Abstract
As one of the most diverse and productive ecosystems known, and one of the first ecosystems to exhibit major climate-warming impacts (coral bleaching), coral reefs have drawn much scientific attention to what may prove to be their Achilles heel, the thermal sensitivity of reef-building corals. Here we show that climate change-driven loss of live coral, and ultimately structural complexity, in the Seychelles results in local extinctions, substantial reductions in species richness, reduced taxonomic distinctness, and a loss of species within key functional groups of reef fish. The importance of deteriorating physical structure to these patterns demonstrates the longer-term impacts of bleaching on reefs and raises questions over the potential for recovery. We suggest that isolated reef systems may be more susceptible to climate change, despite escaping many of the stressors impacting continental reefs.
Keywords: biodiversity, climate change, coral bleaching, resilience, reef fish
Global warming is causing reef corals around the world to expel their photosynthetic symbionts, resulting in “bleaching” and extensive coral mortality (1–3). Widespread impacts of bleaching are predicted (4), although empirical data on the long-term effects on other components of the ecosystem are lacking. Of particular interest are reef fish, which support local fisheries and tourism (5), and are crucial for the resilience of coral reefs (6–8). Existing studies of the indirect effects of bleaching mediated coral mortality on reef fish assemblages have been short-term and indicated limited change (9–11); however, the longer-term implications of coral loss may be much more substantial (12). Long-term responses may result from changes in the physiological condition of species following coral loss (13) or through processes such as the breakdown of the physical matrix of the remaining reef structure.
The global mass bleaching event of 1998 was devastating in the western Indian Ocean, where the El Niño event interacted with the Indian Ocean dipole (14), resulting in 75–99% loss of live coral (15). The Seychelles suffered particularly badly; live coral was reduced by >90% across the entire range of the inner islands (2, 16), with no apparent depth refuge. With widely accepted predictions of all reef regions of the world suffering similar large-scale degradation through bleaching in coming decades (1, 2), the study of such locations provides a unique opportunity to understand longer-term impacts on other components of the ecosystem, with implications for resilience and the persistence of vulnerable species.
We surveyed coral and fish communities at 21 sites across the inner islands of the Seychelles in 1994 (17) and 2005. Over 50,000 m2 of three distinct coral reef habitats were surveyed: fringing reefs with carbonate framework, coral growth on a granitic substrate, and patch reefs on a sand, rock, or rubble base (17). The study specifically aimed to assess changes in benthic variables after the 1998 bleaching event and relate these to changes in the total, taxonomic, size and trophic diversity of reef fish.
Results and Discussion
The coral reef system of the inner Seychelles has undergone a widespread phase shift from a coral-dominated state to a rubble and algal-dominated state (Fig. 1). The reefs in 1994 were characterized by high cover of live branching and massive coral, soft coral, and high structural complexity, whereas the same reefs in 2005 had low complexity, rubble, standing dead branching coral, and algal fields. In 2005, average coral cover was 7.5%, and <1% of the benthos consisted of fast growing, habitat forming branching and plating functional groups of corals. The remaining live corals were massive and encrusting forms offering limited refuge for reef-associated organisms (12). Conversely, macroalgae cover had increased 7-fold and dominated many of the carbonate reefs.
Fig. 1.
Change in the structure of Seychelles reef habitats from 1994 to 2005. Change assessed by correlation-based principle components analysis of log(x + 1) transformed and normalized environmental data. Eigenvectors of each benthic variable are overlaid.
