Abstract
Using GFP to mark recent thymic emigrants (RTEs) in mice carrying a GFP transgene driven by the recombination-activating gene 2 promoter, we demonstrate that RTEs are readily detectable even in 2-year-old mice, despite the fact that the proportion of the peripheral T cell pool comprised of RTEs declines with age. Although the number of RTEs decreases after reaching a peak at 6 weeks of age, thymic output as a function of thymic size is surprisingly age-independent. The CD4:CD8 ratio of RTEs declines with age, partly because of a striking decrease in steady-state proliferation of CD4+ RTEs in older mice. RTEs in aged mice undergo phenotypic maturation in the lymphoid periphery with delayed kinetics compared with young mice. RTEs from aged mice secrete less IL-2, proliferate less well, and achieve only weak expression of early-activation markers compared with more mature naïve peripheral T cells from the same mice. The proportion of GFP− cells in the CD4+ and CD8+ thymic compartments increases with age, partly as a result of leakiness in the aged thymus, allowing reentry of naïve peripheral T cells.
Keywords: aging, recent thymic emigrants, T cell development
Maintenance of the peripheral T cell population throughout life depends on balancing the influx of recent thymic emigrants (RTEs) with the homeostatic regulation of mature peripheral T cells. Although T cell numbers can be sustained by homeostatic proliferation of peripheral T cells after lymphocyte depletion, the thymus is essential for maintaining a diverse antigen receptor repertoire and a substantial pool of naïve peripheral T cells. Hallmarks of the aging immune system include thymic involution, enhanced contribution of memory cells to the peripheral T cell pool, and striking clonal expansions among both CD4 and CD8 T cell populations (reviewed in refs. 1 and 2). These phenomena are interrelated, because shrinkage of the thymus limits the number of newly exported T cells, triggering the gradual decline in the naïve T cell pool, which in turn likely contributes to the expansion of select memory phenotype T cells.
Understanding the contribution of thymic output to the peripheral T cell pool requires identification of RTEs as a population distinct from the bulk of naïve and previously activated peripheral T cells. Over the years, this distinction has been achieved in mice by identifying RTEs that have originated from thymocytes labeled by BrdU (3) or intrathymic injection of FITC (4, 5) by following a wave of thymocyte differentiation and egress from thymic lobes transplanted into congenic hosts (6, 7) and in mice and humans by using T cell receptor (TCR) rearrangement excision circles to identify cells that have not proliferated since rearranging antigen receptor genes (8–13). Although highly useful, these techniques suffer serious disadvantages, including the short time frame over which RTEs can be observed (4–7), the trauma inherent in the labeling technique and its potential to alter thymic output (4–7), the inherent inaccuracy of the tag itself (3, 8–13), and the inability to simultaneously identify RTEs and measure their function (3, 8–13).
We recently used animals (14) carrying a GFP transgene driven by the recombination-activating gene 2 (RAG2) promoter [RAG2p-GFP transgenic (Tg) mice] to tag RTEs in unmanipulated mice. These GFP+ peripheral T cells no longer express RAG2 mRNA (15) and disappear rapidly after thymectomy. We estimate that GFPhi, GFPlow, and GFPneg cells, respectively, have left the thymus 1 week, 2–3 weeks, or >3 weeks previously (16). The CD4:CD8 ratio of RTEs is higher than that of mature naïve T cells, and this ratio is down-modulated both by preferential proliferation of CD8+ RTEs and by loss of CD4+ RTEs as these cells are incorporated into the mature naïve T cell compartment (16). RTEs in young adult mice continue to undergo phenotypic maturation in the periphery, up-regulating Qa2 and down-regulating CD24 surface expression. RTEs are also functionally immature compared with mature naïve CD4+ T cells. Upon activation, RTEs secrete less IL-2, express lower levels of surface CD25, and exhibit reduced proliferative capacity. In vivo stimulated CD8+ RTEs show defects in cell accumulation and IFNγ secretion compared with their mature counterparts. What drives the maturation of RTEs is unclear, although the influence of Egr-1-mediated signals on RTE survival and incorporation into the mature peripheral pool suggests that this process may be active rather than passive (17).
Using GFP to tag RTEs allows analysis of the function and population dynamics of newly emigrated T cells in unmanipulated aged mice. Our current studies document the expected age-dependent decline in RTE numbers but reveal that RTEs are readily detectable even in 2-year-old mice. Thymic output is surprisingly age-independent when normalized to the size of the generative CD4+CD8+ double-positive (DP) compartment. Similar to RTEs from young adults, thymic emigrants from aged mice continue functional and phenotypic maturation in the lymphoid periphery, albeit with slower kinetics. The CD4:CD8 ratio of RTEs, but not that of single-positive (SP) thymocytes, declines sharply with age, and aged thymic tissue becomes permissive for the reentry of mature but still naïve peripheral T cells. What role these cells may play in the aged thymus is unknown.
Results
Whereas the Proportion and Number of RTEs Decrease with Age, Thymic Output as a Function of Thymic Size Is Relatively Age-Independent.
