Abstract
Among mammals, modern cetaceans (whales, dolphins, and porpoises) are unusual in the absence of hind limbs. However, cetacean embryos do initiate hind-limb bud development. In dolphins, the bud arrests and degenerates around the fifth gestational week. Initial limb outgrowth in amniotes is maintained by two signaling centers, the apical ectodermal ridge (AER) and the zone of polarizing activity (ZPA). Our data indicate that the cetacean hind-limb bud forms an AER and that this structure expresses Fgf8 initially, but that neither the AER nor Fgf8 expression is maintained. Moreover, Sonic hedgehog (Shh), which mediates the signaling activity of the ZPA, is absent from the dolphin hind-limb bud. We find that failure to establish a ZPA is associated with the absence of Hand2, an upstream regulator of Shh. Interpreting our results in the context of both the cetacean fossil record and the known functions of Shh suggests that reduction of Shh expression may have occurred ≈41 million years ago and led to the loss of distal limb elements. The total loss of Shh expression may account for the further loss of hind-limb elements that occurred near the origin of the modern suborders of cetaceans ≈34 million years ago. Integration of paleontological and developmental data suggests that hind-limb size was reduced by gradually operating microevolutionary changes. Long after locomotor function was totally lost, modulation of developmental control genes eliminated most of the hind-limb skeleton. Hence, macroevolutionary changes in gene expression did not drive the initial reduction in hind-limb size.
Keywords: cetacea, delphinidae, evo-devo, limb development, whale evolution
The absence of hind limbs in cetaceans can be studied from a paleontological, functional, and developmental perspective. From a paleontological perspective, hind-limb reduction is well documented, and specific morphologies can be linked to specific locomotor modes (1, 2): whereas the earliest cetaceans (pakicetids and ambulocetids) had large feet that were used in swimming, later taxa used their long tails for propulsion in water (remingtonocetids), and hind limbs became rudiments when osteological evidence correlated with fluke origins appeared (in dorudontids and basilosaurids). From a functional perspective, experimental data indicate that cetaceans evolved toward torpedo-shapes, and hind-limb loss enhanced streamlining (3). Otters and their relatives are excellent functional models for the evolutionary stages of cetacean locomotion (1, 4), and it is clear that reduction of the hind limbs during swimming led to more efficient swimming. Selection for the loss of hind limbs must have been strong when cetaceans became fluked-pursuit predators in the late Eocene (1, 5).
From a developmental perspective, the descriptive embryology of hind-limb reduction in cetaceans has been studied (6, 7). However, the genetically regulated mechanism underlying this developmental pattern remains unknown, even though the early stages of genetic control of limb development in tetrapods are well understood from experiments in chicks and mice. In amniote embryos, limb outgrowth is controlled by two signaling centers that are both located in the limb bud. The first of these centers, the apical ectodermal ridge (AER), is situated along the distal margin of the bud and presents morphologically as a thickening of the epithelium. Fgf4, 8, 9, and 17 mediate the signaling activity of the AER in amniotes (8, 9). The second signaling center is the zone of polarizing activity (ZPA), which is located in the posterior mesenchyme of the limb bud (10). Although it is not morphologically distinct from the rest of the limb mesenchyme, the ZPA is characterized at the molecular level by the expression of Sonic hedgehog (Shh). Expression of Shh at the posterior margin of the limb bud is regulated, in part, by expression of the transcription factor Hand2. In chick and mouse embryos, Hand2 is initially expressed widely throughout the mesenchyme of the limbs and flanks but then becomes restricted to the posterior edge of the fore- and hind-limb bud, where it is a necessary condition for the expression of Shh (11–13).
Here, we investigate the molecular basis for hind-limb loss during cetacean evolution by studying gene expression during early development of hind-limb buds in embryos of the pantropical spotted dolphin, Stenella attenuata. We report that the molecular cascade that controls limb development deviates from that described for other tetrapods. Combined with paleontological data documenting the changing limb proportions through the early evolution of cetaceans, these findings allow us to propose an evolutionary mechanism at the developmental genetic level can account for loss of hind limbs during cetacean evolution.
Results
The AER.