The change in habitat had a profound impact on reef-associated fish assemblages, in particular, and diversity. To describe these impacts, we used robust measures of biodiversity, average taxonomic distinctness (AvTD) and variation in taxonomic distinctness (VarTD), which incorporate species’ taxonomic relatedness (18, 19). Low AvTD and VarTD values indicate degraded locations (19). The 2005 data demonstrate reduced AvTD and slightly reduced VarTD, with four sites showing a significant departure from expected values, suggesting that they were taxonomically depauperate (Fig. 2A). Although only four sites depart from expected values in the bivariate plot, there was a significant decline in AvTD for all sites by habitat type (F1,36 = 9.33, P < 0.01). The declines are greatest for the carbonate and patch reef habitats, for both AvTD and VarTD, indicating fish taxonomic composition on granitic reefs is more stable (Fig. 2 B and C). As taxonomic distinctness is related to trophic diversity, the observed reduction in AvTD represents a possible loss of functionality in the fish assemblage and may have serious repercussions for ecosystem stability (20–22).
Fig. 2.
Change in taxonomic diversity of Seychelles reef fish assemblage from 1994 to 2005. (A) Bivariate plot of AvTD on the x axis and VarTD on the y axis. 95% confidence ellipses, based on M counts of 40, 60, and 80, are constructed from a simulation distribution of random subsets of the master taxonomy list. Four sites that depart from expected values are labeled A–D in both 1994 and 2005. (B) Mean average taxonomic distinctness by habitat ± SE. (C) Mean variation in taxonomic distinctness by habitat ± SE.
The three main families that have been heavily impacted through bleaching are the monacanthids, chaetodontids, and pomacentrids. Furthermore, given the spatial scale of our surveys, we identify the possible local extinction of four fish species (Labrichthys unilineatus, Chaetodon lineolatus, Plectroglyphidodon johnstonianus, and Thalassoma hardwicke) and a reduction in abundance to critically low levels for six species (Oxymonacanthus longirostris, Chaetodon trifascialis, Chaetodon melannotus, Chaetodon meyeri, Plectroglyphidodon dickii, and Chromis ternatensis), all of which rely on live coral for key life processes, such as recruitment, shelter, or diet.
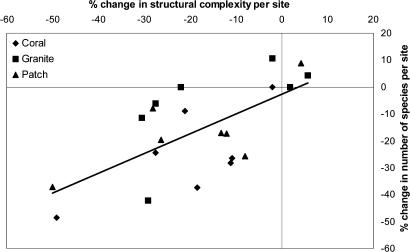
The influence of change in structural complexity and habitat type on changes in species richness (S) of fish communities was investigated by using analysis of covariance, where habitat type was a categorical predictor and change in structure a covariate. Habitat type did not significantly affect species richness (F2,17 = 2.63, P = 0.10); however, there was a significant effect of complexity (F1,17 = 14.75, P < 0.001). Combined, changes in structural complexity and habitat explain ≈50% (r2 = 0.57) of the changes in species richness (Fig. 3). There was no significant impact on species richness due to loss in live coral cover per se (r2 = 0.13). Consistent with studies of other forms of disturbance, this finding suggests that loss in physical structure is the main driving force in species richness declines on coral reefs after disturbance (23–25), and explains why the short-term impacts of bleaching on fish assemblages may appear to be negligible (9–11). Furthermore, because changes in species richness were correlated linearly with the extent of habitat complexity loss, there is no evidence of a threshold level of structural complexity below which species richness was maintained.
Fig. 3.
Relationship between the change in structural complexity and the change in fish species richness of Seychelles reef from 1994 to 2005.
To understand which components of the fish assemblage are most affected by habitat loss, we assessed changes by using size and trophic categorization. Small species have been lost at all sites, whereas larger species have only declined as more structure has been lost (Fig. 4A). The reef matrix offers a host of niches and varying habitat characteristics for small species, often resulting in high specialization (26). Loss of this habitat results in increased competition over the remaining space and increased susceptibility to predation (26), and may also increase the efficiency of fishing. Similarly, corallivorous and planktivorous species, reliant on live coral for food and shelter, have been generally lost at all sites, whereas species from other trophic groups have declined only as the complexity of the reef has been reduced (Fig. 4B). The most plausible explanations for this pattern are increased rates of predation and competition in smaller size classes due to loss of refuge space (27) and because many reef fish species require this habitat to recruit from the plankton (12).
