Abstract
Chondrocyte anabolic activity has been shown to decline with aging, but catabolic activity has received little attention. In this study, the effect of aging on the chondrocyte catabolic response was determined by stimulating isolated human chondrocytes with fibronectin fragments (FN-f) or interleukin-1β and measuring matrix metalloproteinase-13 (MMP-13) production as a catabolic response. A significant age-related increase in chondrocyte MMP-13 production was noted. FN-f stimulation of MMP-13 expression was blocked using a nuclear factor kappa-B (NFκB) inhibitor suggesting a role for NFκB in this chondrocyte catabolic response. Chondrocyte production of the NFκB-regulated cytokine interleukin-1β was also found to increase with donor age in unstimulated cells. These results demonstrate a significant age-related increase in chondrocyte catabolic responsiveness which could contribute to the development of osteoarthritis in older adults.
THE hallmark of osteoarthritis (OA) is progressive loss of articular cartilage to a much greater extent than that which characterizes the normal “wear and tear” of aging. However, although it is known that the single greatest risk factor for the development of OA is age, exactly how cartilage aging processes contribute to the development of OA is unknown [reviewed in (1)]. In general, aging is characterized by progressive loss of tissue physiologic function due to an imbalance favoring catabolic processes. Increasing evidence supports a role for reactive oxygen species (ROS) and the proinflammatory cascade regulated by the transcription factor nuclear factor kappa-B (NFκB) in driving this aging-related catabolic imbalance (2,3).
The chondrocyte is the only cell type found in articular cartilage and is the primary mediator of the anabolic and catabolic processes that determine cartilage extracellular matrix composition (4,5). In OA, articular cartilage extracellular matrix is lost due to a progressive imbalance between synthesis and degradation resulting in unchecked degradation of cartilage. Although multiple enzymes are certainly involved in the degradation of cartilage in OA, substantial evidence now supports a key role for matrix metalloproteinase-13 (MMP-13; collagense-3) (6–9). Therefore, changes in chondrocyte function that result in decreased anabolic activity and increased catabolic activity, and in particular an increase in MMP-13 production, could play a central role in the development of OA.
Several studies have provided evidence that aging results in a reduction in the ability of chondrocytes to respond to anabolic stimuli [reviewed in (1)]. Perhaps the best documented evidence is the aging-related decline in proteoglycan synthesis stimulated by insulin-like growth factor I (IGF-I) (10–12). Much less is known about the catabolic response of aging chondrocytes. Because IGF-I can be anticatabolic (13) and has been shown to reduce the chondrocyte production of MMP-13 (14), an aging-related loss in responsiveness to IGF-I could not only result in reduced chondrocyte anabolic activity but could potentially contribute to increased catabolic activity.
In support of the hypothesis that the catabolic activity of chondrocytes may increase with age are studies that have shown an age-related accumulation of collagen neoepitopes representing denatured or cleaved collagen (15,16) as well as increased staining for MMP-3 and MMP-13 in cartilage with aging (17). However, there is a lack of data, particularly in adult human articular cartilage, to determine if the response to catabolic stimuli changes with aging. When comparing cells from a young and an old adult, a cell-labeling experiment suggested that the response to tumor necrosis factor-α (TNF-α) but not interleukin (IL)-1α was greater in the cells from the older person, but the lack of sufficient numbers of samples or quantitation of the data limited the significance of the results (18).
Our laboratory has recently performed a series of studies focused on defining the cell signaling pathways by which fibronectin fragment (FN-f) stimulation of the α5β1 integrin increases MMP-13 production by human chondrocytes (14,19,20). FN-f were chosen as a stimulus because they have been shown to be present in osteoarthritic cartilage and synovial fluid (21) and are known to stimulate catabolic activity (22) including an increase in MMP-13 production (19). IL-β was used as a positive control in some experiments because it is a well characterized mediator of catabolic (including MMP-13 production) and antianabolic activity and is thought to play an important role in the development of OA (4,5,23). In the course of these studies, we observed that the amount of MMP-13 produced in response to FN-f or IL-1β appeared to be greater when chondrocyte cultures were established from older aged donors. Therefore, we determined if there was a relationship between donor age and MMP-13 production in vitro to test the hypothesis that catabolic responsiveness increases with age in isolated human articular chondrocytes.
