiple abnormalities were noted. Approximately 200 mL of serous fluid was present. The liver was small, firmer than normal, and pale. There were numerous abnormal vessels. A single large (7 mm in diameter) vessel arose from the left renal vein and terminated beyond the area visible from the celiotomy incision. In addition, there were several abnormal vessels ranging from 1 to 3 mm in diameter, within the mesentery between the spleen and the major shunt vessel and between the pancreas and the shunt vessel. Various other anomalous vessels were present in the mesentery of the colon, extending between the left kidney and the shunt. Intraoperative portography was performed to see if the largest shunt vessel did, in fact, by-pass the liver. Positive contrast media injected through a catheter into the shunt did not end up in the liver. Due to the existence of multiple shunt vessels, the dog’s condition was deemed inoperable. As an owner had never been found and life-long medical management made the dog a poor candidate for adoption, euthanasia was the best solution. Sodium pentobarbital (Euthanyl; Bimeda-MTC; Kirkland, Quebec), 120 mg/kg BW, was administered.
Necropsy confirmed the surgical findings. The largest shunt vessel terminated within the liver parenchyma. Histopathologic examination of the liver revealed subacute, generalized severe hepatitis. The hepatocytes were separated and surrounded by both fibrous tissue and a moderate number of inflammatory cells, including lymphocytes, plasma cells, and neutrophils. Histopathologic examination of the brain revealed Alzhemier type II astroctyes. In hepatic failure, astrocytes proliferate without eosinophilia developing. These cells, known as Alzheimer Type II astrocytes, are characterized by irregularly shaped nuclei with an exaggerated vesicular appearance. In addition, the dog’s brain showed mild to moderate spongy change or polymicrocavitation of the white matter in the brain stem and internal capsule.
Portosystemic shunts are anomalous vessels that divert portal blood from the abdominal viscera to the heart, bypassing the hepatic sinusoids and carrying nutrients and toxicants from intestines directly into systemic circulation. Portosystemic shunts can be classified as extrahepatic, located outside the hepatic parenchyma, or intrahepatic, located within the liver. Extrahepatic PSS may be congenital or acquired.
Congenital extrahepatic PSS are irregular connections between the vitelline and cardinal venous systems. The condition usually results in a single, large anomalous vessel and is rarely associated with hepatic disease or portal hypertension.
Acquired PSS develop secondary to portal hypertension. They are defined as multiple, tortuous anastomotic vessels that represent the recanalization of preexistent portosystemic collateral vessels (1). Such shunts are most commonly seen in adult dogs with chronic, severe hepatic disease (cirrhosis). Younger dogs can acquire PSS subsequent to hepatoportal fibrosis resulting from diseases such as lobular dissecting hepatitis (2,3).
The existence of both congenital and acquired shunts in the same dog has been debated among veterinary specialists (4,5). This explanation is most often proposed when one large anomalous branch of the portal vein and multiple small tortuous collateral vessels are identified in the same dog, which describes the anatomic anomalies found in the dog in this case. Basic hemodynamic principles dictate that fluids flow through vessels presenting the lowest resistance. The pressures required to recanalize additional, acquired shunts would only occur if the existing congenital shunt became occluded or was too small to handle the entire blood volume being delivered. In the current case, the large shunt identified on ultrasonography was patent. Although acquired PSS are usually small and tortuous, they can also be wide veins (5). With this possibility, combined with the histological finding of severe liver disease, the dog’s final diagnosis was multiple PSS, acquired secondarily to portal hypertension.
The etiology of the dog’s primary hepatic disease is unknown. Chronic hepatitis is the current terminology encompassing a heterogenous group of mixed inflammatory-necrotizing disorders of the liver (1). Hepatoxicity, hypoxic injury, and chronic inflammation can all produce chronic hepatitis through a pathogenesis that is poorly understood. A liver with chronic inflammation is typically characterized by lymphocytic and plasmacytic infiltrates with varying degrees of biliary hyperplasia and fibrosis. Long-standing inflammation progresses to cirrhosis and endstage liver disease. Cirrhosis is characterized by fibrosis and destruction of the hepatic lobular architecture, which results in portal hypertension. Possible ingestion of toxins or previously existing medical conditions of this dog were unknown, so the etiology or original insult to the liver remains a mystery.
