Abstract
Objective
To determine the effects of basic fibroblast growth factor (bFGF) on the chondrocyte anabolic activity promoted by insulin-like growth factor 1 (IGF-1) and osteogenic protein 1 (OP-1).
Methods
Human articular chondrocytes were cultured in alginate beads or as cartilage explants in serum-free medium with or without IGF-1 (100 ng/ml), OP-1 (100 ng/ml), or bFGF (0–100 ng/ml). Cell survival, proliferation, proteoglycan synthesis, and total proteoglycan accumulation were measured after 21 days of culture in alginate beads, and proteoglycan synthesis was measured in explants.
Results
Cell survival was not altered by bFGF at any dose, and chondrocyte proliferation was stimulated only at doses above 1 ng/ml. When combined with IGF-1, 1 ng/ml of bFGF stimulated proliferation to 170% of control, but when combined with IGF-1 and OP-1, proliferation increased to 373% of control. Doses of bFGF of 100 ng/ml decreased total proteoglycan levels accumulated per cell by 60% compared with control and also inhibited the ability of IGF-1 or OP-1 to increase proteoglycan production. Likewise, sulfate incorporation in response to IGF-1 and OP-1 alone or together was completely inhibited by 50 ng/ml bFGF in both alginate and explant cultures.
Conclusion
The anabolic activity of IGF-1 and OP-1, alone and in combination, is significantly inhibited by bFGF. The results suggest that excessive release of bFGF from the cartilage matrix during injury, with loading, or in arthritis could contribute to increased proliferation and reduced anabolic activity in articular cartilage.
Maintenance of the integrity of articular cartilage and its ability to react to mechanical loads and injury requires a properly orchestrated response of the chondrocyte to cell signals generated by growth factors, cytokines, and the extracellular matrix. Growth factors are important positive regulators of cartilage homeostasis due to their ability to stimulate chondrocyte anabolic activity and, in some cases, inhibit catabolic activity. A number of growth factors have been found to be present and active in adult articular cartilage, including insulin-like growth factor 1 (IGF-1), osteogenic protein 1 (OP-1; or bone morphogenetic protein 7 [BMP-7]), transforming growth factor β, BMP-2, basic fibroblast growth factor (bFGF; or FGF-2), cartilage-derived morphogenetic proteins, and human cartilage glycoprotein 39 (1–5). The basic biologic function of these various growth factors has most often been studied in vitro by testing individual factors in isolation. In vivo, growth factors most likely act in an environment in which multiple factors work in concert. Therefore, a better understanding of growth factor function requires additional studies using combinations of key growth factors.
In the present study, we chose to focus on the growth factor response of chondrocytes from normal adult human articular cartilage. Although growth factor activity is vital for cartilage during development and maturation, it is also important to study growth factor activity using chondrocytes from older adult articular cartilage. This is the tissue that is most susceptible to the development of osteoarthritic (OA) changes, including chondrocyte proliferation and increased anabolic activity in early phases of the disease and increased catabolic activity and cell death in later phases (for review, see refs. 6–8). Older adult articular cartilage is also the tissue that is considered to be a potential target for growth factor therapy designed to boost cartilage matrix production and prevent cartilage loss during the development of arthritis.
The growth factors chosen for this study were IGF-1, OP-1, and bFGF. IGF-1 is a well-known cartilage growth factor that is found in synovial fluid at concentrations of ~50 ng/ml and is produced by chondrocytes and stored in the cartilage matrix at concentrations of ~10 ng per gram of normal cartilage and ~50 ng per gram of OA cartilage (9). IGF-1 is thought to be the major stimulator of chondrocyte proteoglycan synthesis found in serum and synovial fluid (10,11), although to be active, it needs to be released from its binding proteins. OP-1 is a potent anabolic factor that is also produced by chondrocytes and is found in normal cartilage matrix at concentrations of 50 ng per gram of dry tissue and ~5 ng per gram of OA cartilage (12,13). In a previous study (14), it was found that neither IGF-1 nor OP-1 alone acted as a mitogen, but the combination of IGF-1 and OP-1 stimulated a 2-fold increase in chondrocyte numbers, as measured by DNA quantitation after 21 days of alginate culture using cells that had been isolated from older adults. Importantly, IGF-1 plus OP-1 also resulted in a 3-fold increase in proteoglycan production, normalized for changes in cell numbers, so that the total amount of matrix produced by the combination of growth factors was more than additive of that produced by each factor alone, suggesting that these anabolic factors may work in concert.
Unlike the activity of IGF-1 and OP-1, it has been recognized for quite some time that bFGF is a potent mitogen for adult articular chondrocytes (15–17), but its effect on adult articular chondrocyte matrix production has been less clear. In some studies it has been shown to stimulate proteoglycan synthesis (17–19), while in others, it was inhibitory or had no effect (15,16,20). Few, if any, studies of bFGF have examined articular chondrocytes from older adult humans. It is important to better define the action of bFGF in adult articular cartilage in light of recent studies which have shown that bFGF stored in the adult cartilage matrix is released with mechanical injury (21) or with loading (22). In these studies, addition of bFGF was not found to stimulate proteoglycan synthesis, but rather, stimulated production of matrix metalloproteinases (MMPs) 1 and 3 as well as tissue inhibitor of metalloproteinases 1 (TIMP-1).
