Abstract
The mRNA levels of NKCC1, an inwardly directed Na+, K+-2Cl− cotransporter that facilitates the accumulation of intracellular Cl−, and of KCC2, an outwardly directed K+-Cl− cotransporter that extrudes Cl−, were studied in surgically resected brain specimens from drug-resistant temporal lobe (TL) epilepsy (TLE) patients. Quantitative RT-PCR analyses of the mRNAs extracted from the human TLE-associated brain regions revealed an up-regulation of NKCC1 mRNA and a down-regulation of KCC2 mRNA in the hippocampal subiculum, compared with the hippocampus proper or the TL neocortex, suggesting an abnormal transcription of Cl− transporters in the TLE subiculum. In parallel experiments, cell membranes isolated from the same TLE-associated brain regions were injected into Xenopus oocytes that rapidly incorporated human GABAA receptors into their surface membrane. The GABA currents elicited in oocytes injected with membranes from the subiculum had a more depolarized reversal potential (EGABA) compared with the hippocampus proper or the neocortex. The NKCC1 blocker bumetanide or a temperature decrease of 10°C shifted the GABA-current EGABA more negative in oocytes injected with membranes from TLE hippocampal subiculum, matching the EGABA of TL neocortex-injected oocytes. We conclude that the anomalous expression of both Cl− transporters, KCC1 and NKCC2, in TLE hippocampal subiculum probably causes altered Cl− transport in the “epileptic” neurons, as revealed in the microtransplanted Xenopus oocytes, and renders GABA aberrantly “exciting,” a feature that may contribute to the precipitation of epileptic seizures.
Keywords: chloride transporter, GABAA receptors, Xenopus oocytes
Epilepsy affects ≈1% of the world population and is a devastating neurological disorder. Of many forms of epilepsy, the refractory human temporal lobe (TL) epilepsy (TLE) is successfully treated surgically by resection of epileptic foci, thus reducing or eliminating seizures. Despite a large body of experimental work, TLE epileptogenesis is still an open question (1, 2), with the notable hypothesis that the hippocampal subiculum may serve as an origin for synchronous firing spreading to other regions of the TL during seizures (3). Also, it has been shown that GABA is excitatory in a significant percentage of pyramidal neurons from the TLE subiculum, and this feature, probably because of altered Cl− homeostasis, is involved in epileptogenesis (4).
There is ample evidence that the neuron-specific K+/Cl− cotransporter KCC2, which maintains low intracellular Cl− concentration by extruding Cl− from the cell down the outward K+ gradient (5–7), is down-regulated in animal models after epileptiform activity and leads to impaired neuronal Cl− extrusion (8–10). The consequent increase in internal Cl− may result in a decreased GABAergic inhibition. Furthermore, it has been shown that the NKCC1 transporter, which plays the opposite function of facilitating the accumulation of Cl− in neurons (5), is up-regulated in the rat cortex after amygdala kindling (11), again producing an increase in internal Cl− and a reduction of GABAergic inhibition. Finally, the NKCC1 transporter facilitates seizures in the developing brain because of its high expression in perinatal vs. adult rat and human cortices (12).
To investigate directly the role played by Cl− transporters in the human TLE, we analyzed their mRNA expression pattern throughout the TLE epileptic regions and found that KCC2 is down-regulated, whereas NKCC1 is up-regulated, in the subiculum vs. the hippocampus proper or temporal neocortex. To determine whether these mRNA changes are sufficient to render the GABA-current reversal potential (EGABA) less negative and consequently favor the conversion of GABAergic responses from inhibitory to excitatory, we microtransplanted the human “epileptic” GABAA receptors to Xenopus oocytes by injecting them with membranes isolated from the same surgically resected TLE brain tissues (13). We confirmed further (14) that the EGABA values were shifted to more positive potentials in oocytes injected with membranes from the subiculum, compared with those injected with membranes from the hippocampus proper or the TL. This suggests that the altered mRNA levels of Cl− transporters that we found lead to the neurotransmitter GABA becoming excitatory and render the subiculum particularly susceptible to seizures.
Results and Discussion
Quantitative RT-PCR (qRT-PCR) Analysis.