We found that embryos of the pantropical spotted dolphin (S. attenuata) display a hind-limb bud with a morphologically distinct AER at their tip around embryonic stage Carnegie 13 (7). The AER persists and hind-limb bud outgrowth is sustained through Carnegie 15 (Fig. 1A and B). Shortly thereafter, distal ectodermal cells lose their columnar shape, and the AER is lost (Fig. 1 C and D). After this degeneration, the hind-limb bud diminishes in size.
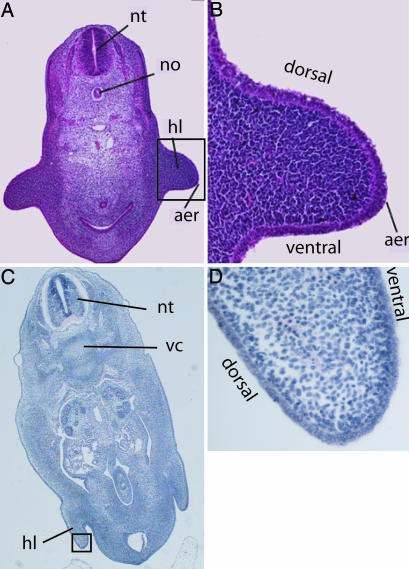
Fig. 1.
Hind-limb loss in embryos of the dolphin S. attenuata. (A and B) Embryo (LACM 94706, section 183a, coronal section) at the stage of largest hind-limb development (Carnegie Stage 15). Hind-limb bud (hl) with apical ectodermal ridge (aer) is visible on either side of the abdomen of the embryo, with notochord (no) and neural tube (nt) in the median plane. (C and D) Embryo (LACM 94747, section 238, cross section) with reduced hind limbs and missing AER (Carnegie Stage 16). Chondrification is taking place in the vertebral column (vc). Boxes in A and C indicate location of enlargements found in B and D, respectively.
To determine whether the AER of the dolphin hind limb is functional at a molecular level, we next investigated whether it expresses Fgf8. Fgf8 protein localizes to the AER in both fore- and hind-limb buds of Stenella at Carnegie 14 (Fig. 2C–F), consistent with the expression pattern in chick and mouse embryos (14, 15). By Carnegie 16, however, Fgf8 is undetectable in the hind-limb bud ectoderm. These results suggests that the dolphin hind-limb bud initially has a functional, albeit transient, AER.
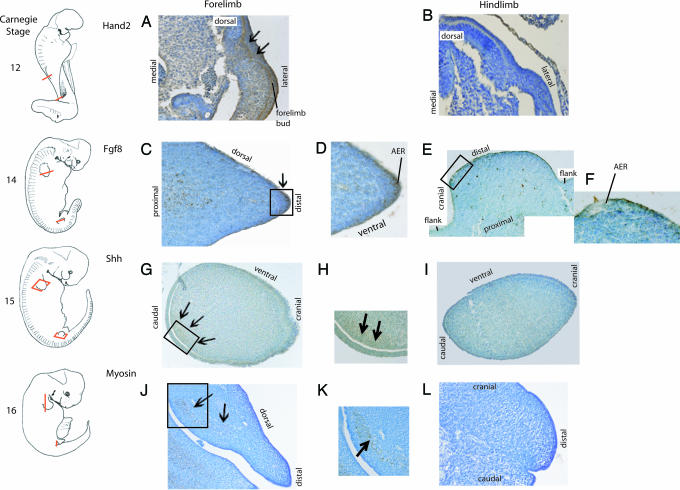
Fig. 2.
Gene expression in Stenella embryos. Drawings of embryos at Carnegie Stages 12, 14, 15, and 16 are shown, and red lines indicate location of sections. Sections through fore-limb bud (A, C, D, G, H, J, and K) and hind-limb bud area (B, E, F, I, and L). (A and B) Hand2 expression in embryo LACM 94789 (sections 208c and 286b, respectively). (C–F) Fgf8 expression in embryo LACM 94594 (sections 72c, detail of 72c, 208a, detail of 208b, respectively) region labeled flank in E shows low epithelial background staining. (G–I) Shh expression in embryo LACM 94746 (three views of section 4b). (J and K) Myosin expression in embryo LACM 94770 (section 214a with detail and 481a, respectively). Boxes indicate areas enlarged in adjacent image, and arrows indicate areas of high expression.