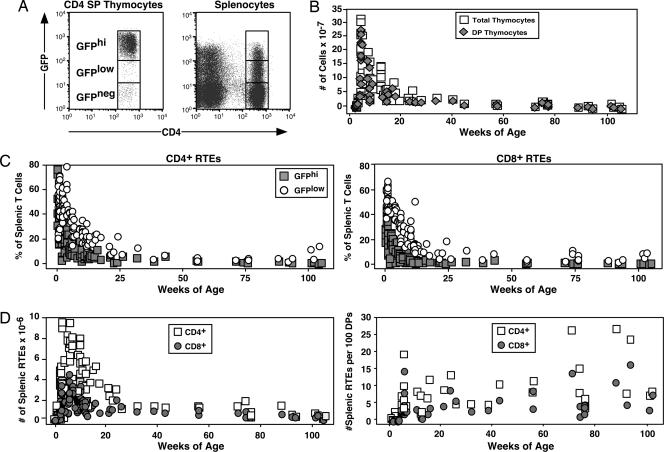
By using GFP as a marker for RTEs in RAG2p-GFP Tg mice (Fig. 1A), it is possible to analyze the population dynamics of RTEs as a function of age. From these time point data, it is clear that the entire peripheral CD4+ and CD8+ T cell pool is comprised of GFPhi and GFPlow RTEs in neonatal mice. The proportion of the peripheral pool comprised of RTEs decreases thereafter, leveling off at ≈3% by 6 months of age. RTEs are clearly detectable, and the thymus is therefore functional, even in 2-year-old mice (Fig. 1C).
Fig. 1.
RTEs remain readily detectable even in 2-year-old mice, and thymic output as a function of thymic size is relatively age-independent. Splenocytes and thymocytes from individual RAG2p-GFP Tg mice of the indicated ages were stained for CD4 and CD8 surface expression. (A) GFPhi and GFPlow gates were defined on SP thymocytes (Left) and applied to splenocytes (Right). (B) The number of total and DP thymocytes was determined as a function of age. (C) The percentages of GFPhi (filled squares) and GFPlow (open circles) cells among CD4+ and CD8+ splenocytes were determined in mice of the indicated ages. (D) By using organ cell counts and the percentage of DP thymocytes and RTEs (GFPhi plus GFPlow), the total number (Left) and the number per 100 DP thymocytes (Right) of splenic CD4+ (open squares) and CD8+ (filled circles) RTEs were calculated.
Whereas the proportion of splenocytes that are RTEs declines steadily during the first 6 months of age, the number of splenic RTEs increases from birth, peaks at ≈6 weeks of age, and then decreases to a plateau of ≈1–1.5 × 106 each of CD4+ and CD8+ RTEs at ≈6 months of age (Fig. 1D). The total number of thymocytes decreases by ≈30-fold as mice age from 6 weeks to 2 years (Fig. 1B). To control for this striking age-dependent involution, thymic output was quantified as the number of splenic RTEs per 100 DP thymocytes, the generative compartment for thymocytes that seed the periphery. By this measure, thymic output is relatively age-independent (Fig. 1D). Similar results are obtained when thymic output is measured against the size of the GFPhi SP thymocyte pool (data not shown).
The CD4:CD8 Ratio of RTEs Decreases with Age, Due in Part to a Relative Dampening of the Steady-State Proliferation of CD4+ RTEs.
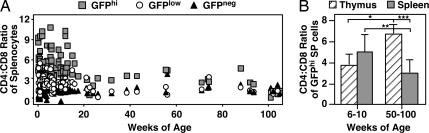
In young adult mice, the CD4:CD8 ratio of GFPhi RTEs is greater than that of GFPlow RTEs, which is in turn greater than that of naïve mature peripheral T cells (16). We now show that the CD4:CD8 ratio of GFPhi splenic RTEs decreases dramatically with age, reaching a low plateau at ≈5–6 months of age (Fig. 2A). In contrast, the CD4:CD8 ratio of GFPhi SP thymocytes significantly increases with age (Fig. 2B).
Fig. 2.
The CD4:CD8 ratio of RTEs decreases with age, whereas that of SP thymocytes increases. Splenocytes and thymocytes from individual RAG2p-GFP Tg mice of the indicated ages were stained for CD4 and CD8 surface expression. (A) The CD4:CD8 ratio of peripheral CD4+ and CD8+ T cells was determined among GFPhi (gray squares), GFPlow (open circles), and GFPneg (black triangles) T cells. (B) The CD4:CD8 ratio was calculated for GFPhi SP thymocytes (striped bars) and GFPhi splenocytes (gray bars). Bars represent the average CD4:CD8 ratios for 6–26 mice within the indicated age ranges, and error bars represent standard deviations of the means. Using a two-tailed Student t test with equal variance, P = 0.0028 (∗), 0.00052 (∗∗), and 0.00043 (∗∗∗) for the bracketed values.