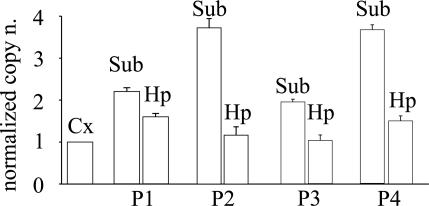
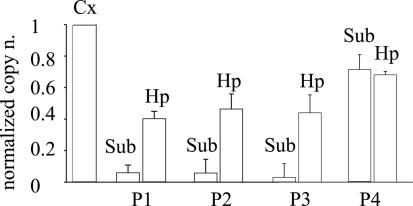
To determine whether the transcription of genes encoding Cl− transporters is altered in the human epileptic brain, as occurs in animal models (10, 12), we performed qRT-PCR analyses of the mRNAs encoding the NKCC1 and KCC2 transporters in the TLE subiculum and compared them with that in the hippocampus proper and TL neocortex of four patients. As illustrated in Figs. 1 and 2, it was found that the levels of mRNAs encoding the transporters were significantly different between neocortex and subiculum. Specifically, (i) the level of NKCC1 mRNA was significantly higher (2.9 ± 0.4-fold, mean ± SEM), whereas that of the KCC2 mRNA was significantly lower (0.21 ± 0.01-fold), in the subiculum compared with the neocortex; (ii) the amount of the NKCC1 mRNA level in the hippocampus proper was slightly higher than that in the neocortex in two of the four patients examined (Fig. 1); and (iii) the level of KCC2 mRNA in the hippocampus proper was reduced, compared with the neocortex, although to a smaller extent than that of the subiculum (Fig. 2). Moreover, the levels of expression of either KCC2 or NKCC1 mRNA were very similar in the cortex of all four patients (normalized to control mRNAs from each of the two patients; see Materials and Methods; data not shown).
Fig. 1.
Real-time quantitative analysis of the relative expression of NKCC1 mRNAs from the neocortex (Cx), subiculum (Sub), and hippocampal proper (Hp) of four TLE patients (Table 1). Each column represents the mean value obtained from 18 determinations per patient. For each tissue (as indicated), the relative mRNA copy number is normalized to the mRNA copy of TL cortex of the same patient (Pn). Bars represent standard error. P < 0.001 between Sub and Hp data sets. Data sets between Cx and Hp: P < 0.001 for P1 vs. P4; P > 0.05 for P2 vs. P3.
Fig. 2.
Real-time quantitative analysis of the relative expression of KCC2 mRNAs from the neocortex (Cx) subiculum (Sub) and hippocampal proper (Hp) of the same four TLE patients shown in Fig. 1. Columns and symbols are as described in the legend to Fig. 1. P < 0.001 between Sub and Hp data sets. P > 0.05 for data sets between Cx and Hp or Sub.
To investigate whether the different patterns of mRNA expression have a functional impact on the Cl−-dependent current responses to GABA, we injected Xenopus oocytes with membranes isolated from the same brain regions that were used for the mRNA analyses and studied the GABA-current responses by using electrophysiological techniques.
GABA-Current Rundown.
Application of GABA (1 mM) to control (noninjected) oocytes did not elicit a significant current. In contrast, all of the oocytes injected with membranes from the subiculum elicited inward membrane currents within a few hour of injection (see ref. 13). These currents were caused by activation of bona fide GABAA receptors transplanted from the human brain, because they were activated by the neurotransmitter GABA and were blocked by the GABAA-receptor antagonist bicuculline (100 μM; data not shown). We tested whether the GABA currents, elicited by activation of the “epileptic” receptors transplanted from the brains of the patients listed in Table 1, which is published as supporting information on the PNAS web site, exhibited the current rundown previously discovered for the TLE brain (15). It was found that the excessive rundown of GABA currents was a feature shared by GABAA receptors from the epileptic brain regions examined, namely hippocampal subiculum and TL neocortex (data not shown; see ref. 14). In a number of experiments, we also examined whether the brain-derived neurotrophic factor (BDNF, 500 ng/ml) reduced the GABA-current rundown in oocytes injected with TLE subiculum membranes, as shown previously for oocytes injected with membranes from the temporal neocortex (16). In 18 oocytes examined (two frogs and two patients), pretreating the oocytes with BDNF for 1 h reduced the current rundown (data not shown). Specifically, after the stimulation protocol used (see Materials and Methods), repetitive applications of GABA reduced the GABA current to 30 ± 3%, whereas after BDNF treatment, it was reduced to only 61 ± 9%, a finding similar to that reported for TL neocortex and hippocampus proper (16). Finally, GABA concentration–current response relationships were analyzed to see whether the affinity of the GABAA receptors for the neurotransmitter GABA was smaller for subiculum compared with temporal neocortex (see ref. 14). We confirmed that oocytes injected with subicular membranes showed a smaller apparent affinity (EC50 = 191 μM; nH = 1.24; two patients, two frogs, and seven oocytes) than oocytes injected with TL membranes (EC50 = 98.3 μM; nH = 2.1). All these findings are in good agreement with data previously reported for different TLE patients (14, 16).