Given that the AER initially forms in dolphin hind-limb buds and that the maintenance of the AER requires signaling by the underlying mesenchyme, we hypothesized that degeneration of the AER in dolphins is not the primary defect responsible for absence of hind limbs, but instead it may reflect an underlying deficiency in mesenchymal cell signaling, perhaps in the ZPA.
The ZPA.
To study signaling in the ZPA, we examined expression of Shh, the polarizing signal of the ZPA, before loss of the AER in dolphin hind-limb buds (Fig. 2 G–I). Although Shh was expressed posteriorly in the fore limb of Stenella embryos during Carnegie 14 and 15 (Fig. 2 G and H), it was not detected in the posterior hind-limb bud mesenchyme of the same embryos (Fig. 2I). Hence, whereas the fore limb has a Shh-producing ZPA, similar to the pattern in chicks and mice (16, 17), absence of Shh suggests that Stenella lacks a functional ZPA in the hind-limb bud. Our finding that Shh is undetectable at stages when an AER is present in hind-limb buds suggests that it is the initial establishment, rather than maintenance, of the ZPA that is perturbed in dolphins.
Establishment of the ZPA.
To identify the cause of the lack of Shh expression in the hind limb, we examined expression of the transcription factor Hand2. Hand2 is one of the upstream regulators of Shh transcription that is expressed posteriorly in the prospective limb buds and in the flank (interlimb region) and is required both for Shh expression and cell survival in the limb buds of mouse embryos (11, 18). In Stenella, we found that Hand2 expression in the fore-limb region at Carnegie 12 follows the generalized pattern (Fig. 2A), but Hand2 was undetectable in the area of the developing hind limb (Fig. 2B). Thus, absence of Hand2 may underlie the failure of the hind limb to express Shh and establish a ZPA.
Loss of Hind-Limb Musculature.
During normal limb development, hind-limb muscle is derived from myoblasts that invade the limb buds from the adjacent somites. These myogenic cells are attracted to the limb bud by factors that are regulated, in part, by signals from the AER and ZPA (19). To study this relationship in dolphin embryonic development, we investigated the ability of the truncated hind-limb bud to induce somitic myoblasts to migrate. Using myosin as a marker for myogenic cells, we found myosin-positive cells in the fore-limb buds but not in the hind-limb buds of Stenella embryos at Carnegie 16 (Fig. 2 J–L). This finding suggests that myoblasts fail to migrate into the dolphin hind-limb bud, consistent with our conclusions that the AER degenerates soon after bud initiation (Fig. 1 C and D) and that the ZPA does not form. We recognize that this finding does not exclude the possibility that some myoblasts may colonize the bud but fail to survive. There is a complete halt of further hind-limb development at this point, including the lack of induction of somitic tissue to provide limb musculature.
Discussion
Major Morphological Shift in Whale Evolution.
Modern cetaceans have a strongly reduced hind-limb skeleton embedded in the ventral abdominal wall (Fig. 3). It consists, at most, of innominate, femur, and tibia (25), and at least just the innominate (e.g., in Stenella). Interestingly, mice lacking Shh expression are strikingly similar to the cetacean pattern: Both exhibit loss of distal limb structures, but retain parts of the remaining limb skeleton embedded within the body wall (26). Our hypothesis is that the reduction of the hind limbs in Stenella is due to elimination of Hand2 and Shh, accompanied by the early loss of the AER. Given that other modern cetaceans have similarly developed hind limbs and given the hind-limb morphology of fossil whales, we hypothesize that a mechanism involving Shh and Hand2 was responsible for hind-limb absence in the last common ancestor of modern cetaceans. Such changes in gene expression in early developmental stages can lead to a sudden and major morphological shift (27), and these shifts may drive evolutionary transitions (28). However, in the cetacean case, fossil evidence suggests that the macroevolutionary loss of the ZPA did not drive hind-limb reduction and, instead, occurred after substantial reduction of hind-limb size and after the complete loss of the hind limb as a locomotor organ.