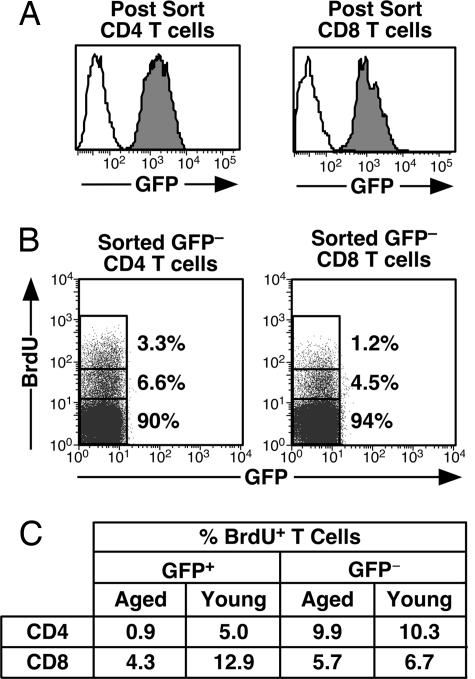
To explore the mechanism for the decline in the CD4:CD8 ratio of RTEs in aged mice, BrdU uptake during an 8-day labeling period was assessed to measure the steady-state proliferation of CD4+ and CD8+ RTEs and mature peripheral T cells (Fig. 3). GFP− CD4+ and CD8+ T cells show comparable steady-state proliferation in old and young mice (Fig. 3C). In contrast, GFP+ CD4+ cells in aged mice are severely compromised in their ability to undergo steady-state proliferation relative to their counterparts in young animals. CD8+ RTEs also proliferate less in aged mice than in young adults, but this defect is less severe than that experienced by CD4+ RTEs (Fig. 3C).
Fig. 3.
Age dampens the steady-state proliferation of CD4+ and, to a lesser extent, CD8+ RTEs. (A) Peripheral T cells from eight 6-week-old and eight 50- to 60-week-old mice fed BrdU in their drinking water for 8 days were stained for CD4 and CD8 and sorted into four populations: CD4+ GFP+ (Left, filled histograms), CD4+ GFP− (Left, open histograms), CD8+ GFP+ (Right, filled histograms), and CD8+ GFP− (Right, open histograms). (B) Sorted cells were stained with anti-BrdU and analyzed by using the illustrated gates into BrdUhi, BrdUlow, and BrdUneg populations. Percentages of cells falling into these populations are shown in representative dot plots for the sorted GFP− CD4+ (Left) and CD8+ (Right) populations. Cytoplasmic GFP is lost during the procedure. (C) Percentages of BrdU+ (BrdUhi plus BrdUlow) CD4+ and CD8+ populations that are GFP+ and GFP− are shown. Data are representative of three independent experiments.
Phenotypic Maturation of RTEs Occurs with Delayed Kinetics in Aged Mice.
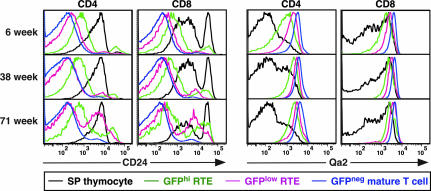
RTEs in young adult animals continue their development in the lymphoid periphery. The surface phenotypes of immature GFPhi, more mature GFPlow, and mature GFPneg T cells change progressively, as assessed by surface expression of several molecules, including CD24, Qa2, CD28, CD3, and IL-7Rα (Fig. 4Top) (16). A similar pattern of phenotypic maturation in Qa2 (and IL-7Rα; unpublished data) expression levels occurs in both CD4+ and CD8+ RTEs from mice at 10 and 18 months of age (Fig. 4 Middle and Bottom). However, the down-regulation of CD24 surface expression that characterizes RTE maturation in young adults is delayed as mice age. Whereas 35–36% of GFPlow RTEs remain CD24hi in the CD4+ and CD8+ splenic compartments at 71 weeks, in 6-week-old mice only 6–7% of GFPlow CD4+ and CD8+ splenic RTEs remain CD24hi (Fig. 4). The CD24hi CD8 SP thymocytes seen in all age groups are TCRlow/neg and are therefore immature cells.
Fig. 4.
Phenotypic maturation of RTEs occurs with delayed kinetics in aged compared with young mice. Splenocytes and thymocytes from mice of the indicated ages were stained for CD4, CD8, CD24, and Qa2 surface expression. GFP+ CD4+ and CD8+ SP thymocytes (black histograms) and splenocytes gated as GFPhi (green histograms), GFPlow (pink histograms), and GFPneg (blue histograms) were analyzed for CD24 and Qa2 expression. Data are representative of seven or more mice analyzed from each age group in five independent experiments.
Functional Maturation Occurs in CD4+ RTEs from Aged Mice.