Because the goal of these studies was to determine the effects of normal aging in cartilage samples which had not already developed obvious degenerative changes or changes consistent with OA, we chose to study chondrocytes isolated only from normal appearing ankle cartilage obtained from tissue donors who had no known history of arthritis. Degenerative changes in knee cartilage are very common in the older adult population making it difficult to obtain sufficient numbers of completely normal knee cartilage samples, whereas similar changes in ankle cartilage are much less frequent (24). Preliminary experiments were performed to demonstrate a similar response between knee and ankle chondrocytes.
Methods
Reagents
The 110 kd FN-f was obtained from Upstate Biotechnology (Lake Placid, NY). The protein kinase C (PKC) inhibitor rottlerin and the NFκB cell-permeable inhibitor peptide (SN50) and matching inactive control peptide were purchased from Calbiochem (San Diego, CA). IL-1β and enzyme-linked immunosorbent assay (ELISA) kits for IL-1β and active MMP-13 were obtained from R&D Systems (Minneapolis, MN). The sheep polyclonal MMP-13 antibody L29/6 has been previously described (25). Control plasmids for transfection studies, phRL-SV40, pGL2-Enhancer, and pGL2-control, were obtained from Promega (Madison, WI) All cell culture plates were obtained from Corning (Corning, NY), and cell culture reagents including fetal bovine serum were obtained from Gibco/Invitrogen (Carlsbad, CA).
Chondrocyte Culture
Normal human ankle (talocrural joint) and knee (tibiofemoral joint) cartilage were obtained from tissue donors through an agreement between Rush Medical College and the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL). Tissue was obtained only from donors without a known history of arthritis or joint symptoms. Each donor specimen was graded for gross degenerative changes based on a modified version of the 5-point scale of Collins as previously described (26). Samples used for this study were grade 0 or 1. Chondrocytes were isolated by enzymatic digestion using pronase followed by overnight digestion with collagenase-P as previously described (19). Isolated cells were resuspended in medium at 1 × 106 cells/ml, and 2 ml were added to each well of a six-well plate. Cells were cultured in 50:50 Dulbecco’s modified Eagle’s medium: Ham’s F12 medium containing 10% fetal bovine serum and antibiotics (complete medium) for 5–7 days before use. Media were changed to serum-free Dulbecco’s modified Eagle’s medium: Ham’s F12 medium with antibiotics 18 hours (overnight) and again 2 hours before each experiment. For inhibitor studies, cells were preincubated with inhibitor for 30 minutes before stimulation. FN-f (1 μM final, unless noted otherwise) or IL-1 (5 ng/ml) was added directly to the culture medium for the indicated time periods. Experiments were terminated with removal of media. The conditioned media were aliquoted and stored at −70°C or 4°C with 0.1% NaN3. The protein concentration of conditioned media (supernatant) was determined with BCA reagent (Pierce, Rockford, IL).
Analysis of MMP-13 Production by Immunoblotting and ELISA
Supernatant samples containing equal amounts of total protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 4%/10%) and transferred to nitrocellulose for immunoblotting using the enhanced chemiluminescence system (Amersham, Piscataway, NJ). Where indicated (Figures 1 and 2), conditioned media were concentrated (10:1) as described (19). All other immunoblots and ELISAs were carried out using unconcentrated conditioned media. Densitometric analysis was performed on designated immunoblots using a Kodak Image Station 1000 and Kodak 1D Image Analysis software (Eastman Kodak Co., Rochester, NY). Exact-fit outlining of each area to be determined was drawn using the densitometry imaging software, and background was set as the edge of each best-fit area drawn and is subtracted by the software to arrive at a value for the outlined area. The densitometric results obtained from immunoblots of sets of cell samples from each donor (unstimulated control and FN-f- or IL-1β-stimulated cells) were used to calculate a ratio of the proMMP-13 (60 kd band) in lanes from stimulated cells to the proMMP-13 band in lanes from control cells. This was done to normalize the data and account for the day-to-day variability in band intensity that occurs with immunoblotting and enhanced chemiluminescence detection. ELISAs were performed according to manufacturers’ specifications using duplicate wells for each sample and, after preliminary testing (when needed), were diluted with medium to remain within the linear range of the assays.