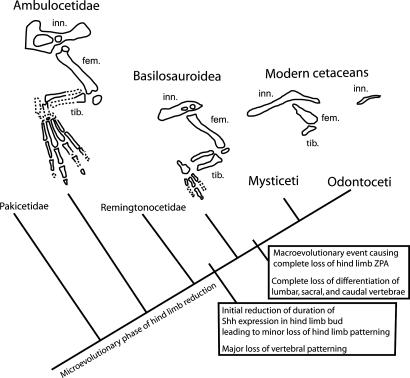
Fig. 3.
Simplified phylogeny of cetaceans discussed here with evolutionary events indicated. Hind limbs represent fossil ambulocetid Ambulocetus (20, 21), fossil basilosauroid Basilosaurus (22, 23), and two modern mysticetes (Bowhead Whale and Sei Whale, respectively; ref. 24). In most odontocetes, the only hind-limb element preserved is the innominate, as in the Sei Whale (see supporting information). Labeled bony elements of the hind limb are innominate (inn.), femur (fem.), and tibia (tib).
The hypothesis that duration of Shh expression led to unusual morphologies in cetaceans is consistent with the morphology of the fore limb. Whereas most mammals have two phalanges in the thumb and three in each of the fingers, cetacean fingers are commonly hyperphalangeous. Based on the observation of a persistent AER at the distal tip of the dolphin fore-limb, it has been suggested that an extension of the growth and segmentation program in the cetacean hand was achieved by prolonged Shh expression (29).
The phylogeny of Eocene cetaceans is stable at the family level (refs. 30 and 31; Fig. 3), and relatively complete skeletons are known for most families (2, 20, 32–34). The fossil record shows that cetaceans originated ≈50 million years ago, and their hind limbs retained the original patterning of a complete limb skeleton with four toes and three phalanges each for the next 9 million years (31, 32). However, there is a gradual reduction in relative limb length during this period, even before the fluke develops (2, 20, 32–34; see also supporting information, which is published on the PNAS web site). This reduction closely matches locomotor function: Initially the thigh and leg reduce in relative length, but the foot remains large. This reduction leads to shortened lever arms, with retention of a large propulsive surface in ambulocetids and protocetids (32, 33), functioning as an oar. Subsequently, the entire limb reduces as the tail comes to dominate propulsive function in basilosauroids. These cetaceans are the oldest that display osteological evidence for a fluke (34, 35). This phase of hind-limb reduction did not involve a major developmental overhaul (1, 36) and followed a gradual microevolutionary pattern of reduced ontogenetic growth. Approximately 41 million years ago, tail-propelled basilosauroid cetaceans display some loss of patterning of the distal hind limb: They lose one metatarsal and several phalanges (22). The resulting foot is very similar to that in some skinks, where the shorter duration (in developmental time) of Shh expression in the ZPA results in the formation of fewer digits (37). Shh plays a central role in hind-limb loss in cetaceans and skinks, and we propose that the duration of Shh expression in the basilosauroid hind limb may have been an important factor determining their hind-limb patterning. Development of the most anterior digit, digit 1, is independent of Shh, and digit 2 is specified by a low dose of Shh signal. The formation of digits 3 to 5 is determined by a temporal mechanism: the duration of exposure to Shh (38). Our hypothesis that early whales underwent a temporal shift in the duration of Shh exposure is consistent both with these experimental results and the patterns of hind-limb reduction seen in the whale fossil record.
The complete loss of the ZPA occurred above the basilosauroid node in cetacean phylogeny (Fig. 3). Given that both modern suborders have similarly reduced hind limbs, we suggest that it occurred at ≈34 million years ago. This macroevolutionary event did not drive the evolutionary loss of the hind limb but codified developmentally what had been an established pattern of reduced hind-limb function for several million years.
Changes in the Body Axis.