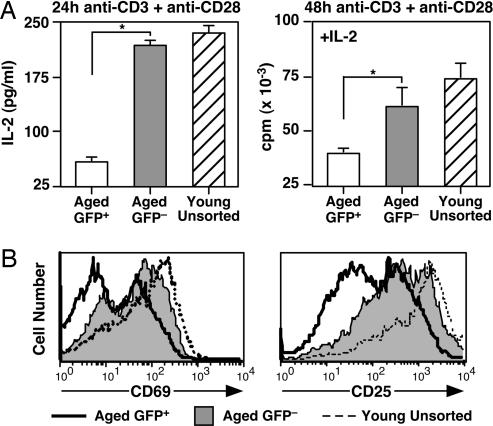
In young adult mice, RTE phenotypic maturation is mirrored by the continued acquisition of mature T cell functions by RTEs, including proliferation and cytokine production (16). CD4+ RTEs from mice over 1 year of age secrete less IL-2 after stimulation with a suboptimal dose of anti-CD3 and anti-CD28 than do CD4+ GFP− mature naïve T cells from the same pool of animals (Fig. 5A). IL-2 secretion is not the sole functional defect exhibited by RTEs, because proliferation by stimulated RTEs is also dampened relative to their mature T cell counterparts, even in the presence of exogenous IL-2 (Fig. 5A). Uptake of IL-2 is also likely defective in RTE populations, because CD25 (IL-2Rα) surface expression is poorly up-regulated 24 h (unpublished data) and 48 h (Fig. 5B) after activation. Although the activation-induced up-regulation of CD69 surface expression is indistinguishable in RTEs and mature naïve T cells in young adults (16), CD69 expression is dampened in CD4+ RTEs compared with their mature naïve counterparts in mice over 1 year of age (Fig. 5B).
Fig. 5.
Functional maturation occurs in CD4+ RTEs from aged mice. (A) CD4+ RTEs from aged mice secrete less IL-2 and proliferate less even in the presence of exogenous IL-2 than do CD4+ GFP− naïve T cells from the same animals. Sorted GFP+ (white bars) and naïve GFP− (gray bars) CD4+ peripheral T cells from eight 68- to 69-week-old mice and purified, unsorted total CD4+ T cells (striped bars) pooled from two 7-week-old mice were stimulated for 24 or 48 h with 30 ng/ml anti-CD3 plus 1 μg/ml anti-CD28 in the presence of APCs. (Left) The amount of IL-2 in the culture supernatants is shown as means ± SD. P = 0.00005 for the bracketed values using a two-tailed Student t test with equal variance. (Right) [3H]Thymidine incorporation during an 18-h pulse was measured after 48 h of stimulation in the presence of 25 units/ml exogenous IL-2. Mean values are shown for triplicate wells, and error bars represent standard deviation of the mean cpm. P = 0.0028 for the bracketed values using a two-tailed Student t test with equal variance. (B) Stimulated CD4+ GFP+ T cells from aged mice express lower levels of CD69 and CD25 than do their GFP− counterparts. The same populations as in A were stained after 48 h of stimulation for CD4 and CD69 or CD25. Histograms were gated on CD4+ T cells.
The Proportion of GFP− SP Thymocytes Increases with Age, Partly as a Result of Recirculation to the Aged Thymus of Naïve Peripheral T Cells.
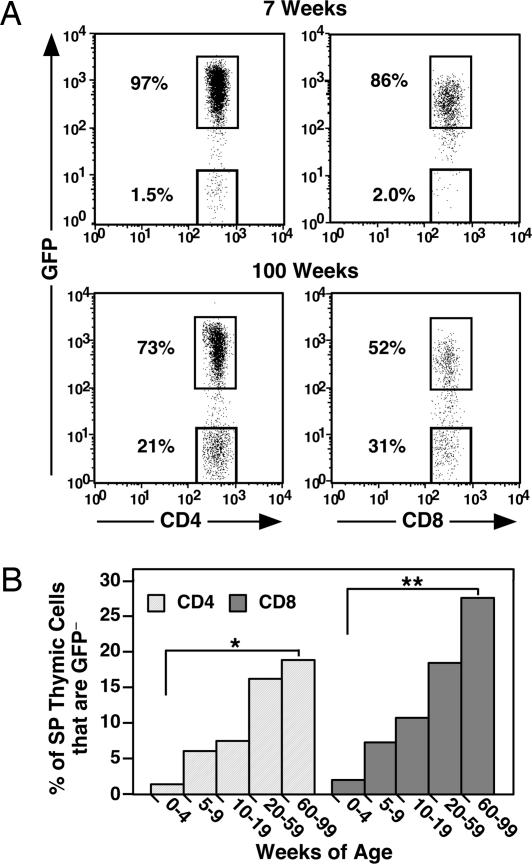
In young adult RAG2p-GFP Tg mice, only 1–2% of SP cells in the thymus are GFP− (Fig. 6A) (16). The proportion of GFP− cells in both the thymic CD4+ and CD8+ SP compartments increases dramatically with age, particularly within the CD8+ population, which includes an average of 25% GFP− cells in mice over 1 year of age (Fig. 6B). Activated peripheral T cells have been shown to recirculate to the thymus of young adults (18–21). To determine whether recirculation into the thymus of naïve peripheral T cells also contributes to the increased proportion of GFP− cells in the old thymus, Ly-5-marked naïve CD8+ peripheral T cells from OT-1 TCR Tg donors were injected into young and old RAG2p-GFP Tg recipients, and the distribution of CD8+ GFP− Ly5.1+ donor T cells was determined by flow cytometry. Donor cells are readily detectable in the spleens of both young and old hosts and appear in approximately the same proportion and number in both sets of recipients (Table 1). In contrast, the donor cells are excluded from the thymuses of the young recipients but gain entry into the old thymic tissue whether donor cell contribution is measured by percent or number (Table 1). Thus, the aged thymus allows entry of naïve peripheral T cells that are excluded by the young thymus.