Figure 1.
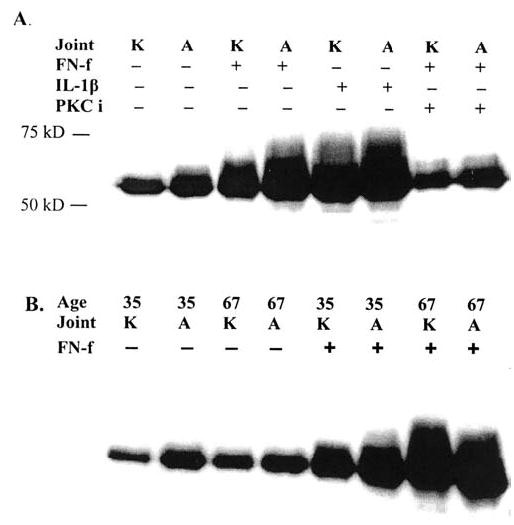
Comparison of matrix metalloproteinase-13 (MMP-13) production in knee and ankle chondrocytes from the same donors. Culture media from equal numbers of normal knee and ankle chondrocytes from the same 58-year-old donor (A) or from young (35-year-old) and old (67-year-old) donors (B) were assessed for MMP-13 production by immunoblotting. Cells were made serum-free for 18 hours prior to each experiment. Samples are from concentrated (10X) serum-free culture media without (–) and with fibronectin fragment (FN-f) treatment for 24 hours with 1 μM 110 kd fibronectin fragment (FN-f) or interleukin-1β (IL-1β) at 5 ng/ml and were equalized for total protein loaded per lane. Cells preincubated with the protein kinase C inhibitor rottlerin at 4 μm (PKCi) were also tested.
Figure 2.
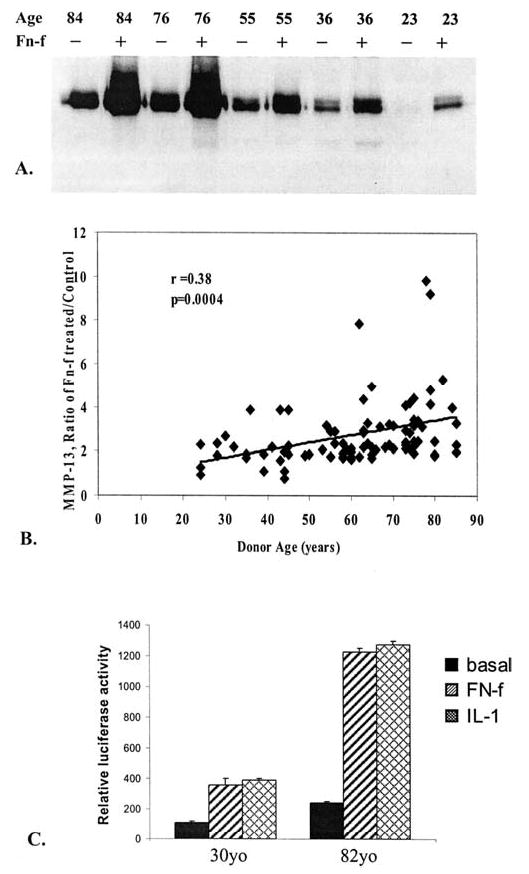
Fibronectin fragment (FN-f)-stimulated matrix metalloproteinase-13 (MMP-13) production in ankle chondrocytes from young and old donors. A, MMP-13 was assessed by immunoblotting culture media samples from cells isolated from donors of different ages and stimulated in serum-free media with FN-f as described in Figure 1. Media were collected from wells with equal cell numbers (2 × 106 cells/well) and normalized for equal total protein loaded per lane (20 μg/lane). B, MMP-13 immunoblots from 89 different donors were scanned, and densitometric data were obtained for control (unstimulated) and FN-f-stimulated MMP-13 bands and expressed as the ratio of proMMP-13 after FN-f treatment/baseline proMMP-13. These results were regressed versus donor age (r = 0.38, p = .003). C, A full-length MMP-13 promoter–luciferase reporter and a control Renilla luciferase reporter were transiently cotransfected into cells from a young and an old donor followed by treatment with 1 μM FN-f or interleukin-1β (IL-1β) at 5 ng/ml or left untreated (basal activity). After 24 hours, the cells were lysed and subjected to dual luciferase assay. Results are means of duplicate transfections and are representative of experiments performed with two sets of young and old donors with each set performed in parallel.