Reversal Potential of GABA Currents.
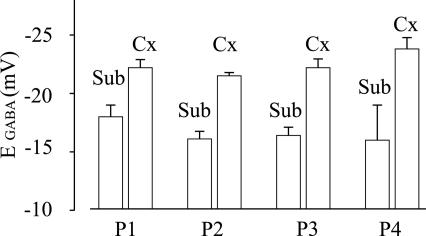
To see whether the cell Cl− homeostasis was altered after injection of epileptic membranes into the oocytes, we measured EGABA in oocytes injected with subicular or TL membranes. In oocytes injected with subicular membranes the GABA currents decreased in amplitude as the oocytes were depolarized and, in all four tested patients (Table 1), the current inverted direction at −17.5 ± 0.4 mV (63 oocytes, 5 donors; see, e.g., Fig. 3). These EGABA values were significantly different (P < 0.01) from those of oocytes injected with neocortical (−24.0 ± 0.5 mV; Fig. 3) or hippocampus proper membranes (−25 ± 1 mV; data not shown). It is noteworthy that these EGABA values were significantly similar (P > 0.2) to those previously reported for subicular membranes from a different group of TLE patients (14). Therefore, the EGABA in oocytes injected with subicular membranes is significantly depolarized compared with the EGABA in oocytes injected with hippocampal or neocortical membranes, presumably because of differences in the Cl− transporters.
Fig. 3.
Reversal potential of GABA currents recorded in oocytes injected with membranes extracted from the same epileptic neocortex (Cx) and subiculum (Sub) of the four patients shown in Figs. 1 and 2. Each column represents the mean value obtained from 12 to 20 oocytes (five frogs). GABA concentration, 1 mM. The values for described in the legends to Sub vs. Cx of each patient are significantly different (P < 0.001). Symbols are as described in the legends to Figs. 1 and 2. Note the positive shift of the reversal potential in Sub vs. Cx.
Effect of Blocking Cl− Transporters.
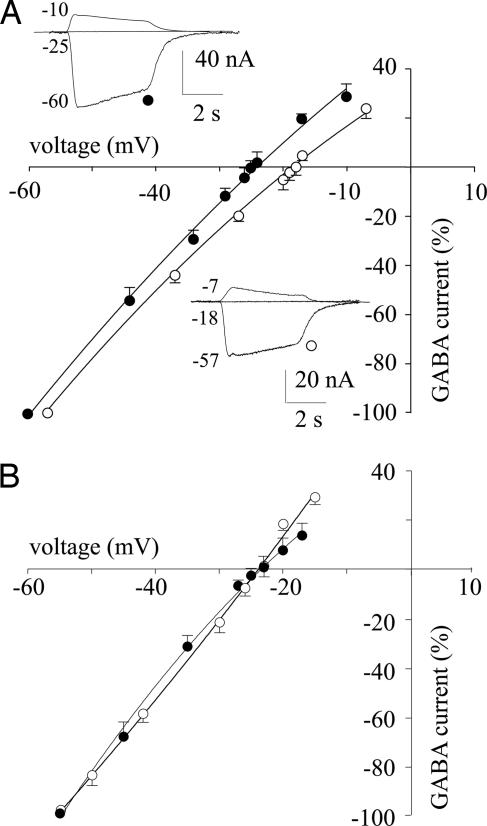
To ascertain that the altered Cl− transporter systems were responsible for the positive shift of EGABA, we measured it after inhibiting the Cl− transporters. First, we lowered the bathing medium temperature from 22°C to 12°C. Under these conditions, we found that the mean value of EGABA in oocytes injected with temporal neocortex membrane (EGABA = −27.7 ± 0.3 mV; 2 patients, 2 frogs, 16 oocytes) matched that determined in oocytes injected with subicular membranes (EGABA = −27.2 ± 0.4 mV, with the same patients, frogs, and number of oocytes). Second, we studied the effect of bumetanide, a selective inhibitor of NKCC1 (12), on EGABA. Oocytes previously injected with subiculum membranes and treated with bumetanide (12 μM; see ref. 12) showed an EGABA value of −23.3 ± 0.6 mV (4 patients, 5 