Fig. 6.
The proportion of GFP− thymocytes increases with age in both the CD4 and CD8 SP compartments. Thymocytes from 7-week-old and 100-week-old RAG2p-GFP Tg mice were stained for CD4 and CD8 surface expression. (A) Shown are the percentages of GFPhi and GFPneg cells gated as CD4 SP (Left) and CD8 SP (Right). (B) The mean percentages of GFP− CD4 SP (light gray bars) and CD8 SP (dark gray bars) thymocytes are shown for eight or more mice of the indicated age ranges. P = 7.2 × 10−6 (∗) and 1.8 × 10−12 (∗∗) for bracketed values using a two-tailed Student t test with equal variance.
Table 1.
Naïve T cells selectively enter the aged thymus
| Organ | Host mice | Donor CD8 SP GFP− cells |
Mean no. of donor cells ± SD | |
|---|---|---|---|---|
| % | No. | |||
| Thymus | Young | 0.16–2.0 | 0.1–1.3 × 103 | 6.1 ± 4.7 × 102 |
| Old | 4.7–12.4 | 3.6–9.3 × 103 | 6.5 ± 2.5 × 103 | |
| Spleen | Young | 5.4–11.3 | 2.3–8.4 × 105 | 4.8 ± 2.9 × 105 |
| Old | 5.4–11.6 | 4.0–7.8 × 105 | 5.8 ± 1.8 × 105 | |
Naïve CD8+ T cells from 8-week-old OT-1 TCR Tg Ly5.1 mice were injected into six young (8- to 10-week-old) and five aged (70- to 76-week-old) Ly5.2 congenic RAG2p-GFP Tg hosts. Recipient thymocytes and splenocytes were analyzed for donor cells 4 days after adoptive transfer by staining for Ly5.1, CD4, CD8, CD44, and CD62L surface expression. The donor cells remain CD44low and CD62Lhi. The percentages and number of donor cells among CD8 SP GFP− cells in each organ were tabulated. P = 0.00033 comparing the number of donor cells in the old and young thymuses; P = 0.513 comparing the number of donor cells in the old and young spleens (two-tailed Student t test with equal variance).
Discussion
Using GFP to tag RTEs in RAG2p-GFP Tg mice without the need for surgical manipulation allows analysis of the phenotype and function of T cells during the first few weeks of their residence in the lymphoid periphery. Taking advantage of this system, we have shown that, although the proportion of the peripheral T cell pool comprised of RTEs declines steadily during the first 6 months of life, the number of splenic RTEs reaches a peak at 6 weeks of age and declines thereafter (Fig. 1 C and D). The striking involution of the thymus follows this same pattern (Fig. 1B), but thymic output, measured as a function of thymic size, is surprisingly age-independent (Fig. 1D). These data point to the resilient generative capacity of the immune system even in 2-year-old mice. This measure of thymic function is supported by more indirect tags for RTEs that have suggested that the thymus is active in both aged mice (reviewed in ref. 1) and humans (22).
As evidenced by the high CD4:CD8 ratio of RTEs and the preferential loss of CD4+ RTEs, the thymus of the young adult mouse exports more CD4+ T cells than can be successfully incorporated into the mature peripheral T cell pool (16). However, also correlated with thymic involution is the progressive decline in the CD4:CD8 ratio of GFPhi RTEs (Fig. 2A). This decrease is at least partly due to the diminished steady-state proliferation of CD4+ RTEs (and perhaps the SP thymocytes that give rise to these RTEs) in aged mice relative to young adult mice (Fig. 3C). However, the higher CD4:CD8 ratio among GFPhi SP thymocytes in aged mice compared with young mice (Fig. 2B) suggests that CD4 SP thymocytes may not emigrate as efficiently as their CD8+ counterparts. In support of this notion, CD69 expression is slightly decreased in CD4 but not CD8 SP thymocytes from 60-week-old mice relative to 6-week-old mice (data not shown).