Chondrocyte Transfection
Primary chondrocytes cultured in six-well plates in medium with 10% serum were transfected with a plasmid containing the −1600MMP-13 promoter–luciferase reporter using the FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN) as previously described (20). Cells were cotransfected with a Renilla luciferase construct (phRL-SV40) to control for transfection efficiency. Cells in additional control wells were transfected with the promoter-less vector, pGL2-Enhancer, to test for nonspecific basal luciferase activity. Twenty-four hours after transfection, the cultures were switched to serum-free medium and then stimulated overnight with FN-f or IL-1β as described above. Cell lysates were prepared, and luciferase activity was measured using the dual luciferase reporter assay system (Promega). Relative luciferase activity was calculated using the firefly luciferase reading from the −1600MMP-13 promoter–luciferase reporter corrected for the control Renilla luciferase reading. We also performed an additional experiment using cells from one young (age 30 years) and two old (ages 79 and 82 years) donors to test for age-related differences in transfection efficiency by transfecting cells with the plasmid pGL2-control in which luciferase expression is driven by an SV40 promoter with an enhancer. The luciferase activity was found to be very similar in the three different cultures, suggesting equal transfection efficiency.
Statistical Analysis
Linear regression was used to assess the association between MMP-13 or IL-1 and the age, in years, of the donors. Assumptions of the models were evaluated graphically and analytically. Models were fit using SAS version 8 (SAS Institute Inc., Cary, NC). A p value of < .05 was considered significant.
Results
Ankle and Knee Chondrocytes From the Same Donor Exhibit Similar FN-f Stimulation of MMP-13 Production
Chondrocytes isolated from knee and ankle cartilage of the same tissue donors were compared for MMP-13 production in response to FN-f and IL-1β. In preliminary studies, we noted that maximal MMP-13 production occurred after 24 hours of stimulation, so we chose that time point for additional comparisons. A representative MMP-13 immunoblot, shown in Figure 1A, compares the MMP-13 production by ankle and knee chondrocytes isolated from normal cartilage (grade 0) from a 58-year-old donor. The ankle and knee chondrocyte cultures produced similar amounts of MMP-13 in response to either IL-1β or FN-f. Because previous work had demonstrated a requirement for activation of PKCδ in FN-f-induced MMP-13 expression (20), we compared the ability of the PKCδ inhibitor rottlerin to block FN-f-induced MMP-13 in knee and ankle chondrocytes. Similar inhibition was noted between knee and ankle cells providing further evidence for the relevance of the ankle cartilage model.
Additional matched pairs of knee and ankle chondrocytes from three donors showed similar results. Matched pairs of knee and ankle chondrocytes from a young adult (age 35 years) and an older adult (age 67 years) were obtained at the same time and stimulated with FN-f in parallel (Figure 1B). Consistent with the other samples, a similar increase in MMP-13 production was seen when comparing knee and ankle cells and, importantly, the amount of MMP-13 produced by the older donor appeared to be greater. These results suggested that ankle cartilage could be used to further study the effects of aging on the chondrocyte response to catabolic stimuli. Similar results were obtained whether sample loading was normalized for total protein in the conditioned medium or for cell numbers determined by manual counting with a hemocytometer. Therefore, for further experiments equal protein loading was used.