frogs, 49 oocytes). This value was equivalent to the EGABA determined in oocytes injected with TL neocortex membranes (EGABA = −23.4 ± 0.3 mV, with the same patients, frogs, number of oocytes, and bumetanide treatment; Fig. 4). Furthermore, the EGABA recovered to the original value after bumetanide withdrawal, whereas the amplitude of the GABA currents changed as expected for changes in Cl− transporter activity (Fig. 4). Altogether, these findings indicate that blockage of the Cl− transporters in oocytes possessing the human subicular GABAA receptors produced a negative shift in EGABA, rendering it similar to that found in oocytes injected with TL neocortex membranes (see also ref. 14).
Fig. 4.
Current–voltage (I–V) relationships from oocytes injected with membranes from the brain of a patient (no. 3 in Table 1). (A) Points represent means (±SEM) of peak GABA currents normalized to Imax (at −60 mV membrane holding potential): −107 ± 25 nA (●, TLE neocortex); −49 ± 11 nA (○; TLE subiculum). Currents inverted at −25 mV (●) and at −18 mV (○). The GABA concentration applied was 1 mM (12 oocytes, 3 frogs). (Inset) Sample currents from the same experiments, at the holding potentials indicated (in millivolts). (B) I–V curves and symbols are as A. Oocytes were incubated for 3 h with 12 μM bumetanide (nine oocytes, three frogs). Imax (at −60 mV membrane holding potential): −99 ± 7 nA (●); −41 ± 10 nA (○). Currents inverted at −23 mV are shown.
During early neuronal development, GABAA-mediated responses are depolarizing (17), and this excitatory action of the neurotransmitter GABA is attributed mainly to low expression of the Cl− extruder KCC2 (18).
In two experiments, we injected membranes from the cerebral cortex of six 1-day-old rats and found that the EGABA value was more depolarized (−20.0 ± 0.5 mV; 16/2) than that of a 60-day-old rat (−26.0 ± 0.4 mV 16/2; P < 0.001). These results suggest strongly that GABAA receptors and transporters are both present in the transplanted brain membrane vesicles. This issue is supported by the Western blotting analyses made in human subicular and TL membranes from two epileptic patients (nos. 1 and 2; see Table 1) by using an anti-rat NKCC1 antibody (Chemicon; see ref. 12) and an anti-human KCC2 Ab (gift of Claudio Rivera, University of Helsinki, Helsinki), which showed expression of both Cl− transporters (not illustrated; methods as in ref. 14). Thus, it is likely that the equilibrium potential for GABA action is modulated locally by the presence of the transporters.