Therefore, the present study was designed to test the effects of bFGF alone and in combination with IGF-1 and OP-1 using cartilage from older adult humans. Chondrocytes were cultured in suspension in alginate beads in order to maintain the differentiated chondrocyte phenotype (23) or were maintained in situ as explant cultures. A 21-day culture period was chosen for the alginate experiments in order to quantify effects of the growth factors on cell survival, proliferation, and matrix production, as was done in previous work with IGF-1 and OP-1 (14). Cartilage from the ankle joint was used in order to study the response of normal human adult chondrocytes that are difficult to obtain from the knee due to the high prevalence of degenerative-type changes in the knee joints of older adults.
MATERIALS AND METHODS
Chondrocyte isolation and culture
Normal human ankle (talocrural joint) cartilage was obtained from tissue donors through an agreement between Rush Medical College and the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL) or through the National Disease Research Interchange (Philadelphia, PA). The donors had no known history of arthritis or joint symptoms, and only donor specimens graded as 0 or 1 for gross degenerative changes were used, as previously described (14). Tissue was obtained from a total of 9 donors with a mean ± SEM age of 62 ± 2.5 years (range 47–73 years).
For alginate cultures, cartilage was removed with a scalpel, and chondrocytes were isolated by enzymatic digestion using Pronase, followed by overnight digestion with collagenase P, as previously described (24). Cells were resuspended in alginate, and beads were formed using a CaCl2 solution, as previously described (24). Alginate beads were cultured at 8 beads per well in 24-well plates in 0.5 ml/well of Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 medium (1/1) supplemented with 1% mini-ITS+ (insulin–transferrin–selenium). This serum-free medium was used to examine growth factors without influence of those present in serum. Mini-ITS+ contains 5 nM insulin (a miniature dose of insulin so that the IGF-1 receptor is not stimulated), 2 μg/ml transferrin, 2 ng/ml selenous acid, 25 μg/ml ascorbic acid, and bovine serum albumin/linoleic acid at 420/2.1 μg/ml (25). Insulin was purchased from Sigma (St. Louis, MO), l-ascorbic acid phosphate magnesium salt n-hydrate was obtained from Wako (Richmond, VA), and the other mini-ITS+ supplements were obtained from BD Biosciences (Bedford, MA).
For explant cultures, full-thickness cartilage discs were obtained using a 4-mm biopsy punch. Wet weight was measured, and explants were cultured at 1 explant per well in 48-well plates in 0.4 ml/well of DMEM/Ham’s F-12 (1/1) supplemented with 1% mini-ITS+. After a 5–7-day recovery period, growth factors were added to the culture medium, which was exchanged with fresh medium with growth factors every 48 hours. Recombinant human bFGF (PeproTech, Rocky Hill, NJ) was used at doses of 0, 1, 10, 50, and 100 ng/ml. IGF-1–treated wells received 100 ng/ml of recombinant human IGF-1 (a gift from Chiron, Emeryville, CA) or 100 ng/ml human des(1–3) IGF-1 (GroPep, Adelaide, South Australia, Australia), OP-1–treated wells received 100 ng/ml recombinant human OP-1 (provided by Stryker Biotech, Hopkinton, MA), and the combination treatment wells received 100 ng/ml of each growth factor. Triplicate wells were used for each condition for alginate cultures, and for explants, 8 discs were used for each condition.
Cell survival assay
Cell survival was measured as previously described (26), using Calcein AM to stain live cells and ethidium bromide homodimer 1 to stain dead cells. These reagents were obtained from Molecular Probes (Eugene, OR). At least 100 cells were counted in triplicate for each data point.
Particle exclusion assay for matrix assessment
The cells with their pericellular matrix were visualized using the particle exclusion assay, as previously described (27,28). Briefly, after day 21 of culture in alginate, the beads were solubilized with sodium citrate, the cells were pelleted by centrifugation, resuspended in DMEM, and then placed in the bottom of a multiwell plate. The cells were allowed to settle and attach to the plates by incubating for 6–12 hours, followed by washing with calcium- and magnesium-free phosphate buffered saline. Formalin-fixed erythrocytes were then added and allowed to settle for 10–15 minutes. Cells were then observed and photographed with an inverted phase-contrast microscope (Nikon, Melville, NY).