Fig. 4.
Net number of species lost or gained between 1994 and 2005. (A) Species categorized by maximum attainable size. (B) Species categorized by trophic grouping. Least loss of structural complexity is shown to the left of plots, and greatest loss is shown to the right.
The decline of herbivorous fish species diversity on carbonate and patch reefs (Table 1) is of particular concern; theory predicts that bioeroders, scrapers, and grazers have complementary functions in reef recovery to coral-dominated states (6, 20). However, it is not just the presence or absence of herbivorous species that determines resilience, because resilience is also a function of the diversity of responses and the abundance of the remaining species (22). Indeed, previous phase shifts have been preceded by reduced abundance of herbivores (7), and a reduction in macroalgae cover in marine reserves has been associated with increases in parrotfish biomass (8). Although the abundances of individuals within herbivorous functional groups has declined in the Seychelles (significantly for grazers), the biomass has remained relatively stable (Table 1). Because many of the component species are long lived (28), the large individuals currently contributing to the biomass were potentially recruited before 1998. Therefore, changes in Seychelles are analogous to those in the Caribbean in the 1980s (7), where key ecosystem functions were performed by few species, leaving a more fragile and less stable system (20, 22).
Table 1.
Change in diversity, abundance, and biomass per site of key functional groups of herbivorous fish within the three habitats surveyed
| Bioeroders (n = 4) | Scrapers (n = 12) | Grazers (n = 23) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Carbonate | Granitic | Patch | Carbonate | Granitic | Patch | Carbonate | Granitic | Patch | |
| Mean no. of species in 1994 | 2.43 | 2.57 | 2.71 | 6.57 | 6.43 | 6 | 11 | 10 | 11 |
| Mean no. of species in 2005 | 1.86 | 2.43 | 2.14 | 5.57 | 6.86 | 5.57 | 8.43 | 10.7 | 8.57 |
| Change, % | −24 | −6 | −21 | −15 | 7 | −7 | −23 | 7 | −22 |
| Mean abundance in 1994 | 53 | 46.1 | 48.1 | 78.9 | 41.1 | 58.9 | 318.7 | 256.4 | 174.3 |
| Mean abundance in 2005 | 43.3 | 29.9 | 30.6 | 48.3 | 48.3 | 37.6 | 125.4 | 100.7 | 90.3 |
| Change, % | −18 | −35 | −36 | −39 | 17 | −36 | −61 | −61 | −48 |
| Mean biomass in 1994, kg | 11.2 | 12.8 | 11.3 | 14.3 | 12.3 | 11.6 | 21.4 | 20.7 | 19.7 |
| Mean biomass in 2005, kg | 13.3 | 10.8 | 11.6 | 25.3 | 29.1 | 11.9 | 20.8 | 23.7 | 10.9 |
| Change, % | 19 | −16 | 3 | 77 | 137 | 3 | −3 | 14 | −44 |
Total number of species in surveys for each group given in brackets. Two-factor orthogonal ANOVAs indicated a significant decline in number of species for bioeroders (P < 0.05) and in abundance for grazers (P < 0.02).
Although there has been debate over the speed at which reefs recover from disturbance (29), our data raise questions as to why there seems to be at most a slow recovery in the Seychelles, when other reef systems have recovered more quickly (9, 30). Stochastic variation in recruitment can have limiting effects. However, across the number of species and spatial and temporal scales surveyed, the lack of recovery is most likely due to determinate factors, such as physical isolation, loss of habitat complexity and the inability of herbivorous groups to control algae. Long-distance dispersal of external larvae to isolated reef systems is rare (3, 31). Indeed, assuming dispersal limits are ≈600 km (32), with the majority of settlers dispersing <100 km (33), the inner Seychelles will be largely reliant on self-recruitment. The small and disconnected brood stocks of many species of coral (particularly fast growing branching species) and specialized fish species will likely reduce the rate of any recovery. Although key functional groups of herbivores may be expected to control algae (8) and encourage coral recruitment (6), sustaining and increasing their biomass is complicated. This is because fishing has already reduced the biomass of large herbivores (17) and the loss of habitat complexity is impacting smaller size classes. Synergistic effects of other disturbances will also likely impede recovery, ultimately “ratcheting down” coral cover (7, 34).