What then triggered ZPA loss in cetaceans? We hypothesize that ZPA loss is linked to evolutionary changes in the main body axis. The body axis of modern cetaceans is very different from that of their Eocene relatives. In the modern forms, there is no morphological difference between lumbar, sacral, and anterior caudal vertebrae, and, in the past, these vertebrae could only be homologized with those in generalized mammals based on indirect evidence, such as the position of the pudendal nerve (ref. 39, see supporting information). The position of the hind-limb bud in our embryos of S. attenuata can be directly compared to somite levels: The hind-limb bud is located near Somite 43 at Carnegie Stage 13, indicating that the 39th vertebra is homologous to the first sacral vertebra of land mammals and confirms the earlier inferences (39). This pattern implies that the lumbar/sacral boundary is well posterior to that in other mammals, where it is near vertebra 26 (40, 41).
Unlike modern cetaceans, late Eocene basilosauroids do retain morphologically distinct sacral vertebrae, differing from lumbar vertebrae by thick distal transverse processes (34). This morphology suggests that the genes that specify vertebral identity in basilosauroids retain the expression patterns of generalized mammals and are unlike those of modern cetaceans. Changes in body axis patterning, post-basilosauroids, thus coincided with the loss of patterning of the hind limb, not with the reduction in length of the hind limb. Our results are consistent with previous findings in snakes, which indicated that loss of vertebral patterning and loss of limb patterning are developmentally coupled and may be controlled by the same genes (42). It is tempting to speculate that modulation of Hox gene expression along the craniocaudal axis underpins the altered expression of Hand2 in the hind limb and posterior flank of Stenella. This hypothesis is supported by work showing that ZPA position in the fore limb is specified by the anterior boundary of Hoxb8 and by the recent finding that modulation of Hoxd gene expression in mice can shift the boundary of Hand2 in the early limb bud (43). This finding provides a mechanistic link between hind-limb reduction and homogenization of the posterior axial skeleton in whale evolution and can be tested by studies of Hoxd expression.
The pattern of gradual limb reduction in the cetacean fossil record makes it unlikely that Hand2 expression was lost as a sudden event before the origin of basilosauroids, because it would have led to more severe truncation of the limb (Fig. 3). It is possible that the duration of Hand2 expression was shortened gradually, which could have lead to shortening the time of Shh expression, thereby causing gradual reduction of digit number. However, we cannot exclude the possibility that the Hand2 domain was eliminated from the hind-limb bud after the leg had been reduced via other mechanisms, which would have relaxed the selective pressure to maintain an active signaling pathway in the hind-limb bud. Finally, the correlation in evolutionary time between loss of vertebral patterning and loss of hind-limb patterning in cetaceans is consistent with our hypothesis that evolutionary changes to the axial and appendicular skeleton during cetacean evolution may be linked by a common developmental mechanism.
Materials and Methods
Embryonic Specimens.
All embryonic specimens of S. attenuata were from the museum collection of the Los Angeles Museum of Natural History (LACM). Embryos were immersion-fixed and preserved in 70% ethanol. Specimens were stored without refrigeration for time periods ranging from 15 to 32 years. The embryos were staged by using a modified version of the Carnegie system (44) as designed originally for human embryos. Immunohistochemical data presented in this paper are based on four embryos (LACM 94594, 94617, 94746, and 94789), ranging from Carnegie Stage 12–16 (see details in supporting information), but additional embryos were used to optimize protocols and test antibodies.
General Immunohistochemistry.
Each embryo was embedded in paraffin, and 6-μm sections were prepared. Protocols for each antibody were optimized by using immersion-fixed, ethanol-preserved chick embryos and then tested and further optimized on nonlimb embryonic dolphin tissue. After optimal protocols were determined, we carried out experiments on our staged Stenella embryos. Because of the variance in fixation and storage times for the different embryos, slightly different procedures were used for different specimens to obtain optimal results (see supporting information). However, results for each embryo always were compared with identically treated reference sections from the same embryo, usually the normally developing fore limb, which served as positive controls (see supporting information). In addition, negative control samples (minus primary antibody) were used to determine the level of background staining for all experiments.
The following antibodies were used in this study: anti-fibroblast growth factor-8 (Fgf-8; Santa Cruz Biotechnology; sc-6958); anti-Sonic Hedgehog (Shh; Santa Cruz Biotechnology; sc-6958); anti-Hand2 (Santa Cruz Biotechnology; sc-9409); and anti-myosin antibody A4.1025. The latter antibody recognizes all isoforms of fast and slow myosin heavy chain protein (kindly provided by S. Hughes and L. Robson, King's College, London).