Dimethylmethylene blue (DMMB) assay for proteoglycan production and DNA assay for cell numbers
At the end of the culture period, the medium was removed, and the alginate beads were collected and processed for proteoglycan assays using the DMMB binding method, as previously described (24). The proteoglycan levels measured in the alginate matrix (further removed matrix) and those measured in the cell-associated matrix were combined to give the total amount of proteoglycans produced and retained in the alginate beads. Using PicoGreen (Molecular Probes), cell numbers were determined by assay of total DNA in the cell pellets, as previously described (24).
Sulfate incorporation assay for proteoglycan synthesis
Radiolabeled sulfate was added during the final 4 hours of culture, and sulfate incorporation was measured using the Alcian blue precipitation method, as previously described (25). For alginate cultures, sulfate incorporation was measured in the whole beads and normalized for DNA content using Hoechst 33258, as previously described (25). For explant cultures, sulfate incorporation was measured in the whole explant. Following incubation with radiolabeled sulfate, the explants were extensively washed and then digested overnight in 20 μg/ml papain (250 μl/10 mg wet weight). The Alcian blue precipitation method was performed the next day. Because the sulfate incorporation results for explants were the same whether the cells were treated for 10 or 21 days, the results were combined.
Statistical analysis
Analysis of variance was performed using StatView 5.0 software (SAS Institute, Cary, NC). P values less than 0.05 were considered significant.
RESULTS
Effects of growth factors on cell proliferation and survival
Human articular chondrocytes isolated from normal-appearing ankle cartilage were cultured for 21 days in alginate. Growth factor treatment began after a 5–7-day recovery period and then continued throughout the culture period. Tested alone, bFGF increased the amount of DNA on day 21 when used at doses >1 ng/ml (Figure 1A), consistent with stimulation of cell proliferation. The difference in cell numbers compared with control was significant at the 50 ng/ml and 100 ng/ml doses, with a maximal 2-fold stimulation at 100 ng/ml of bFGF. IGF-1 alone at 100 ng/ml did not stimulate proliferation, but proliferation was increased with the addition of as little as 1 ng/ml bFGF (Figure 1B). Cell numbers with higher doses of bFGF added to IGF-1 were similar to those seen with bFGF alone. OP-1 increased cell numbers to 166% of control, and there was no significant change when it was combined with bFGF (Figure 1C). However, when bFGF was added to the combination of IGF-1 and OP-1, increased cell numbers (373% of control) were noted with the addition of the lowest dose of bFGF (1 ng/ml), compared with 253% of control with IGF-1 plus OP-1 alone (P = 0.007) (Figure 1D). Cell survival was 100% of control survival with bFGF alone, and the addition of bFGF to IGF-1 or OP-1, either alone or in combination, did not change survival (data not shown).
Figure 1.
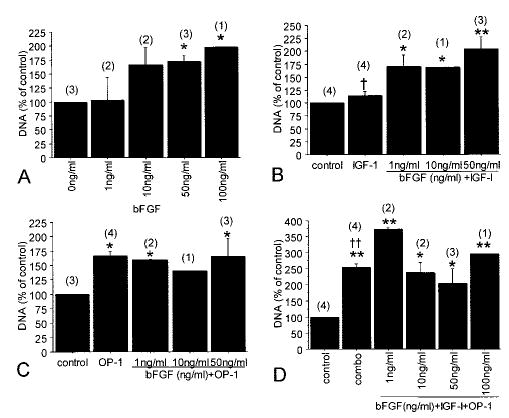
Chondrocyte cell number during culture in the presence of basic fibroblast growth factor (bFGF), insulin-like growth factor 1 (IGF-1), and osteogenic protein 1 (OP-1). Human articular chondrocytes were cultured in alginate in serum-free medium with mini-ITS+ (insulin–transferrin–selenium) (control) or the control medium plus bFGF (0–100 ng/ml), IGF-1 (100 ng/ml), and/or OP-1 (100 ng/ml). Cell numbers at the end of 21 days of culture were measured in triplicate samples using a DNA assay and are expressed as a percentage of the control cultures (mean and SEM). Results are for cultures with bFGF alone (A), bFGF plus IGF-1 (B), bFGF plus OP-1 (C), and bFGF plus IGF-1 and OP-1 (“combo”) (D). Numbers in parentheses are the number of donors used for each culture condition.* = P < 0.05 versus control; ** = P < 0.001 versus control; † = P = 0.02 versus 1 ng/ml bFGF plus IGF-1; †† = P = 0.007 versus 1 ng/ml bFGF plus IGF-1 and OP-1.
Effects of growth factors on matrix and proteoglycan production
Culture in alginate for 21 days with 50 ng/ml of bFGF resulted in the formation of tightly packed cell clusters (Figure 2). When examined using the particle exclusion assay to visualize the pericellular matrix, these cells had little to no visible matrix. The size of the cells did not appear to differ between treatment groups, but the amount of pericellular matrix did vary. We have previously shown that the addition of IGF-1 plus OP-1 to alginate-cultured chondrocytes stimulates the formation of clusters of chondrocytes that are more spread out and have an abundant pericellular matrix as visualized by particle exclusion (14). In the present study, when bFGF was combined with IGF-1 plus OP-1, compact clusters of cells were observed (Figure 2). When these cells were disassociated and examined by particle exclusion, it was found that the addition of bFGF completely inhibited the ability of IGF-1 and OP-1 to stimulate the formation of an abundant pericellular matrix, which is responsible for keeping the cells further apart in cultures treated with IGF-1 plus OP-1 (Figure 2). This suggested that bFGF was either inhibiting the matrix production promoted by IGF-1 and OP-1 or was stimulating degradation of the newly formed matrix.