The severity and extent of the 1998 bleaching event in the Seychelles and the isolation of these islands may explain why recovery is faster in continental reef systems such as East Africa (9) and the Great Barrier Reef (30), where larval replenishment from less impacted reefs is expected (3). This finding suggests that isolated reefs, including those of many small island developing states, where reefs are critical for coastal protection, food supply, income, and employment (5), may be the most susceptible to climate change-driven reef degradation, despite escaping many of the stressors impacting continental reef systems. Offering hope, some habitats are less vulnerable than others; in the Seychelles, the granitic reefs are more resistant to algal overgrowth with stable herbivorous fish assemblages, which may explain the apparent increase in coral recruits within this habitat. Subsequent recovery of coral brood stocks on granitic reef sites may facilitate coral recruitment to carbonate and patch reefs. Minimizing the range of additional stresses to reefs and promoting biomass increases of key functional groups will be fundamental to any recovery. However, if the probability of 1998-scale thermal anomalies increases as predicted for the Indian Ocean (2), the medium-term prospects for recovery of Seychelles’ reefs are not good.
Methods
Other than bleaching, Seychelles reefs have experienced little change in other stressors in the period from 1994 to 2005. Fishing pressure and biomass of catches have remained relatively stable (Seychelles Fishing Authority technical reports from 1989 to 2004), a small crown-of-thorns starfish outbreak before 1998 has not been repeated (16), sedimentation on near-shore reefs remains a chronic problem at a few sheltered locations (J.R., unpublished data), and the December 2004 tsunami has had negligible impact to date (35). Although historical fishing pressure and other stressors will likely have exerted stress on the system in the past and may act synergistically to inhibit recovery, available evidence indicates that the changes we demonstrate are principally caused by climate anomaly mediated coral loss.
Over 50,000 m2 of coral reef habitat was surveyed at the same time of year in both 1994 and 2005. At each site, 16 7-m-radius point counts were conducted by using underwater visual census at the bottom of the reef slope (17). This technique was most appropriate because spear fishing is banned in the Seychelles and it maximized area coverage and replication. Replicates were placed a random number of fin kicks apart, with the proviso that boundaries of adjacent counts were at least 15 m apart. The individual size and abundance of 134 noncryptic, diurnally active, reef-associated fish species that could be positively identified and censused was quantified; this included species within the following families: Acanthuridae, Balistidae, Chaetodontidae, Haemulidae, Labridae, Lethrinidae, Lutjanidae, Monacanthidae, Mullidae, Nemipteridae, Pomacanthidae, Pomacentridae, Scaridae, Serranidae, Siganidae, and Zanclidae. Species were surveyed sequentially, with the most active being recorded first and any subsequent movements of that species in or out of the count area ignored to avoid bias. The time required to complete a census varied according to the number and diversity of fish present in the area.
Once a fish count was complete, the percent cover (based on plan view) of sand, rock, rubble, macroalgae, dead and live branching coral, and massive and soft coral was estimated, and accuracy was assessed by using the line intercept method (no significant difference P = 0.639). The topographic structural complexity of the reef inside each count area was then described by using a six-point scale (36) that was checked for accuracy by using the linear versus contour chain method (significantly correlated P < 0.001).