Supplementary Material
Acknowledgments
We thank Norihiro Okada for early encouragement, Lisa Cooper and Sandra Madar for discussions, Sirpa Nummela for preparing the Clear-and-Stain specimens, Brooke Garner for technical assistance, and Simon Hughes and Leslie Robson for the 1045 antibody. Financial support was provided by the U.S. National Science Foundation (J.G.M.T.), the National Institutes of Health (M.J.C.), and the Indian Department of Science and Technology, Delhi, India (S.B.).
Abbreviations
- AER
apical ectodermal ridge
- ZPA
zone of polarizing activity.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Thewissen J. G. M., Fish F. E. Paleobiology. 1997;23:482–490. [Google Scholar]
- 2.Bajpai S., Thewissen J. G. M. Curr. Sci. 2000;79:1478–1482. [Google Scholar]
- 3.Fish F. E. Aust. J. Zool. 1993;42:79–101. [Google Scholar]
- 4.Fish F. E. Am. Zool. 1996;36:628–641. [Google Scholar]
- 5.Thewissen J. G. M., Williams E. M. Annu. Rev. Ecol. Syst. 2002;33:73–90. [Google Scholar]
- 6.Guldberg G., Nansen F. On the Development and Structure of the Whale, Part 1. On the Development of the Dolphin. Bergen, Norway: Bergens Museum; 1894. [Google Scholar]
- 7.Sedmera D., Misek I., Klima M. Eur. J. Morphol. 1997;35:25–30. doi: 10.1076/ejom.35.1.25.13058. [DOI] [PubMed] [Google Scholar]
- 8.Boulet A. M., Moon A. M., Arenkiel B. R., Capecchi M. R. Dev. Biol. 2004;273:361–372. doi: 10.1016/j.ydbio.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Sun X., Mariani F. V., Martin G. R. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- 10.Tickle C., Musterberg A. Curr. Opin. Genet. Dev. 2001;11:476–481. doi: 10.1016/s0959-437x(00)00220-3. [DOI] [PubMed] [Google Scholar]
- 11.Charité J., McFadden D. G., Olsen E. N. Development (Cambridge, U.K.) 2000;127:2461–2470. doi: 10.1242/dev.127.11.2461. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Teran M., Piedra M. E., Kathiriya I. S., Rodriguez-Rey J. C., Ros M. A. Development (Cambridge, U.K.) 2000;127:2133–2142. doi: 10.1242/dev.127.10.2133. [DOI] [PubMed] [Google Scholar]
- 13.Yelon D., Ticho B, Halpern M. E., Ruvinsky I., Ho R. K., Silver L. M., Stainier D. Y. Development (Cambridge, U.K.) 2000;127:2573–2582. doi: 10.1242/dev.127.12.2573. [DOI] [PubMed] [Google Scholar]
- 14.Crossly P. H., Minowada G., MacArthur C. A., Martin G. R. Cell. 1996;84:126–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 15.Vogel A., Rodriguez C., Izpisua-Belmonte J. C. Development (Cambridge, U.K.) 1996;122:1737–1750. doi: 10.1242/dev.122.6.1737. [DOI] [PubMed] [Google Scholar]
- 16.Riddle R. D., Johnson R. L., Laufer E., Tabin C. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 17.Chang D. T., Lopez A., Von Kessler D. P., Chiang C, Simandl B. K., Zhao R., Seldin M. F., Fallon J. F., Beachy P. A. Development (Cambridge, U.K.) 1994;120:3339–3353. doi: 10.1242/dev.120.11.3339. [DOI] [PubMed] [Google Scholar]
- 18.Aiyer A. R., Honarpour N., Herz J., Srivastava D. Dev. Biol. 2005;278:155–162. doi: 10.1016/j.ydbio.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Scaal M., Bonafede A., Dathe V., Sachs M., Cann G., Christ B., Brand-Saberi B. Development (Cambridge, U.K.) 1999;126:4885–4895. doi: 10.1242/dev.126.21.4885. [DOI] [PubMed] [Google Scholar]