Figure 2.
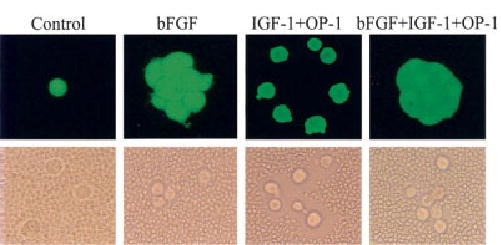
Chondrocyte appearance and pericellular matrix production after alginate culture in the presence of bFGF, IGF-1, and OP-1. Chondrocytes were cultured for 21 days in alginate beads in serum-free mini-ITS+ medium (control) or the control medium supplemented with 50 ng/ml bFGF, 100 ng/ml each of IGF-1 and OP-1, or the combination of all 3. At the end of the culture period, the beads were incubated with Calcein AM and ethidium bromide homodimer, as described in Materials and Methods, to assess survival. The beads were dissolved in sodium citrate, and a sample of the cells from each condition was observed using a fluorescence microscope (top). Dissolved alginate beads from separate cultures were pelleted by centrifugation, resuspended in Dulbecco’s modified Eagle’s medium, and placed in the bottom of microwell plates, followed by the addition of fixed erythrocytes, as described in Materials and Methods. A representative sample was photographed using an inverted phase-contrast microscope (bottom). The cell-associated (pericellular) matrix can be seen excluding the erythrocytes from the chondrocyte plasma membrane in the cells treated with IGF-1 plus OP-1. The clusters of cells treated with bFGF revealed by Calcein AM staining were partially broken up by the processing for the particle exclusion assay. See Figure 1 for definitions. (Original magnification × 400.)
Further experiments were performed to quantify proteoglycan production in the alginate cultures. After 21 days of culture in serum-free medium supplemented only with mini-ITS+, a relatively small amount of proteoglycan was produced, most likely the result of endogenous autocrine and paracrine growth factor activity (see Figure 4 for day 0 versus day 21 control). The addition of bFGF to the control medium reduced the amount of total proteoglycan per cell that was produced and retained in the alginate beads in a dose-dependent manner (Figure 3A). At 100 ng/ml bFGF, the amount of proteoglycan per cell was ~60% below day 21 control.
Figure 4.

Chondrocyte proteoglycan production when human des(1–3) IGF-1 was substituted for IGF-1 in bFGF- and OP-1–treated cultures. Experiments were performed as described in Figure 3, except that equal amounts of human des(1–3) IGF-1 were compared with IGF-1. Samples from 1 donor were measured (in triplicate) by the dimethylmethylene blue (DMMB) assay and normalized to cell numbers using DNA measurements (DMMB/DNA) (mean and SEM). * = P < 0.0001 versus day 21 control; † = P < 0.0001 for comparison of each growth factor with and without bFGF. See Figure 1 for other definitions.
Figure 3.
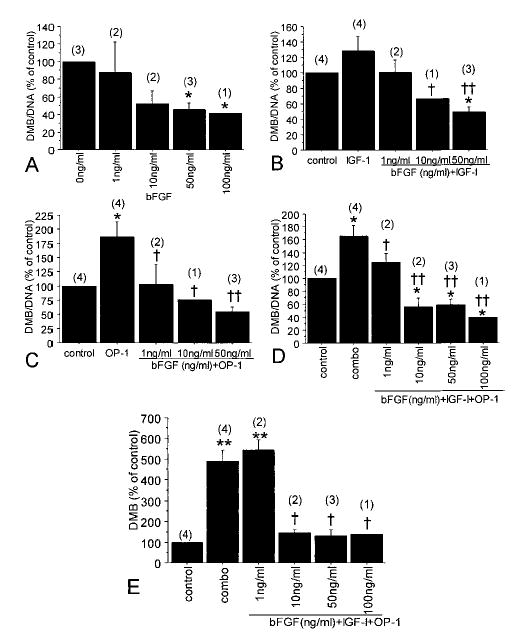
Chondrocyte proteoglycan production after culture in the presence of bFGF, IGF-1, and OP-1. Human articular chondrocytes were cultured in alginate in serum-free medium with mini-ITS+ (control) or the control medium plus bFGF (0–100 ng/ml), IGF-1 (100 ng/ml), and/or OP-1 (100 ng/ml). The amount of proteoglycan in the alginate beads (cell-associated and further-removed matrix) was measured by the dimethylmethylene blue (DMMB) assay and normalized to cell numbers using DNA measurements (DMMB/DNA). Samples were measured in triplicate and are expressed as a percentage of the day 21 control cultures (mean and SEM). Numbers in parentheses are the number of donors used for each culture condition. Results are for cultures with A, bFGF alone (* = P < 0.05 versus control); B, bFGF plus IGF-1 (* = P = 0.02 versus control; † = P = 0.04 versus IGF-1; †† = P = 0.002 versus IGF-1); C, bFGF plus OP-1 (* = P < 0.008 versus control; † = P = 0.02 versus OP-1; †† = P = 0.001 versus OP-1); and D, bFGF plus IGF-1 and OP-1 (“combo”) (* = P < 0.05 versus control; † = P < 0.05 versus combo; †† = P < 0.001 versus combo). E, Results of the same experiments shown in D, but for the total DMMB in the beads, without DNA correction (** = P < 0.0001 versus control; † = P < 0.001 versus combo). See Figure 1 for other definitions.
