Abstract
To elucidate whether the role of leptin in regulating neuroendocrine and immune function during short-term starvation in healthy humans is permissive, i.e., occurs only when circulating leptin levels are below a critical threshold level, we studied seven normal-weight women during a normoleptinemic-fed state and two states of relative hypoleptinemia induced by 72-h fasting during which we administered either placebo or recombinant methionyl human leptin (r-metHuLeptin) in replacement doses. Fasting for 72 h decreased leptin levels by ≈80% from a midphysiologic (14.7 ± 2.6 ng/ml) to a low-physiologic (2.8 ± 0.3 ng/ml) level. Administration of r-metHuLeptin during fasting fully restored leptin to physiologic levels (28.8 ± 2.0 ng/ml) and reversed the fasting-associated decrease in overnight luteinizing hormone pulse frequency but had no effect on fasting-induced changes in thyroid-stimulating hormone pulsatility, thyroid and IGF-1 hormone levels, hypothalamic–pituitary–adrenal and renin–aldosterone activity. FSH and sex steroid levels were not altered. Short-term reduction of leptin levels decreased the number of circulating cells of the adaptive immune response, but r-metHuLeptin did not have major effects on their number or in vitro function. Thus, changes of leptin levels within the physiologic range have no major physiologic effects in leptin-replete humans. Studies involving more severe and/or chronic leptin deficiency are needed to precisely define the lower limit of normal leptin levels for each of leptin’s physiologic targets.
Keywords: fasting, reproductive
Deficiency of the adipocyte-secreted hormone leptin (1) is associated with distinct abnormalities in energy-demanding processes such as neuroendocrine and immune function. Leptin-deficient ob/ob mice and humans with congenital complete leptin deficiency have abnormal neuroendocrine function, including hypogonadotropic hypogonadism, hypothalamic hypothyroidism, and/or growth-hormone-axis abnormalities (2–6) and impaired cell-mediated immunity (4, 7), which are improved with leptin replacement (4, 8). Similarly, starvation-induced decline of circulating leptin to very low levels in normal mice (9) and lean men (10) causes comparable neuroendocrine (9, 10) and immune defects (11, 12) that are significantly blunted or reversed with exogenous leptin.
We have shown that an 80% decline of leptin levels from ≈2 to 0.3 ng/ml in men mediates the fasting-induced suppression of gonadotropin and thyroid-stimulating hormone (TSH) pulsatility as well as sex steroid, insulin-like growth factor-1 (IGF-1), and thyroid hormone levels (10). Importantly, although observational studies have proposed that leptin regulates the hypothalamic–pituitary–gonadal axis only when serum leptin levels fall below a “threshold” of ≈2 ng/ml (13), the role of decreasing leptin levels to approximately, but not below, this threshold in leptin-replete humans with higher baseline leptin levels (e.g., normal-weight women) has not yet been directly studied.
To elucidate whether such a threshold exists, below which leptin has a “permissive” effect to regulate neuroendocrine and immune function [including peripheral blood mononuclear cell (PBMC) subpopulations, T cell proliferation, and cytokine production], we assessed pituitary hormone pulsatility and hormone levels of several neuroendocrine axes and markers of immune function in normal-weight women during a normoleptinemic-fed condition and two hypoleptinemic 72-h fasting states, with administration of either placebo (to achieve a low leptin level close to the proposed threshold) or recombinant methionyl human leptin (r-metHuLeptin) (to replace leptin to physiologic levels). To further investigate the question of a threshold leptin level in regulating immune function, we studied the effect of a range of leptin levels on T cell proliferation in vitro.
Results
Seventy-Two-Hour Fasting Suppresses Serum Leptin Levels out of Proportion to Changes in Body Weight and Fat Mass, and r-metHuLeptin Replacement Restores Leptin Levels Without Affecting Metabolic Parameters.