The phenotypic maturation that characterizes RTEs as they move from the GFPhi through the GFPlow and eventually to the GFPneg compartment is delayed in mice beginning at 5–6 months of age (Fig. 4 and data not shown). Thus, although the up-regulation of Qa2 and IL-7Rα expression and the down-regulation of CD3 expression all occur with similar kinetics in aged and young mice, the progressive down-regulation of CD24 expression in a fraction of maturing RTEs from aged mice is strikingly retarded relative to that in young mice. One-third of GFPlow RTEs in both the CD4 and CD8 compartments in aged mice maintain high levels of CD24 expression (Fig. 4). Given the relationship between homeostatic proliferation and CD24 expression (23), it is possible the CD24hi GFPlow RTEs peculiar to aged mice are also characterized by altered population dynamics. As further evidence that RTEs from aged mice differ from those in young mice, stimulation with anti-CD3 and anti-CD28 drives defective up-regulation of CD69 expression in RTEs from aged mice (Fig. 5B), whereas expression of this early-activation marker in stimulated RTEs from young adults is indistinguishable from that of mature peripheral T cells from the same animals (16). Stimulated CD4+ RTEs from aged mice show defects in IL-2 secretion, CD25 expression (and therefore presumably IL-2 uptake), and proliferation, even in the presence of exogenous IL-2 (Fig. 5). The extent of these functional defects is similar to that exhibited by RTEs from young adults (16). Thus, although age may retard the kinetics of RTE maturation, the long-term functional consequences of this impairment are unclear, as further suggested by the observation that newly generated (mature, non-RTE) T cells in aged mice function as well as those in young mice (24). In agreement with work from many laboratories (reviewed in refs. 1 and 2), these experiments also reveal that, even within the GFP− compartment, T cells from aged mice are defective in proliferative capacity and the up-regulation of early-activation markers relative to T cells from younger individuals.
Thymic tissue from aged mice does display one characteristic (accessibility to naïve peripheral T cells) that distinguishes it from the young adult thymus. In young adult RAG2p-GFP Tg mice, 1–2% of SP cells in the thymus are GFP− (Fig. 6). These anomalous cells are large and CD44hi (ref. 16 and data not shown) and are therefore likely to be previously activated peripheral T cells that are known to recirculate to the young adult thymus (18–21). The proportion of GFP− cells in both the thymic CD4+ and CD8+ SP compartments increases dramatically with age, particularly within the CD8+ population (Fig. 6B). This unusual population of GFP− cells is intrathymic, because neighboring lymph nodes were excluded by staining with India ink injected i.p., and the exclusion was confirmed by the paucity of B cells. Although the proportion of peripheral T cells that have been previously activated is known to increase with age (reviewed in refs. 2 and 25), not all GFP− cells within the aged thymus bear an activated phenotype (data not shown). Adoptive-transfer experiments showed clearly that naïve T cells can selectively reenter the aged thymus but not the young adult thymus (Table 1) and that this reentry is not a function of the age of the recirculating T cells. Activation is not a requirement for gaining access to the aged thymus, because the transferred donor cells within the thymus of old recipients maintain the surface antigen phenotype of CD44lowCD62Lhi naïve T cells (data not shown). It is unknown what triggers the leakiness of aged thymic tissue, but young thymic lobes maintain the barrier to naïve T cells even after thymic atrophy is induced by irradiation and cortisone injection (20). Activated allogeneic T cells reside in the medulla of the young thymus (20, 26, 27) and have been shown to induce alloantigen-specific tolerance (21, 28). It has also been suggested that activated T cells persisting in the thymus may serve as a repository of memory in the young adult (18, 19). Such a role has also been suggested for activated T cells recruited to the young adult bone marrow, another generative organ (29–32). However, it remains unclear what function, if any, mature naïve T cells may serve in the aged thymus.
Materials and Methods
Mice.
RAG2p-GFP Tg breeders (NG-BAC mice) were originally provided by M. Nussenzweig (The Rockefeller University, New York) (14). C57BL/6 (B6) and B6.SJL-Ptprca Pep3b/BoyJ (B6.Ly5.1) breeders were purchased from The Jackson Laboratory. OT-1 TCR Tg mice (33) were bred on site. For in vivo BrdU incorporation studies, mice were given sterile drinking water containing 0.8 mg/ml BrdU (Sigma–Aldrich) made fresh and changed daily for 8 days. All experiments were performed in compliance with the University of Washington Institutional Animal Care and Use Committee.
Flow Cytometry.
Red blood cell-depleted single-cell suspensions from spleen and thymus were prepared, and Fc receptors were blocked with anti-CD16/32 (clone 2.4G2; BD Pharmingen). Cells were stained as previously described (34), and live cells were analyzed on a FACSCalibur or FACSCanto with cellquest (Becton Dickinson) or flowjo (Tree Star, Ashland, OR) software. Allophycocyanin (APhC)-labeled or peridinin chlorophyll protein-labeled anti-CD4 (RM4-5), biotin-labeled anti-Qa2 (1-1-2), and phycoerythrin (PE)-labeled anti-Ly5.1 (clone A20) were purchased from BD Pharmingen. APhC-labeled anti-CD44 (clone IM7) and PE-labeled anti-CD24 (M1/69), anti-CD25 (PC61), and anti-CD69 (H1.2F3) were purchased from eBioscience (San Diego). APhC-Alexa Fluor 750-labeled anti-CD8α (5H10) was purchased from Caltag. Bound biotin-labeled antibodies were detected with streptavidin-APhC (BD Pharmingen). GFPhi and GFP− gates were defined by using CD4+ and CD8+ SP thymocytes stained on the same day. Those splenocytes falling between GFPhi and GFPneg gates were designated GFPlow. Intracellular staining for BrdU was performed as previously described (3) by using PE-labeled anti-BrdU (3D4, BD Pharmingen).