Treatment with 100 ng/ml IGF-1 by itself increased the amount of proteoglycan per cell by 29%, but this was not statistically significant compared with the control (P = 0.1). The addition of increasing doses of bFGF along with 100 ng/ml of IGF-1 resulted in decreasing levels of proteoglycan, similar to what was observed with bFGF alone (Figure 3B). The highest dose of bFGF tested (50 ng/ml) decreased proteoglycan levels per cell to ~50% of control, as compared with the results of IGF-1 treatment alone, which was 129% of control. Perhaps even more striking was the reduction in proteoglycan levels noted when bFGF was added with OP-1 (Figure 3C). OP-1 treatment alone increased proteoglycan levels to 186% of control, but the addition of bFGF at 1 ng/ml resulted in levels equal to control, while higher doses of bFGF decreased proteoglycan levels to below control (55% of control with 50 ng/ml bFGF). Similar results were obtained when bFGF was added to the combination of IGF-1 and OP-1 (Figure 3D). The total amount of proteoglycan per cell was only 40% of control when 100 ng/ml bFGF was added with IGF-1 plus OP-1.
Because the combination of IGF-1 and OP-1 also stimulates cell proliferation and this was modified by the addition of bFGF (as shown in Figure 1D), we examined the effect of the combination of growth factors on the total amount of proteoglycan produced per bead in addition to the amount per cell. Only the lowest dose of bFGF tested (1 ng/ml) did not decrease the total amount of proteoglycan per bead, which was nearly 500% of control with IGF-1 plus OP-1 but <150% of control when bFGF at 10–100 ng/ml was included with IGF-1 plus OP-1 (Figure 3E).
In some cell types, bFGF has been reported to stimulate production of IGF binding proteins (IGFBPs) (29,30), and we have noted stimulation of IGFBP-4 and IGFBP-5 messenger RNA levels after bFGF treatment of chondrocytes (Im HJ, Loeser RF: unpublished observations). An increase in IGFBP levels by bFGF could, in turn, inhibit the response to IGF-1 or IGF-1 plus OP-1. Therefore, an additional experiment was performed using human des(1–3) IGF-1 in place of IGF-1. This truncated form of IGF-1 has significantly reduced the affinity for IGFBPs, so if the response is different from IGF-1, it suggests an effect of the IGFBPs. In this experiment, a control at the start of growth factor treatment (day 0) was also included, and all treatment groups that did not include bFGF were significantly greater than the day 0 control (Figure 4). Compared with the day 21 control, the results with human des(1–3) IGF-1 were similar to those with IGF-1, suggesting that bFGF stimulation of IGFBPs is an unlikely mechanism for inhibition of IGF-1 activity.
Effects of growth factors on proteoglycan synthesis
As indicated above, a reduction in the amount of proteoglycan found in 21-day alginate cultures treated with bFGF could be due to inhibition of proteoglycan synthesis and/or degradation of newly synthesized proteoglycans. In order to determine if bFGF inhibited proteoglycan synthesis, sulfate incorporation assays were performed in cultures treated with 50 ng/ml bFGF, with or without IGF-1 and OP-1. In the experiment in alginate, only the combination of IGF-1 and OP-1 was sufficient to stimulate significant sulfate incorporation, and this stimulation was completely blocked by the addition of bFGF (Figure 5A). Additional experiments were performed using explants in place of alginate beads. In explants, levels of proteoglycan synthesis were below controls with the addition of bFGF, and bFGF blocked the ability of IGF-1, OP-1, or IGF-1 plus OP-1 to stimulate proteoglycan synthesis (Figure 5B).
Figure 5.