A possible cause of epileptogenesis in human TLE is a dysfunction of the inhibitory GABAergic system (19). In a previous work, we reported a reduced affinity to GABA in the subiculum and an excessive rundown of the GABAA receptors throughout the epileptic tissues (15). Both events would result in a diminished action of GABA and are therefore presumably involved in epileptogenesis. Another likely contributor to the neurological disorders in TLE is the EGABA, which in some pyramidal neurons of the hippocampal subiculum is abnormally depolarized (4) and renders GABA excitatory. This shift in EGABA, which has been tentatively explained by altered expression of Cl− transporters (3, 20), has been consistently observed in the Xenopus oocytes injected with subicular membranes isolated from the TLE brain (see ref. 14 and present data). Nevertheless, until now, a link between an abnormal expression of transporters and a switch of GABA action, from inhibitory to excitatory, had not been demonstrated directly. Strong evidence that abnormal expression of transporters makes GABA excitatory is provided by the present finding that oocytes that have incorporated membranes isolated from the hippocampal subiculum, an area that exhibits up-regulation of NKCC1 and down-regulation of KCC2, yield GABA currents with a depolarized EGABA. Furthermore, as would be expected by an involvement of GABA transporters, the shift in EGABA is removed by lowering the temperature, very likely reducing the ion transport across the oolemma, and also by treating the oocytes with bumetanide, a human NKCC1 blocker. It seems likely that in the oocytes, the activity of the up-regulated NKCC1, rather than of the down-regulated KCC2 carries more weight in altering the value of EGABA. In native neurons instead, probably both Cl− transporters are important for rendering GABA excitatory in some subicular neurons (4).
In conclusion, it appears that human GABA receptors and transporters are in the same patches of brain membranes microtransplanted to the oolemma, and that the transporters efficiently control the receptor microenvironment. It is also shown that the Cl− transporters are abnormally expressed in the neuronal membranes of the epileptic hippocampal subiculum. We speculate that this anomalous expression of Cl− transporters decreases the inhibitory GABAergic transmission and can thus contribute to the increased susceptibility of human TLE patients to seizures. The reduced efficacy of inhibitory GABAergic neurotransmission is because of the GABAA receptor rundown that occurs in the TL neocortex and hippocampus proper (15), as well as the EGABA shift in hippocampal subiculum (see refs. 4 and 14 and the present data). It should be noted that data reported here and previously (16) suggest that the action of brain-derived neurotrophic factor is proexcitatory in the hippocampal subiculum, because it reduces the excitatory GABA-current rundown but is antiexcitatory in the neocortex, where it reduces the inhibitory GABA-current rundown. Further studies of the mechanisms that regulate the expression and action of Cl− transporters in TLE tissues are essential to better understand human epilepsies and to develop medical treatments that could be substituted for surgery.
Materials and Methods
Patients.
Surgical specimens were obtained from the hippocampus and temporal neocortex of four patients with drug-resistant mesial TLE (see Table 1); all were operated on at the Neuromed Neurosurgery Center for Epilepsy (Pozzilli, Italy). Informed consent was obtained from all patients to use part of the biopsy material for our experiments, and the Ethics Committees of Neuromed and the University of Rome “La Sapienza” approved the selection processes and procedures. The histopathology of all specimens showed the typical neuropathological features of Ammon’s horn sclerosis and did not show obvious sclerosis in the TL. For more details, see refs. 14 and 16.
Quantitative RT-PCR Analysis.
Expression levels of human NKCC1 and KCC2 transporter mRNAs were determined from resected human tissues of four TLE patients. Total RNAs (Ambion, Austin, TX) from two human temporal cortices were used as controls. Procedures were as described in Supporting Text, which is published as supporting information on the PNAS web site. For more details, see refs. 14 and 16.
Membrane Preparation and Injection Procedures.
Membranes were prepared as described by using tissues from human epileptic brain regions (hippocampus proper, hippocampal subiculum, and TL; see refs. 13, 14, and 16).
Electrophysiology.
From 12 to 48 h after injection, membrane currents were recorded from voltage-clamped oocytes by using two microelectrodes filled with 3 M KCl (21). The oocytes were placed in a recording chamber (volume, 0.1 ml) perfused continuously (9–10 ml/min) with oocyte Ringer at room temperature (20–22°C). GABA-current rundown was defined as the decrease (in percent) of the current peak amplitude after six 10-s applications of GABA at 40-s intervals. Current–voltage (I–V) curves were obtained as reported in ref. 14 and in Supporting Text. For some experiments, oocytes were pretreated with bumetanide for 1–3 h before examining the I–V curves in oocytes injected with subicular or neocortical membranes.
Chemicals and Solutions.
Oocyte Ringer had the following composition: 82.5 mM NaCl/2.5 mM KCl/2.5 mM CaCl2/1 mM MgCl2/5 mM Hepes, adjusted to pH 7, with NaOH. Except when otherwise indicated, all drugs were purchased from Sigma.