Cell Enrichment and Sorting.
For cell culture experiments, T cells were enriched from combined spleen and lymph nodes from aged (68–85 weeks of age) or young (6–13 weeks of age) RAG2p-GFP Tg mice by negative selection by using antibodies against MHC class II (AF6-120.1), Fc receptors (2.4G2), and CD8 (2.43) followed by anti-mouse and anti-rat IgG-coated magnetic beads (Qiagen, Valencia, CA). The resulting 78% pure CD4+ population from the aged mice was then stained with APhC-labeled CD62L (MEL-14) and PE-labeled anti-CD8 (53.6-7), anti-B220 (CD45R, clone RA3-6B2), anti-CD11b (M1/70), anti-Ly76 (Ter119), and anti-NK1.1 (PK136), all from BD Pharmingen. Stained cells were further purified by sorting on a FACSAria cytometer (Becton Dickinson) as PE−, CD62L+, and either GFP+ or GFP−. Purity was verified at 93–98% by staining for CD4.
For BrdU intracellular staining experiments, T cells were enriched by negative bead selection by using antibodies against MHC class II and Fc receptors. The resulting 77–80% pure T cells were stained with APhC-labeled anti-CD4 and APhC-Alexa Fluor 750-labeled anti-CD8 and sorted into four populations: GFP+ CD4+ (91–98% pure), GFP− CD4+ (95–98% pure), GFP+ CD8+ (91–95% pure), and GFP− CD8+ (96–98% pure).
Adoptive Transfer.
Naïve Vα2+Vβ5+ CD8+ T cells from young OT-1 TCR Tg mice were isolated by negative bead selection as described above, and purity was assessed by staining for CD8, Vα2, and CD44 surface expression. A total of 6–8 × 106 naïve CD8+ T cells were injected into the lateral tail veins of young (8- to 10-week-old) and old (70- to 76-week-old) RAG2p-GFP Tg mice. Spleens and thymic lobes from recipients were collected 4 days after transfer. Recipients were injected i.p. 30 min before euthanasia with 100 μl of a 1:10 dilution of particulate India ink in PBS to mark the draining parathymic lymph nodes to ensure their exclusion. Splenocytes and thymocytes were stained for CD4, CD8, CD44, and Ly5.1 surface expression to quantify the donor cell contribution.
Cell Culture and IL-2 Measurement.
Sorted CD4 T cells were cultured in the presence of antigen-presenting cells (APCs), 30 ng/ml anti-CD3 (clone 145-2C11), and 1 μg/ml anti-CD28 (clone 37.51), both from BD Pharmingen, with or without 25 units/ml recombinant human IL-2 (originally from Cetus). APCs were irradiated (3,000 rad) B6 splenocytes depleted of T cells by incubation with antibodies against Thy-1, CD4, and CD8 and lysis with rabbit complement (Cedarlane Laboratories). Triplicate wells of 96-well plates were seeded with 5 × 104 T cells and 1.5 × 105 APCs. At 48 h, 1 μCi (1 Ci = 37 GBq) of [3H]thymidine was added per well, and plates were harvested 18 h later. Separate cultures in 48-well plates (2.5 × 105 T cells, 7.5 × 105 APCs, 30 ng/ml anti-CD3, and 1 μg/ml anti-CD28) were established to assess IL-2 secretion and activation marker expression. At 24 and 48 h, cells were stained for CD4 and CD25 or CD69. IL-2 in serially diluted supernatants collected from triplicate wells at 24 and 48 h was quantified in duplicate by using the mouse IL-2 OptEIA ELISA set from BD Pharmingen according to the manufacturer's protocol.
Acknowledgments
We thank Dr. M. Nussenzweig for his generous gift of RAG2p-GFP Tg breeders and D. Soper and J. Golob for their help in the early phases of this work. This work was generously supported by National Institutes of Health Grants R01 AI064318 and R21 AG023781 (to P.J.F.) and a Cancer Research Institute predoctoral training grant (to J.S.H.).