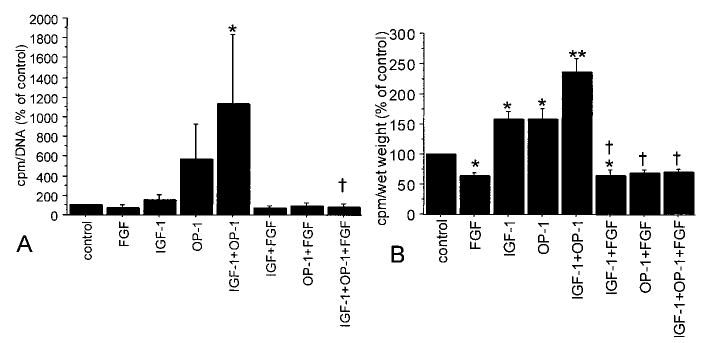
Chondrocyte proteoglycan synthesis after culture in the presence of bFGF, IGF-1, and OP-1. Human articular chondrocytes were cultured in alginate for 21 days or as cartilage explants for 10–21 days in serum-free medium with mini-ITS+ (control) or the control medium plus bFGF (50 ng/ml), IGF-1 (100 ng/ml), and/or OP-1 (100 ng/ml). Proteoglycan synthesis was measured during the last 4 hours of culture using sulfate incorporation and was normalized to cell numbers by DNA assay (for alginate cultures) or by wet weight (for explants). Results are expressed as a percentage of control for triplicate samples (mean and SEM); n = 2 donors for alginate cultures and 3 donors for explants. A, Alginate cultures (* = P < 0.05 versus control; † = P = 0.03 versus IGF-1 + OP-1). B, Cartilage explants (* = P < 0.05 versus control; ** = P < 0.001 versus control; † = P < 0.0001 versus each growth factor without bFGF). See Figure 1 for definitions.
DISCUSSION
The major new findings from this study were the significant inhibitory effect of bFGF on proteoglycan synthesis and proteoglycan matrix accumulation in adult human articular chondrocytes, and the inability of IGF-1, OP-1, or the combination of IGF-1 and OP-1 to stimulate proteoglycan production in the presence of bFGF. Previous studies have demonstrated the inhibition of proteoglycan synthesis by bFGF in rabbit articular chondrocytes (16,31) and in human OA cartilage (20). In contrast, modest stimulation of proteoglycan synthesis by bFGF in adult bovine cartilage was reported (17,18). The present results clearly demonstrate the inhibition of proteoglycan synthesis by bFGF in alginate-cultured chondrocytes and cartilage explants established from older adult human articular cartilage. The findings suggest that conditions that increase the amount of bFGF available to the adult human chondrocyte will result in reduced matrix production and be detrimental to the maintenance of cartilage homeostasis.
It has been fairly well accepted that IGF-1 and OP-1 function as anabolic factors in adult cartilage; however, the function of bFGF in adult cartilage is not as clear. Outside of the joint, bFGF is known to stimulate angiogenesis and, among other functions, plays a role in wound repair (for review, see ref. 32) and the regulation of longitudinal bone growth (33). Also, bFGF has been shown to be a chondrocyte mitogen (15–17), and our results using bFGF at concentrations >10 ng/ml were consistent with this function.
In alginate cultures treated with 50–100 ng/ml of bFGF, compact clusters of cells were observed. The morphology of these clusters most likely resulted from the lack of sufficient pericellular matrix production, which would normally serve to separate the cells after cell division has occurred. The cells were cultured in low-viscosity alginate, which should not significantly retard cell movement. Evidence for this is provided by the cultures treated with IGF-1 plus OP-1 in which the cells were found to be separated by abundant pericellular matrix. We cannot rule out the possibility that bFGF stimulated degradation of the matrix as it was formed, although we think this is less likely than a decrease in matrix synthesis based on the sulfate incorporation results. Also, in the sulfate incorporation experiments, a significant increase in sulfate release into the medium fraction of bFGF cultures, indicative of proteoglycan degradation, was not seen (data not shown). Perhaps this finding is related to the ability of bFGF to stimulate production of TIMP-1, which could serve to inhibit any increase in MMP production (21,22). The clusters resulting from bFGF treatment were somewhat reminiscent of the clusters of chondrocytes commonly observed in OA cartilage, suggesting that release of bFGF from the cartilage matrix could contribute to chondrocyte proliferation during the development of OA.
A low dose of 1 ng/ml bFGF did not significantly stimulate proliferation; however, it did increase cell numbers when combined with 100 ng/ml IGF-1, and the greatest number of cells (3.7-fold above control) was noted in cultures treated with 1 ng/ml bFGF in combination with IGF-1 plus OP-1. Combining OP-1 with bFGF at any of the concentrations tested did not appreciatively increase cell numbers above those treated with either growth factor alone. In most culture conditions, OP-1 is not mitogenic by itself, but in the present study, it modestly increased cell numbers. This was possibly due to the relatively low cell density used for the alginate cultures (~20,000 cells/bead) and because the control cultures used for comparison were serum free. These findings suggest that the regulation of chondrocyte proliferation by the growth factors studied here is dependent on several factors, including the concentration and combination of factors as well as cell density. This is consistent with previous studies of BALB/c 3T3 cells, which suggests that bFGF and IGF-1 work in concert to regulate proliferation, where bFGF is considered a factor required for competence and IGF-1 is required for progression to S phase in order to obtain the full mitogenic response (34).