Greater Donor Age Is Associated With Increased Production of MMP-13 in Response to FN-f or IL-1β
Further analysis was performed using an MMP-13 immunoblot that contained samples from chondrocytes isolated from donors of different ages that had been stimulated for 24 hours with the same amount of FN-f. Again, the amount of MMP-13 produced by cultured chondrocytes appeared to be greater in cultures established from older donors (Figure 2A). We wished to compare data from additional donors to confirm this finding and so retrospectively analyzed immunoblot data for 89 normal donors already assessed under the same conditions but not compared on the same blot. To compare the data from multiple blots that had been performed over a period of about 2 years, it was necessary to normalize the MMP-13 densitometry data to an internal value present on each blot. For this, we divided the total proMMP-13 (60 kd band, see Figure 1) after stimulation with FN-f by the densitometric value for unstimulated control proMMP-13 and expressed this as a ratio. Remarkably, even though the previous data support an aging-related increase in the numerator (FN-f-stimulated MMP-13) and the denominator (control MMP-13), the aging-related response to FN-f expressed as a ratio was found to increase significantly with age (r = 0.38, p= .0004) (Figure 2B).
In further support of the immunoblotting data, cells from two young and two old donors were transiently transfected, in parallel, with an MMP-13 promoter–luciferase reporter construct and then stimulated with FN-f or IL-1β. As previously reported (20), luciferase activity was increased by both stimuli compared to untreated controls. Importantly, the activity appeared to be much greater in the cells from older donors relative to the younger donors (Figure 2C). A sample from an additional older donor was tested; it did not respond to FN-f or IL-1β, consistent with the heterogeneity noted in the immunoblotting results.
An additional set of experiments was performed using a commercial ELISA that measures active MMP-13. This assay captures MMP-13 from media samples by incubation in wells coated with anti-MMP-13 antibody, then activity is measured by the fluorescence generated after cleavage of a fluorogenic collagenase peptide substrate. Chondrocytes from eight donors of different ages were assayed for production of active MMP-13 after 24 hours of culture in serum-free medium. A significant (r = 0.65, p = .01) age-related increase in basal (unstimulated) production of active MMP-13 was detected (data not shown).
We also retrospectively determined, in cells isolated from 29 donors, the relationship between donor age and chondrocyte production of MMP-13 in response to IL-1β at 5 ng/ml. Similar to the results with FN-f stimulation, increased production of MMP-13 was noted with greater donor age (r = 0.57, p = .0001) when the ratio of IL-1β-stimulated to unstimulated control MMP-13 was measured by immunoblotting (Figure 3).
Figure 3.
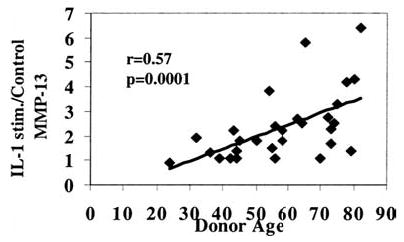
Interleukin-1β (IL-1β)–stimulated matrix metalloproteinase-13 (MMP-13) production increases with donor age. Experiments were performed as described in Figure 2B, except that cells were stimulated with IL-1β at 5 ng/ml.
Inhibition of NFκB Blocks FN-f-Stimulated MMP-13 Production
In previous work, we had provided evidence that NFκB was activated in chondrocytes in response to FN-f and IL-1β stimulation (14,19). We therefore sought to determine if NFκB activity was required for the FN-f stimulation of MMP-13 production. Preincubation of cells with a peptide inhibitor which blocks NFκB translocation (27) was found to significantly reduce FN-f-stimulated MMP-13 production, whereas a control peptide had no effect (Figure 4).
Figure 4.