Change in the benthos was analyzed by using correlation-based principle components analysis. Variables were log(x + 1) transformed to improve the spread of the data and normalized. Change in taxonomic diversity was assessed with a bivariate plot of AvTD (the degree to which species in a sample are related taxonomically to each other, measuring the average path length between every pair of species through a taxonomic tree; ref. 18) and VarTD (the evenness to which the taxa are spread across the tree, ref. 19). The bivariate approach allows 95% confidence ellipses, molded to account for any mechanistic correlation between AvTD and VarTD, to be constructed from a simulation distribution of random subsets of the master taxonomy list (constructed following ref. 37). Here, ellipses based on M counts of 40, 60, and 80 are constructed to reflect the number of species seen at each site. Within-site change in AvTD and VarTD was also assessed at the habitat level by using two-way ANOVAs. The relationship between percent loss of species richness (total number of species, S) and percent loss of benthic variables including live coral cover and structural complexity was tested by using linear regression and analysis of covariance. Each species was categorized by maximum attainable size and trophic group according to regional fish identification guides, dietary literature, and fishbase (www.fishbase.org). The net number of species lost or gained between 1994 and 2005 within each of the size or trophic categories was quantified for each of the 21 sites surveyed. Sites were ordered from least to most species lost, which reflects the amount of physical structure lost at each site from regression analysis. Size estimates of fish were converted to biomass by using published length–weight relationships (ref. 38 and www.fishbase.org). Changes in diversity, abundance, and biomass of the three key functional groups of herbivores were tested by using two-way ANOVAs.
Acknowledgments
We thank the Seychelles Centre for Marine Research and Technology–Marine Parks Authority, Seychelles Fishing Authority, and Nature Seychelles for technical and logistical assistance. The manuscript was improved through the comments of K. R. Clarke, T. R. McClanahan, and M. A. MacNeil. This work was supported by grants from the British Overseas Development Administration (now Department for International Development), the Leverhulme Trust, and the Western Indian Ocean Marine Science Association (WIOMSA).
Abbreviations
- AvTD
average taxonomic distinctness
- VarTD
variation in taxonomic distinctness.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hoegh-Guldberg O. Mar. Freshwater Res. 1999;50:839–866. [Google Scholar]
- 2.Sheppard C. R. C. Nature. 2003;425:294–297. doi: 10.1038/nature01987. [DOI] [PubMed] [Google Scholar]
- 3.Hughes T. P., Baird A. H., Bellwood D. R., Card M., Connolly S. R., Folke C., Grosberg R., Hoegh-Guldberg O., Jackson J. B. C., Kleypas J., et al. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 4.Walther G.-R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J.-M., Hoegh-Guldberg O., Bairlein F. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 5.Moberg F., Folke C. Ecol. Econ. 1999;29:215–233. [Google Scholar]
- 6.Bellwood D. R., Hughes T. P., Folke C., Nyström M. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 7.Hughes T. P. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 8.Mumby P. J., Dahlgren C. P., Harborne A. R., Kappel C. V., Micheli F., Brumbaugh D. R., Holmes K. E., Mendes J. M., Broad K., Sanchirico J. N., et al. Science. 2006;311:98–101. doi: 10.1126/science.1121129. [DOI] [PubMed] [Google Scholar]