Perhaps more important, the results also suggest that the presence of bFGF in amounts >1 ng/ml will have a significant inhibitory effect on chondrocyte proteoglycan synthesis. Also, the addition of IGF-1, OP-1, or IGF-1 plus OP-1 was not able to stimulate proteoglycan production in the presence of >1 ng/ml bFGF. Increased release of bFGF from matrix stores has recently been demonstrated in vitro after cartilage injury (cutting with a scalpel) (21) or with mechanical loading (22). The exact amounts of bFGF released under these conditions have not been measured, but a reasonable hypothesis could be made that excessive loading or significant damage to the cartilage matrix could release amounts of bFGF that then act to inhibit the anabolic activity of IGF-1 and OP-1 in articular cartilage.
The studies by Vincent and colleagues (21,22) also demonstrated that bFGF treatment of chondrocytes did not stimulate proteoglycan synthesis, but rather, stimulated production of MMP-1 and MMP-3. Others have shown that bFGF can stimulate chondrocyte MMP-13 expression (35). Because bFGF also stimulates production of TIMP-1 (21,22), the overall balance of matrix synthesis and degradation in response to bFGF will likely depend on the state of the cell and signals being generated from other growth factors and cytokines. Together, these studies suggest that excessive release of bFGF from the cartilage matrix may be partly responsible for matrix loss during the development and progression of OA, particularly in the stages in which anabolic activity is falling below catabolic activity.
There are recognized limitations of in vitro studies in the prediction of growth factor activity in vivo. Some of these limitations, such as phenotypic changes induced by chondrocyte culture, were addressed by using the alginate system and explants. It is also important to note that the present studies were performed with cartilage removed from ankle joints, and the response to bFGF could be different in knee cartilage or cartilage from other joints. Clearly, the dose of bFGF used in vitro is also important since we found different effects of a low dose (1 ng/ml) of bFGF compared with higher doses. Additional in vivo studies will need to be performed to determine if bFGF has similar activity in the intact joint. If so, then inhibition of excessive bFGF activity in cartilage may be of therapeutic benefit in conditions such as OA, which are characterized by loss of the normal anabolic–catabolic balance.
Acknowledgments
We gratefully acknowledge the Gift of Hope Organ and Tissue Donor Network, the National Disease Research Interchange, and the donor families for providing tissues, and the assistance of Dr. Arkady Margulis in collecting donor tissues. We are also grateful to Dr. Cheryl Knudson for assistance with the particle exclusion assay.
Footnotes
Supported by the NIH (grants AG-16697 and AR-47654), Stryker Biotech (grant SC-001), and the Falk Foundation.
References
- 1.Trippel SB. Growth factor actions on articular cartilage [review] J Rheumatol Suppl. 1995;43:129–32. [PubMed] [Google Scholar]
- 2.Van der Kraan PM, Buma P, van Kuppevelt T, van den Berg WB. Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering [review] Osteoarthritis Cartilage. 2002;10:631–7. doi: 10.1053/joca.2002.0806. [DOI] [PubMed] [Google Scholar]
- 3.Chubinskaya S, Kuettner KE. Regulation of osteogenic proteins by chondrocytes [review] Int J Biochem Cell Biol. 2003;35:1323–40. doi: 10.1016/s1357-2725(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 4.Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J. 2002;365:119–26. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobacz K, Gruber R, Soleiman A, Graninger WB, Luyten FP, Erlacher L. Cartilage-derived morphogenetic protein-1 and -2 are endogenously expressed in healthy and osteoarthritic human articular chondrocytes and stimulate matrix synthesis. Osteoarthritis Cartilage. 2002;10:394–401. doi: 10.1053/joca.2002.0522. [DOI] [PubMed] [Google Scholar]
- 6.Goldring MB. The role of the chondrocyte in osteoarthritis [review] Arthritis Rheum. 2000;43:1916–26. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis: an introduction: cell biology of osteoarthritis [review] Arthritis Res. 2001;3:107–13. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets [review] Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Schneiderman R, Rosenberg N, Hiss J, Lee P, Liu F, Hintz RL, et al. Concentration and size distribution of insulin-like growth factor-I in human normal and osteoarthritic synovial fluid and cartilage. Arch Biochem Biophys. 1995;324:173–88. doi: 10.1006/abbi.1995.9913. [DOI] [PubMed] [Google Scholar]
- 10.McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington AC. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986;240:423–30. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schalkwijk J, Joosten LA, van den Berg WB, van Wyk JJ, van de Putte LB. Insulin-like growth factor stimulation of chondrocyte proteoglycan synthesis by human synovial fluid. Arthritis Rheum. 1989;32:66–71. doi: 10.1002/anr.1780320111. [DOI] [PubMed] [Google Scholar]