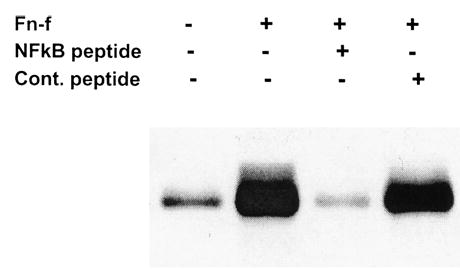
Inhibition of fibronectin fragment (FN-f)-stimulated matrix metalloproteinase-13 (MMP-13) production by a nuclear factor- kB (NFkB) inhibitor peptide. Serum-free, 24-hour unconcentrated media from primary human chondrocytes of a 76-year-old donor, prepared as in Figure 1, were assessed for MMP-13 production by immunoblot. Cells were treated as follows: untreated, 110 kd FN-f (1 μM) alone, FN-f with NKkB inhibitory peptide (12 μM), FN-f with manufacturer-supplied control peptide (12 μM). Cells were counted and judged >95% viable by trypan blue, and loading in each lane was normalized for total protein loaded. Data are representative of experiments performed using three different donor cultures.
Greater Donor Age Is Associated With Increased Production of IL-1β by Chondrocytes
Several aging models, including the inflammation model of aging, present evidence for age-related increases in expression of proinflammatory genes regulated by the transcription factor NFκB (28–30). Because IL-1β is a proinflammatory cytokine which is under the control of NFκB, we measured basal (unstimulated) production of IL-1β by chondrocytes isolated from donors of different ages to provide further support to the hypothesis that aging increases chondrocyte catabolic activity. Using an IL-1β ELISA, we found a significant (r = 0.64, p = .03) increase with age in the production of IL-1β by chondrocytes cultured in serum-free medium (Figure 5).
Figure 5.
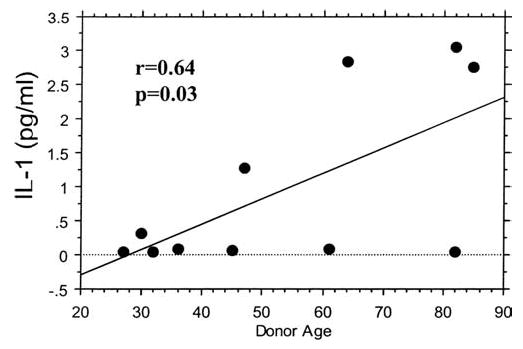
Chondrocyte production of interleukin-1β (IL-1β) increases with donor age. Serum-free culture media from unstimulated primary human chondrocytes cultured as described in Figure 1 were assessed for constitutive (baseline) IL-1β production over 24 hours using 200 μl of unconcentrated medium and an ultrasensitive IL-1β enzyme-linked immunosorbent assay (ELISA). Diamonds represent the means of duplicate wells for each donor.
Discussion
Although an age-related decline in chondrocyte anabolic activity has been described, this study provides results suggesting that catabolic activity may increase with age. This was the first attempt to assess age-related MMP-13 production by human chondrocytes, and the data show not only significant increases in baseline MMP-13, but also an exaggerated production of MMP-13 in response to stimulation by FN-f or IL-1β. Promotion of chondrocyte catabolic activity has been felt to be important in OA pathogenesis (4,23). Therefore, an age-related increase in catabolic responsiveness to mediators which include FN-f and IL-1β could provide a key mechanism linking aging and OA.
This study assessed chondrocytes from a large number of normal donors (n > 100) and, to study normal aging tissue, used cartilage isolated from the talus of the ankle joint. Autopsy studies have revealed significant tibial cartilage fibrillation in the knee joint after age 50–55 making it difficult to obtain sufficient samples of normal knee cartilage from older adults (24,26). Cells from human ankle may represent an excellent model to study age-related changes in human articular cartilage. In contrast to the knee, the ankle joint is rarely affected by severe OA although still undergoing degenerative changes with aging, as evidenced by an age-related increase in Collin’s grade which occurs at a lower level than in the knee (24). The ankle joint will, however, develop OA in aging adults who have had previous trauma (31), and ankle OA is more common in ballet dancers (32) and soccer players (33). Thus, normal ankle joints can serve as a source of cartilage to study chondrocyte aging changes with less influence of disease but with relevance to the development of OA.