Abbreviations
- APC
antigen-presenting cell
- APhC
allophycocyanin
- B6
C57BL/6
- B6.Ly5.1
B6.SJL-Ptprca Pep3b/BoyJ
- DP
CD4+CD8+ double-positive
- PE
phycoerythrin
- RAG2
recombination-activating gene 2
- RTE
recent thymic emigrant
- SP
CD4+ or CD8+ single-positive
- TCR
T cell receptor
- Tg
transgenic.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Linton P. J., Dorshkind K. Nat. Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 2.Nikolich-Zugich J. J. Exp. Med. 2005;201:837–840. doi: 10.1084/jem.20050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tough D. F., Sprent J. J. Exp. Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scollay R. J. Immunol. 1982;128:1566–1570. [PubMed] [Google Scholar]
- 5.Scollay R., Chen W. F., Shortman K. J. Immunol. 1984;132:25–30. [PubMed] [Google Scholar]
- 6.Berzins S. P., Boyd R. L., Miller J. F. A. P. J. Exp. Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berzins S. P., Godfrey D. I., Miller J. F. A. P., Boyd R. L. Proc. Natl. Acad. Sci. USA. 1999;96:9787–9791. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douek D. C., McFarland R. D., Keiser P. H., Gage E. A., Massey J. M., Haynes B. F., Polis M. A., Haase A. T., Feinberg M. B., Sullivan J. L., et al. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 9.Rodewald H. R. Nature. 1998;396:630–631. doi: 10.1038/25251. [DOI] [PubMed] [Google Scholar]
- 10.Kong F. K., Chen C. L., Six A., Hockett R. D., Cooper M. D. Proc. Natl. Acad. Sci. USA. 1999;96:1536–1540. doi: 10.1073/pnas.96.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., Lewin S. R., Markowits M., Lin H. H., Skulsky E., Karanicolas R., He Y., Jin X., Tuttleton S., Vesanen M., et al. J. Exp. Med. 1999;190:725–732. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sempowski G. D., Gooding M. E., Liao H. X., Le P. T., Haynes B. F. Mol. Immunol. 2001;38:841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 13.Ortman C. L., Dittmar K. A., Witte P. L., Le P. T. Int. Immunol. 2002;14:813–822. doi: 10.1093/intimm/dxf042. [DOI] [PubMed] [Google Scholar]
- 14.Yu W., Nagaoka H., Jankovic M., Misulovin Z., Suh H., Rolink A., Melchers F., Meffre E., Nussenzweig M. C. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 15.Cooper C. J., Orr M. T., McMahan C. J., Fink P. J. J. Immunol. 2003;171:226–233. doi: 10.4049/jimmunol.171.1.226. [DOI] [PubMed] [Google Scholar]
- 16.Boursalian T. E., Golub J., Soper D. M., Cooper C. J., Fink P. J. Nat. Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 17.Schnell F. J., Kersh G. J. J. Immunol. 2005;175:2270–2277. doi: 10.4049/jimmunol.175.4.2270. [DOI] [PubMed] [Google Scholar]
- 18.Naparstek Y., Holoshitz J., Eisenstein S., Reshef T., Rappaport S., Chemke J., Ben Nun A., Cohen I. R. Nature. 1982;300:262–264. doi: 10.1038/300262a0. [DOI] [PubMed] [Google Scholar]
- 19.Fink P. J., Bevan M. J., Weissman I. L. J. Exp. Med. 1984;159:436–451. doi: 10.1084/jem.159.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agus D. B., Surh C. D., Sprent J. J. Exp. Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopinathan R., DePaz H. A., Oluwole O. O., Ali A. O., Garrovillo M., Engelstad K., Hardy M. A., Oluwole S. F. Transplantation. 2001;72:1533–1541. doi: 10.1097/00007890-200111150-00011. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson B. D., Douek D. C., Killian S., Hultin L. E., Scripture-Adams D. D., Giorgi J. V., Marelli D., Koup R. A., Zack J. A. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 23.Li O., Zheng P., Liu Y. J. Exp. Med. 2004;200:1083–1089. doi: 10.1084/jem.20040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haynes L., Eaton S. M., Burns E. M., Randall T. D., Swain S. L. J. Exp. Med. 2005;201:845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes L., Swain S. L., Cambier J., Fuldner R. Mech. Ageing Dev. 2005;126:822–825. doi: 10.1016/j.mad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Michie S., Kirkpatrick E. A., Rouse R. V. J. Exp. Med. 1988;168:1929–1934. doi: 10.1084/jem.168.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirokawa K., Utsuyama M., Sado T. Cell. Immunol. 1989;119:160–170. doi: 10.1016/0008-8749(89)90232-3. [DOI] [PubMed] [Google Scholar]
- 28.Tian C., Bagley J., Forman D., Iacomini J. J. Immunol. 2004;173:7217–7222. doi: 10.4049/jimmunol.173.12.7217. [DOI] [PubMed] [Google Scholar]
- 29.Slifka M., Whitmire J., Ahmed R. Blood. 1997;90:2103–2108. [PubMed] [Google Scholar]
- 30.Di Rosa F., Santoni A. Immunology. 2003;108:296–304. doi: 10.1046/j.1365-2567.2003.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klonowski K. D., Williams K. J., Marzo A. L., Blair D. A., Lingenheld E. G., Lefrancois L. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 32.Mazo I. B., Honczarenko M., Leung H., Cavanagh L. L., Bonasio R., Weninger W., Engelke K., Xia L., McIver R. P., Koni P. A., et al. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Hogquist K. A., Jameson S. C., Heath W. R., Howard J. L., Bevan M. J., Carbone F. R. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki I., Martin S., Boursalian T. E., Beers C., Fink P. J. J. Immunol. 2000;165:5537–5543. doi: 10.4049/jimmunol.165.10.5537. [DOI] [PubMed] [Google Scholar]