- 12.Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger DC, Kuettner KE. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1) Biochim Biophys Acta. 2002;1588:126–34. doi: 10.1016/s0925-4439(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 13.Merrihew C, Kumar B, Heretis K, Rueger DC, Kuettner KE, Chubinskaya S. Alterations in endogenous osteogenic protein-1 with degeneration of human articular cartilage. J Orthop Res. 2003;21:899–907. doi: 10.1016/S0736-0266(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 14.Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188–96. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 15.Jones KL, Addison J. Pituitary fibroblast growth factor as a stimulator of growth in cultured rabbit articular chondrocytes. Endocrinology. 1975;97:359–65. doi: 10.1210/endo-97-2-359. [DOI] [PubMed] [Google Scholar]
- 16.Sachs BL, Goldberg VM, Moskowitz RW, Malemud CJ. Response of articular chondrocytes to pituitary fibroblast growth factor (FGF) J Cell Physiol. 1982;112:51–9. doi: 10.1002/jcp.1041120109. [DOI] [PubMed] [Google Scholar]
- 17.Osborn KD, Trippel SB, Mankin HJ. Growth factor stimulation of adult articular cartilage. J Orthop Res. 1989;7:35–42. doi: 10.1002/jor.1100070106. [DOI] [PubMed] [Google Scholar]
- 18.Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308:137–47. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- 19.Veilleux N, Spector M. Effects of FGF-2 and IGF-1 on adult canine articular chondrocytes in type II collagen–glycosaminoglycan scaffolds in vitro. Osteoarthritis Cartilage. 2005;13:278–86. doi: 10.1016/j.joca.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Posever J, Phillips FM, Pottenger LA. Effects of basic fibroblast growth factor, transforming growth factor-β1, insulin-like growth factor-1, and insulin on human osteoarthritic articular cartilage explants. J Orthop Res. 1995;13:832–7. doi: 10.1002/jor.1100130605. [DOI] [PubMed] [Google Scholar]
- 21.Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci U S A. 2002;99:8259–64. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent TL, Hermansson MA, Hansen UN, Amis AA, Saklatvala J. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum. 2004;50:526–33. doi: 10.1002/art.20047. [DOI] [PubMed] [Google Scholar]
- 23.Hauselmann HJ, Fernandes RJ, Mok SS, Schmid TM, Block JA, Aydelotte MB, et al. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 1994;107:17–27. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- 24.Loeser RF, Todd MD, Seely BL. Prolonged treatment of human osteoarthritic chondrocytes with insulin-like growth factor-I stimulates proteoglycan synthesis but not proteoglycan matrix accumulation in alginate cultures. J Rheumatol. 2003;30:1565–70. [PubMed] [Google Scholar]
- 25.Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43:2110–20. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 26.Del Carlo M, Jr, Loeser RF. Nitric oxide–mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum. 2002;46:394–403. doi: 10.1002/art.10056. [DOI] [PubMed] [Google Scholar]
- 27.Knudson CB. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol. 1993;120:825–34. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauselmann HJ, Masuda K, Hunziker EB, Neidhart M, Mok SS, Michel BA, et al. Adult human chondrocytes cultured in alginate form a matrix similar to native human articular cartilage. Am J Physiol. 1996;271:C742–52. doi: 10.1152/ajpcell.1996.271.3.C742. [DOI] [PubMed] [Google Scholar]
- 29.Pons S, Torres-Aleman I. Basic fibroblast growth factor modulates insulin-like growth factor-I, its receptor, and its binding proteins in hypothalamic cell cultures. Endocrinology. 1992;131:2271–8. doi: 10.1210/endo.131.5.1385099. [DOI] [PubMed] [Google Scholar]
- 30.Ververis J, Ku L, Delafontaine P. Fibroblast growth factor regulates insulin-like growth factor-binding protein production by vascular smooth muscle cells. Am J Med Sci. 1994;307:77–81. doi: 10.1097/00000441-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Prins AP, Lipman JM, McDevitt CA, Sokoloff L. Effect of purified growth factors on rabbit articular chondrocytes in monolayer culture. II. Sulfated proteoglycan synthesis. Arthritis Rheum. 1982;25:1228–38. doi: 10.1002/art.1780251012. [DOI] [PubMed] [Google Scholar]
- 32.Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2 [review] Endocr Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- 33.Mancilla EE, De Luca F, Uyeda JA, Czerwiec FS, Baron J. Effects of fibroblast growth factor-2 on longitudinal bone growth. Endocrinology. 1998;139:2900–4. doi: 10.1210/endo.139.6.6032. [DOI] [PubMed] [Google Scholar]
- 34.Stiles CD, Capone GT, Scher CD, Antoniades HN, van Wyk JJ, Pledger WJ. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979;76:1279–83. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12:963–73. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]