The results demonstrating comparable levels of MMP-13 production by ankle and knee chondrocytes isolated from tissue obtained from the same donors (matched pairs) and an age-related increase in MMP-13 production in both, confirmed the utility of the ankle chondrocyte model. In a previous study, we had also found that knee and ankle chondrocytes had a similar increase in mitogen-activated protein kinase activation in response to the 110 kd FN-f (19). The present results clearly demonstrate an age-related difference in response of isolated knee and ankle chondrocytes to FN-f and IL-1. Although these experiments were performed on cultured cells, the results are consistent with in vivo studies which have demonstrated an age-related increase in immunostaining for MMP-13 in articular cartilage (17).
The use of cells removed from their native environment is a recognized limitation; however, it provides an advantage because it allows for the study of aging changes in the cell independent from aging changes that may also be present within the matrix. The serum-free culture conditions can promote oxidative stress which we have shown occurs to a greater degree in chondrocytes from older donors (34). Stimulation of ROS production by FN-f (35) and IL-1 (36) has been linked to increased MMP-1 expression, and we have found that anti-oxidants inhibit FN-f stimulation of MMP-13 production (R. F. Loeser, M. Del Carlo, unpublished data). These findings suggest that a decreased capacity to counteract ROS production due to age-related oxidative stress could have contributed to the increase in MMP-13 observed in cells from older donors.
A second limitation to this study is that the immunoblots used for analysis did not contain a standard known amount of purified recombinant MMP-13 that could have been used to calculate the amounts of MMP-13 in the cell culture samples run on each blot. For this reason a ratio of stimulated to unstimulated MMP-13 was calculated. There appeared to be a subset of older donors who exhibited results closer to those of the younger donors, particularly in the FN-f-stimulated samples. However, these results are consistent with the heterogeneity that is often observed in aging studies in which some individuals appear to age to a greater degree than others (37). One possible explanation for differences in response, both aging-related and among individuals, may be differences in NFκB activity. Activation of NFκB has been shown to regulate, at least in part, MMP-13 expression in chondrocytes stimulated with IL-1β (38). The present studies and previous work from our laboratory (14,19) suggest that NFκB activity is also required for FN-f stimulation of MMP-13. If aging results in increased NFκB activity as has been proposed (29), this could contribute to an increase in response to IL-1β and FN-f and could also explain the observed increase in basal IL-1 production. Heterogeneity could result from differences in basal or stimulated NFκB activity in aging chondrocytes perhaps related to differences in levels of oxidative stress which can modulate NFκB activity (3,29).
In summary, we have provided additional support to the hypothesis that aging-related changes intrinsic to chondrocytes result in dysregulation of chondrocyte metabolic activity. An increase in catabolic responsiveness, measured as MMP-13 production in response to FN-f or IL-1β, was observed as well as an increase in unstimulated production of active MMP-13 and the catabolic cytokine IL-1β. Whether these increases are related to increased NFκB activity and/or age-related oxidative stress will be a subject of further study.
Acknowledgments
This work was supported by the National Institutes of Health (grants AR49003, AG16697, and P50-AR39239), The American Federation for Aging Research, and the Wellcome Trust.
We gratefully acknowledge the Gift of Hope Organ and Tissue Donor Network and the donor families for providing tissue and the assistance of Dr. Arkady Margulis for collecting donor tissues. We thank Todd Beck, MS, for assistance with analytic programming and Carol Pacione for technical assistance.