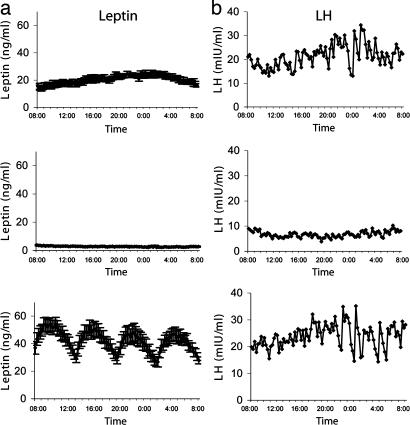
In the baseline fed state, body weight increased slightly without significant changes in percent or total fat mass or fat-free mass, whereas serum leptin levels and insulin levels increased, and free fatty acid (FFA) levels decreased (Table 1). Complete fasting for 72 h significantly decreased serum leptin levels to ≈20% of baseline, out of proportion to the slight decreases in body weight and fat mass (Table 1). Leptin pulsatility on the third day of fasting was markedly suppressed (Fig. 1a) with loss of normal diurnal variation and decreased 24-h mean levels (20.5 ± 1.6 to 2.8 ± 0.2 ng/ml), peak height (23.8 ± 2.1 to 3.4 ± 0.3 ng/ml), valley mean (20.2 ± 1.6 to 7.8 ± 0.2 ng/ml), integrated area (29,286 ± 2,354 to 3,997 ± 277), and pulse mass (2.6 ± 0.8 to 0.5 ± 0.1) (all P < 0.05 vs. fed) but not pulse frequency or interpulse interval. r-metHuLeptin during fasting fully corrected the fasting-induced suppression of leptin to levels that were higher than baseline but within the physiological range for women (24-h mean: 42.4 ± 4.0 ng/ml vs. trough level on day 4 at 8 a.m. in Table 1) (Fig. 1a). Similar decreases in body weight and fat mass were observed as during fasting alone, with a slightly greater decrease in fat-free mass with r-metHuLeptin (Table 1). Resting metabolic rate was not affected by fasting or r-metHuLeptin, and r-metHuLeptin did not alter fasting-induced changes in insulin, respiratory quotient, or FFA (Table 1).
Table 1.
Weight, body composition, resting metabolic rate (RMR), and hormone levels at the beginning (Day 1) and end (Day 4) of a fed state (n = 7), 72-h fasting with placebo (n = 6), and 72-h fasting with r-metHuLeptin (n = 7) mean ± SE
| Baseline fed state (n = 7) |
Fasting + placebo (n = 6) |
Fasting + leptin (n = 7) |
Overall P | Baseline P | ||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 4 | Day 1 | Day 4 | Day 1 | Day 4 | |||
| Weight, kg | 57.0 ± 2.3 | 58.0 ± 2.2* | 56.9 ± 3.1 | 55.4 ± 2.8* | 56.2 ± 2.2 | 54.1 ± 2.0* | 0.01†‡§ | 0.61 |
| Fat mass, % | 29.9 ± 1.5 | 30.0 ± 1.4 | 31.2 ± 1.9 | 29.3 ± 2.2* | 30.0 ± 1.6 | 29.1 ± 1.9 | 0.07†‡ | 0.85 |
| Fat mass, kg | 17.2 ± 1.3 | 17.3 ± 1.2 | 18.0 ± 1.9 | 16.4 ± 1.8* | 17.0 ± 1.4 | 15.9 ± 1.5* | 0.01†‡§ | 0.45 |
| Fat-free mass, kg | 39.9 ± 1.4 | 40.7 ± 1.7 | 38.9 ± 1.6 | 39.0 ± 1.6 | 39.2 ± 1.3 | 38.2 ± 1.3* | 0.03†‡¶ | 0.68 |
| RMR, kcal/d | 1,267 ± 40 | 1,259 ± 36 | 1,285 ± 82 | 1,352 ± 43 | 1,330 ± 52 | 1,344 ± 34 | 0.26 | 0.12 |
| Respiratory quotient | 0.87 ± 0.03 | 0.91 ± 0.01 | 0.88 ± 0.02 | 0.74 ± 0.01* | 0.88 ± 0.03 | 0.72 ± 0.01* | 0.01†‡§ | 0.83 |
| Leptin, ng/ml | 11.4 ± 1.6 | 16.7 ± 1.2* | 14.7 ± 2.6 | 2.8 ± 0.3* | 12.2 ± 1.6 | 28.8 ± 2.0* | 0.002†‡§¶ | 0.85 |
| Insulin, μIU/ml | 6.72 ± 1.21 | 9.28 ± 1.51* | 6.06 ± 0.81 | 1.22 ± 0.24* | 7.91 ± 1.54 | 1.23 ± 0.30* | 0.01†‡§ | 0.85 |
| FFA, mmol/l | 0.07 ± 0.01 | 0.04 ± 0.00* | 0.05 ± 0.01 | 0.94 ± 0.16* | 0.04 ± 0.01 | 0.83 ± 0.08* | 0.01†‡§ | 0.08 |
| Estradiol, pg/ml | 57.0 ± 12.1 | 64.5 ± 11.3 | 60.9 ± 13.1 | 66.1 ± 27.2 | 61.2 ± 11.1 | 52.8 ± 10.2 | 0.31 | 0.61 |
| FSH, mIU/ml | 6.00 ± 0.61 | 5.39 ± 0.90 | 5.83 ± 0.47 | 4.92 ± 0.51 | 5.81 ± 0.44 | 5.66 ± 0.69 | 0.22 | 0.85 |