The diagnosis of extrahepatic PSS is complicated, and distinguishing a single congenital shunt from multiple acquired shunts can be extremely difficult. The clinical signs are variable and typically wax and wane. Certain events, such as the consumption of a large protein meal, gastrointestinal hemorrhage, dehydration, or constipation, can precipitate more severe clinical symptoms. Signs of hepatoencephalopathy often dominate the clinical syndrome because of inadequate hepatic clearance of enteric toxins, such as ammonia, mercaptans, short-chain fatty acids, gamma-aminobutyric acid, and endogenous benzodiazepines (6). Similar biochemical abnormalities can be seen with both congenital and acquired shunts, although the abnormalities are frequently more severe with acquired shunts, because of the underlying primary hepatic disease that precipitated their development. In an Australian study that reviewed several hundred cases of PSS, it was found that dogs with multiple acquired shunts were indistinguishable from those with single congenital shunts, based solely on clinical abnormalities or results of biochemical analysis (7).
Ultrasonography is the most common imaging technique used to diagnose PSS. The majority of affected animals have some degree of microhepatica due to insufficient hepatic perfusion, a deficiency of hepatotrophic factors, such as insulin and glucagons, and functional parenchyma being replaced by fibrous tissue. Finding extrahepatic PSS with ultrasonography is challenging, because the shunts are often obscured by gas-filled intestines. Overlying ribs and lungs may also interfere with a comprehensive evaluation. Finding 1 conspicuous anomalous vessel in a young dog could lead to a premature diagnosis of a congenital PSS and may preclude a thorough search for other shunting vessels. Other advanced imaging techniques, such as nuclear scintigraphy or contrast portal venography, allow more thorough investigation.
The problems from a single, congenital PSS can often be resolved with surgery. However, surgery is ineffective in dealing with multiple acquired shunts resulting from severe hepatitis. Despite advances in modern veterinary medicine, cirrhosis is still an irreversible pathologic state of the liver. Unfortunately, it can be difficult to differentiate inoperable shunts from operable ones prior to laparotomy or necropsy examination.
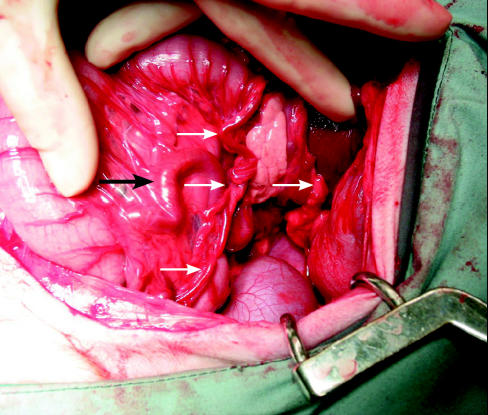
Figure 1.
Intraoperative photograph of the dog’s peritoneal cavity. The large black arrow identifies the largest shunt vessel, which arises from the left renal vein and terminates within the liver. It is also possible to see the numerous tortuous vessels coursing through the mesentery of the colon (smaller arrows).
Acknowledgments
The author acknowledges Drs. Susan Taylor, Kathleen Linn, Cindy Shmon, and especially Dr. Elizabeth Snead for their support with this case. CVJ
Footnotes
Dr. Agg will receive 50 free reprints of her article, courtesy of The Canadian Veterinary Journal.
Dr. Agg’s current address is Parkway Animal Hospital, 2655 Edenbower Boulevard, Roseburg, Oregon 97470, USA.
References
- 1.Fossum TW. Small Animal Surgery, 2nd ed. St. Louis, Mosby, 2002:457–468.
- 2.Bennett AM, Davies JD, Gaskell CJ, Lucke VM. Lobular dissecting hepatitis in the dog. Vet Pathol. 1983;20:179–188. doi: 10.1177/030098588302000205. [DOI] [PubMed] [Google Scholar]
- 3.van den Ingh T, Rothuizen J. Lobular dissecting hepatitis in juvenile and young adult dogs. J Vet Intern Med. 1994;8:217–220. doi: 10.1111/j.1939-1676.1994.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferrell E, Graham JP, Hamel R, Randell S, Farese JP, Castleman WL. Simultaneous congenital and acquired extraheptaic portosystemic shunts in two dogs. Vet Radiol Ultrasound. 2003;44:38–42. doi: 10.1111/j.1740-8261.2003.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 5.Szatmári V. Simultaneous congenital and acquired extrahepatic portosystemic shunts in two dogs (Letter] Vet Radiol Ultrasound. 2003;44:486–487. doi: 10.1111/j.1740-8261.2003.tb00491.x. [DOI] [PubMed] [Google Scholar]
- 6.Maddison JE. Canine congenital portosystemic encephalopathy. Aust Vet J. 1988;65:245–249. doi: 10.1111/j.1751-0813.1988.tb14310.x. [DOI] [PubMed] [Google Scholar]
- 7.Hunt GB. Effect of breed on anatomy of portosystemic shunts resulting from congenital disease in dogs and cats: a review of 242 cases. Aust Vet J. 2004;82:746–749. doi: 10.1111/j.1751-0813.2004.tb13233.x. [DOI] [PubMed] [Google Scholar]