Footnotes
Decision Editor: James R. Smith, PhD
References
- 1.Loeser RF, Shakoor N. Aging or osteoarthritis: which is the problem? Rheum Dis Clin North Am. 2003;29:653–673. doi: 10.1016/s0889-857x(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 3.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 4.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43:1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell PG, Magna HA, Reeves LM, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billinghurst RC, Dahlberg L, Ionescu M, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40:2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- 10.Barone-Varelas J, Schnitzer TJ, Meng Q, Otten L, Thonar EJ. Age-related differences in the metabolism of proteoglycans in bovine articular cartilage explants maintained in the presence of insulin-like growth factor I. Connect Tissue Res. 1991;26:101–120. doi: 10.3109/03008209109152167. [DOI] [PubMed] [Google Scholar]
- 11.Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15:491–498. doi: 10.1002/jor.1100150403. [DOI] [PubMed] [Google Scholar]
- 12.Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43:2110–2120. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 13.Tyler JA. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989;260:543–548. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem. 2003;278:25386–25394. doi: 10.1074/jbc.M302048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aurich M, Poole AR, Reiner A, et al. Matrix homeostasis in aging normal human ankle cartilage. Arthritis Rheum. 2002;46:2903–2910. doi: 10.1002/art.10611. [DOI] [PubMed] [Google Scholar]
- 17.Wu W, Billinghurst RC, Pidoux I, et al. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002;46:2087–2094. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- 18.Dozin B, Malpeli M, Camardella L, Cancedda R, Pietrangelo A. Response of young, aged and osteoarthritic human articular chondrocytes to inflammatory cytokines: molecular and cellular aspects. Matrix Biol. 2002;21:449–459. doi: 10.1016/s0945-053x(02)00028-8. [DOI] [PubMed] [Google Scholar]
- 19.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- 20.Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem. 2003;278:24577–24585. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homandberg GA, Wen C, Hui F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage. 1998;6:231–244. doi: 10.1053/joca.1998.0116. [DOI] [PubMed] [Google Scholar]
- 22.Homandberg GA, Meyers R, Xie DL. Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J Biol Chem. 1992;267:3597–3604. [PubMed] [Google Scholar]
- 23.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 24.Cole AA, Margulis A, Kuettner KE. Distinguishing ankle and knee articular cartilage. Foot Ankle Clin. N. Am. 2003;8:305–316. doi: 10.1016/s1083-7515(03)00012-3. [DOI] [PubMed] [Google Scholar]
- 25.Cowell S, Knauper V, Stewart ML, et al. Induction of matrix metalloproteinase activation cascades based on membrane-type 1 matrix metalloproteinase: associated activation of gelatinase A, gelatinase B and collagenase 3. Biochem J. 1998;331:453–458. doi: 10.1042/bj3310453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 27.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 28.Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- 29.Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY. Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev. 2002;123:1589–1595. doi: 10.1016/s0047-6374(02)00094-5. [DOI] [PubMed] [Google Scholar]
- 30.Franceschi C, Bonafe M. Centenarians as a model for healthy aging. Biochem Soc Trans. 2003;31:457–461. doi: 10.1042/bst0310457. [DOI] [PubMed] [Google Scholar]
- 31.Walter JH, Jr, Spector A. Traumatic osteoarthrosis of the ankle joint secondary to ankle fractures. J Am Podiatr Med Assoc. 1991;81:399–405. doi: 10.7547/87507315-81-8-399. [DOI] [PubMed] [Google Scholar]
- 32.van Dijk CN, Lim LS, Poortman A, Strubbe EH, Marti RK. Degenerative joint disease in female ballet dancers. Am J Sports Med. 1995;23:295–300. doi: 10.1177/036354659502300307. [DOI] [PubMed] [Google Scholar]
- 33.Drawer S, Fuller CW. Propensity for osteoarthritis and lower limb joint pain in retired professional soccer players. Br J Sports Med. 2001;35:402–408. doi: 10.1136/bjsm.35.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Carlo M, Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48:3419–3430. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 35.Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- 36.Lo YY, Conquer JA, Grinstein S, Cruz TF. Interleukin-1 beta induction of c-fos and collagenase expression in articular chondrocytes: involvement of reactive oxygen species. J Cell Biochem. 1998;69:19–29. doi: 10.1002/(sici)1097-4644(19980401)69:1<19::aid-jcb3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 37.Hayflick L. The future of ageing. Nature. 2000;408:267–269. doi: 10.1038/35041709. [DOI] [PubMed] [Google Scholar]
- 38.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]