Supplementary Material
Acknowledgments
We thank Professor Stefano Vicini for critical reading of the manuscript. We thank also Drs. Clotilde Lauro and Cristina Limatola for help with Western blot analysis. We are grateful to epilepsy patients E.V. (no. 1), S.E. (no. 2), T.S. (no. 3), and G.F. (no. 4) for making this work possible. This work was supported by grants from the Ministero della Salute (to F.E.) and the Ministero Università e Ricerca (to F.E.).
Abbreviations
- TL
temporal lobe
- TLE
TL epilepsy
- EGABA
GABA-current reversal potential.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Kullmann D. M. J. Neurol. Neurosurg. Psychiatry. 2002;73(Suppl. II):ii32–ii35. doi: 10.1136/jnnp.73.suppl_2.ii32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vadlamudi L., Scheffer I. E., Berkovic S. F. J. Neurol. Neurosurg. Psychiatry. 2003;74:1359–1361. doi: 10.1136/jnnp.74.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen I., Navarro V., Le Duigou C., Miles R. Biol. Cell. 2003;95:329–333. doi: 10.1016/s0248-4900(03)00081-9. [DOI] [PubMed] [Google Scholar]
- 4.Cohen I., Navarro V., Clemenceau S., Baulac M., Miles R. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 5.Kaila K. Prog. Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 6.Payne J. A., Rivera C., Voipo J., Kaila K. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 7.Fiumelli H., Cancedda L., Poo M. Neuron. 2005;48:773–786. doi: 10.1016/j.neuron.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Rivera C., Li H., Thomas-Crusells J., Lahtinen H., Viitanen T., Nanobashvili A., Kokaia Z., Airaksinen M. S., Voipio J., Kaila K., et al. J. Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera C., Voipio J., Thomas-Crusells J., Li H., Emri Z., Sipila S., Payne J. A., Minichiello L., Saarma M., Kaila K. J. Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera C., Voipio J., Kaila K. J. Physiol. (London) 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okabe A., Ohno K., Toyoda H., Yokokura M., Sato K., Fukuda A. Neurosci. Res. 2002;44:225–229. doi: 10.1016/s0168-0102(02)00093-7. [DOI] [PubMed] [Google Scholar]
- 12.Dzhala V. I., Talos D. M., Sdrulla D. A., Brumback A. C., Mathews G. C., Benke T. A., Delpire E., Jensen F., Staley K. J. Nat. Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 13.Miledi R., Eusebi F., Martinez-Torres A., Palma E., Trettel F. Proc. Natl. Acad. Sci. USA. 2002;99:13238–13242. doi: 10.1073/pnas.192445299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palma E., Torchia G., Limatola C., Trettel F., Arcella A., Cantore G., Di Gennaro G., Manfredi M., Esposito V., Quarato P. P., et al. Proc. Natl. Acad. Sci. USA. 2005;102:1667–1672. doi: 10.1073/pnas.0409442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palma E., Ragozzino D. A., Di Angelantonio S., Spinelli G., Trettel F., Martinez-Torres A., Torchia G., Arcella A., Di Gennaro G., Quarato P. P., et al. Proc. Natl. Acad. Sci. USA. 2004;101:10183–10188. doi: 10.1073/pnas.0403683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palma E., Spinelli G., Torchia G., Martinez-Torres A., Ragozzino D., Miledi R., Eusebi F. Proc. Natl. Acad. Sci. USA. 2005;102:2514–2518. doi: 10.1073/pnas.0409687102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera C., Voipio J., Payne J. A., Ruusuvuori E., Lahtinen H., Lamsa K., Pirvola U., Saarma M., Kaila K. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Ari Y. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 19.Calcagnotto M. E., Paredes M. F., Tihan T., Barbaro N. M., Baraban S. C. J. Neurosci. 2005;25:9649–9657. doi: 10.1523/JNEUROSCI.2687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stell B., Mody I. Neuron. 2003;39:729–733. doi: 10.1016/s0896-6273(03)00528-2. [DOI] [PubMed] [Google Scholar]
- 21.Miledi R. Proc. R. Soc. London Ser. B; 1982. pp. 49l–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.