- 20.Thewissen J. G. M., Madar S. I., Hussain S. T. CFS Cour. Forschungsinst. Senckenberg. 1996;191:1–86. [Google Scholar]
- 21.Madar S. I., Thewissen J. G. M., Hussain S. T. J. Vertebr. Paleontol. 2002;22:405–422. [Google Scholar]
- 22.Gingerich P. D., Smith B. H., Simons A. L. Science. 1990;249:154–157. doi: 10.1126/science.249.4965.154. [DOI] [PubMed] [Google Scholar]
- 23.Lucas F. A. Proc. U.S. Natl. Mus; 1900. pp. 327–331. [Google Scholar]
- 24.Struthers J. J. Anat. Physiol. 1881;15:141–176. 301–321. [PMC free article] [PubMed] [Google Scholar]
- 25.Struthers J. J. Anat. Physiol. 1893;27:291–335. [PMC free article] [PubMed] [Google Scholar]
- 26.Kraus P., Fraidenraich D., Loomis C. A. Mech. Dev. 2001;100:45–58. doi: 10.1016/s0925-4773(00)00492-5. [DOI] [PubMed] [Google Scholar]
- 27.Chiang C., Litingtung Y., Harris M. P., Simandl B. K., Li Y., Beachy P. A., Fallon J. F. Dev. Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- 28.Carroll S. B., Grenier J. K., Weatherbee S. D. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Boston: Blackwell; 2001. [Google Scholar]
- 29.Richardson M. K., Jeffery J. E., Tabin C. J. Evol. Dev. 2004;6:1–5. doi: 10.1111/j.1525-142x.2004.04008.x. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary M. A. Amer. Zool. 2001;41:487–506. [Google Scholar]
- 31.Geisler J. H., Uhen M. D. J. Vertebr. Paleontol. 2005;23:991–996. [Google Scholar]
- 32.Thewissen J. G. M., Williams E. M., Roe L. J., Hussain S. T. Nature. 2001;413:277–281. doi: 10.1038/35095005. [DOI] [PubMed] [Google Scholar]
- 33.Gingerich P. D., Haq M. u., Zalmout I. S., Khan I. H., Malkani M. S. Science. 2001;293:2239–2242. doi: 10.1126/science.1063902. [DOI] [PubMed] [Google Scholar]
- 34.Uhen M. D. Univ. Michigan Papers on Paleontol. 2004;34:1–222. [Google Scholar]
- 35.Buchholtz E. A. In: The Emergence of Whales, Evolutionary Patterns in the Origin of Cetacea. Thewissen J. G. M., editor. New York: Plenum; 1998. pp. 325–352. [Google Scholar]
- 36.Bejder L., Hall B. K. Evol. Dev. 2002;4:445–458. doi: 10.1046/j.1525-142x.2002.02033.x. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro M. D., Hanken J., Rosenthal N. J. Exp. Zool. 2003;297:48–56. doi: 10.1002/jez.b.19. [DOI] [PubMed] [Google Scholar]
- 38.Harfe B. D., Scherz P. J., Nissim S., Tian H., McMahon A. P., Tabin C. J. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Slijper E. J. Die Cetaceen, vergleichend-anatomisch, und systematisch. The Hague, The Netherlands: M. Nijhoff; 1936. [Google Scholar]
- 40.Lessertiseur J., Saban R. In: Traité de Zoologie, Anatomie, Systématique, Biologie. Grassé P. P., editor. Vol. 16, Part 1. Paris: Masson; 1967. pp. 584–708. [Google Scholar]
- 41.Narita Y., Kuratani S. J. Exp. Zool. B Mol. Dev. Evol. 2005;15:91–106. doi: 10.1002/jez.b.21029. [DOI] [PubMed] [Google Scholar]
- 42.Cohn M. J., Tickle C. Nature. 1999;399:474–479. doi: 10.1038/20944. [DOI] [PubMed] [Google Scholar]
- 43.Zákány J., Kmita M., Duboule D. Science. 2004;304:1669–1672. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]
- 44.Thewissen J. G. M., Heyning J. In: Reproductive Biology and Phylogeny of Cetacea, Whales, Porpoises, and Dolphins. Miller D. L., editor. Enfield, NH: Science Publishers; in press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.