- 9.McClanahan T., Maina J., Pet-Soede L. AMBIO. 2002;31:543–550. [PubMed] [Google Scholar]
- 10.Sheppard C. R. C., Spalding M., Bradshaw C., Wilson S. AMBIO. 2002;31:40–48. [PubMed] [Google Scholar]
- 11.Spalding M. D., Jarvis G. E. Mar. Poll. Bull. 2002;44:309–321. doi: 10.1016/s0025-326x(01)00281-8. [DOI] [PubMed] [Google Scholar]
- 12.Jones G. P., McCormick M. I., Srinivasan M., Eagle J. V. Proc. Natl. Acad. Sci. USA. 2004;101:8251–8253. doi: 10.1073/pnas.0401277101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratchett M. S., Wilson S. K., Berumen M. L., McCormick M. I. Coral Reefs. 2004;23:352–356. [Google Scholar]
- 14.Sajl N. H., Goswami B. N., Vinayachandran P. N., Yamagata T. Nature. 1999;401:360–363. doi: 10.1038/43854. [DOI] [PubMed] [Google Scholar]
- 15.Goreau T., McClanahan T., Hayes R., Strong A. Conserv. Biol. 2000;14:5–15. [Google Scholar]
- 16.Linden O., Souter D., Wilhemsson D., Obura D. Coral Reef Degredation in the Indian Ocean, Status Report 2002 284. Kalmar, Sweden: CORDIO; 2002. [Google Scholar]
- 17.Jennings S., Grandcourt E. M., Polunin N. V. C. Coral Reefs. 1995;14:225–235. [Google Scholar]
- 18.Clarke K. R., Warwick R. M. J. Appl. Ecol. 1998;35:523–531. [Google Scholar]
- 19.Clarke K. R., Warwick R. M. Mar. Ecol. Prog. Ser. 2001;216:265–278. [Google Scholar]
- 20.Nyström M., Folke C., Moberg F. Trends Ecol. Evol. 2000;15:413–417. doi: 10.1016/s0169-5347(00)01948-0. [DOI] [PubMed] [Google Scholar]
- 21.Rogers S. I., Clarke K. R., Reynolds J. D. J. Appl. Ecol. 1999;68:769–782. [Google Scholar]
- 22.Chapin F. S., Zavaleta E. S., Eviner V. T., Naylor R. L., Vitousek P. M., Reynolds H. L., Hooper D. U., Lavorel S., Sala O. E., Hobbie S. E., et al. Nature. 2000;405:234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- 23.Risk M. J. Atoll Res. Bull. 1972;193:1–6. [Google Scholar]
- 24.Sano M., Shimizu M., Nose Y. Mar. Ecol. Prog. Ser. 1987;37:191–199. [Google Scholar]
- 25.Syms C., Jones G. P. Ecology. 2000;81:2714–2729. [Google Scholar]
- 26.Munday P. L., Jones G. P. Ocean. Mar. Biol. Ann. Rev. 1998;36:373–411. [Google Scholar]
- 27.Dulvy N. K., Polunin N. V. C., Mill A. C., Graham N. A. J. Can. J. Fish. Aquat. Sci. 2004;61:466–475. [Google Scholar]
- 28.Choat J. H., Robertson D. R. In: Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem. Sale P. F., editor. San Diego: Academic; 2002. pp. 57–80. [Google Scholar]
- 29.Riegl B. Bull. Mar. Sci. 2001;69:595–611. [Google Scholar]
- 30.Halford A., Cheal A. J., Ryan D., Williams D. McB. Ecology. 2004;85:1892–1905. [Google Scholar]
- 31.Ayre D. J., Hughes T. P. Ecol. Lett. 2004;7:273–278. [Google Scholar]
- 32.Bellwood D. R., Hughes T. P., Connolly S. R., Tanner J. Ecol. Lett. 2005;8:643–651. [Google Scholar]
- 33.Cowen R. K., Paris C. B., Srinivasan A. Science. 2006;311:522–527. doi: 10.1126/science.1122039. [DOI] [PubMed] [Google Scholar]
- 34.Birkeland C. Bioscience. 2004;54:1021–1027. [Google Scholar]
- 35.Abdulla A., Bijoux J., Engelhardt U., Obura D., Payet R., Pike K., Robinson J., Russell M., Skewes T. In: Status of Coral Reefs in Tsunami Affected Countries: 2005. Wilkinson C., Souter D., Goldberg J., editors. Townsville, Australia: Australian Institute of Marine Science; 2006. pp. 125–134. [Google Scholar]
- 36.Polunin N. V. C., Roberts C. M. Mar. Ecol. Prog. Ser. 1993;100:167–176. [Google Scholar]
- 37.Helfman G. S., Collette B. B., Facey D. E. The Diversity of Fishes. Oxford: Blackwell Science; 1997. pp. 244–270. [Google Scholar]
- 38.Letourneur Y., Kulbicki M., Labrosse P. Naga. 1998;21:39–46. [Google Scholar]