| Testosterone, ng/dl | 74.6 ± 11.8 | 74.2 ± 10.9 | 78.5 ± 11.9 | 68.5 ± 9.8 | 66.4 ± 8.1 | 67.6 ± 7.1 | 0.85 | 0.61 |
| fT3, pg/ml | 2.81 ± 0.10 | 2.60 ± 0.08 | 2.98 ± 0.17 | 1.66 ± 0.11* | 2.78 ± 0.16 | 1.69 ± 0.08* | 0.01†‡§ | 0.68 |
| Reverse T3, ng/dl | 15.0 ± 0.7 | 12.4 ± 1.0* | 16.9 ± 2.3 | 33.5 ± 3.7* | 15.8 ± 0.9 | 34.1 ± 2.0* | 0.01†‡§ | 0.61 |
| Free T4, ng/dl | 1.19 ± 0.07 | 1.12 ± 0.06 | 1.22 ± 0.09 | 1.18 ± 0.08 | 1.17 ± 0.08 | 1.17 ± 0.08 | 0.83 | 0.54 |
| IGF-1, ng/ml | 265.3 ± 17.1 | 346.9 ± 24.5* | 260.8 ± 19.5 | 162.2 ± 12.5* | 239.3 ± 25.3 | 147.6 ± 10.7* | 0.01†‡§ | 0.31 |
| IGF-BP3, μg/ml | 4.78 ± 0.11 | 5.11 ± 0.22* | 4.94 ± 0.14 | 4.22 ± 0.33 | 4.66 ± 0.25 | 4.06 ± 0.21* | 0.04†‡§ | 0.51 |
| Cortisol, μg/dl | 16.9 ± 3.4 | 16.7 ± 3.1 | 17.5 ± 0.9 | 17.6 ± 3.0 | 16.9 ± 1.9 | 14.5 ± 2.1 | 0.51 | 0.85 |
| PRA, ng/ml/hr | 4.16 ± 0.71 | 2.28 ± 0.75 | 2.45 ± 0.80 | 6.03 ± 2.42 | 6.50 ± 3.18 | 9.42 ± 2.52 | 0.03 | 0.22 |
| Aldosterone, pg/ml | 60.0 ± 11.7 | 38.4 ± 3.0 | 66.0 ± 12.8 | 112.1 ± 23.5 | 66.1 ± 11.7 | 132.6 ± 27.0 | 0.01†‡§ | 1.0 |
| 24-hr urine cortisol, μg | 19.6 ± 1.9 | 28.6 ± 3.3 | 25.0 ± 2.5 | 0.03‡ | — | |||
| 24-hr urine sodium, mEq | 195.4 ± 26.4 | 167.8 ± 9.9 | 153.2 ± 24.6 | 0.51 | — | |||
Overall P value for comparison of change from day 1 to day 4 and baseline P value for comparison of day 1 values across the three conditions by nonparametric ANOVA
*, P < 0.05 vs. Day 1;
†, P < 0.05 by one-way ANOVA;
‡, P < 0.05 for fed vs. fasting + placebo;
§, P < 0.05 for fed vs. fasting + r-metHuLeptin;
¶, P < 0.05 for fasting + placebo vs. fasting + r-metHuLeptin.
Fig. 1.
Twenty-four-hour profile (8 a.m.–8 p.m.) of average (n = 7) leptin (a) and representative LH (b) levels on day 3 of a baseline fed state (Top), 72-h fasting with placebo (Middle), or 72-h fasting with replacement-dose r-metHuLeptin (Bottom).
r-metHuLeptin Restores the Fasting-Induced Decline in Overnight Luteinizing Hormone (LH) Pulse Frequency but Does Not Alter the Suppression of TSH Pulsatility and IGF-1 Levels or Mild Activation of the HPA Axis.
Overnight LH peak frequency decreased significantly during fasting (7.3 ± 0.4 vs. 4.5 ± 1.1 peaks per 12 h, P < 0.05) and was fully corrected with r-metHuLeptin (6.4 ± 0.5 peaks per 12 h, P = 0.04 by ANOVA) (Table 2 and Fig. 1b). A similar trend that did not reach statistical significance was observed for 24 h (see Table 4, which is published as supporting information on the PNAS web site) but not daytime (data not shown) LH pulsatility. There were no changes in estradiol, follicle-stimulating hormone (FSH), or testosterone with fasting or r-metHuLeptin (Table 1).
Table 2.
Pulsatility characteristics (mean ± SE) of frequently sampled (every 15 minutes) overnight LH (8 p.m.–8 a.m.) and 24-h TSH and ACTH (8 a.m.–8 a.m.) on day 3 of a baseline fed state (n = 7), 72-h fasting with placebo (P) (n = 6), or 72-h fasting with r-metHuLeptin (RL) (n = 7)
| Fed | Fast + P | Fast + RL | P | |
|---|---|---|---|---|
| Overnight LH, IU/liter | ||||
| Mean level | 10.57 ± 2.59 | 7.61 ± 0.58 | 11.51 ± 2.76 | 0.31 |
| Peak frequency | 7.29 ± 0.42 | 4.50 ± 1.06 | 6.43 ± 0.48 | 0.04*† |
| Peak interval | 80.86 ± 6.94 | 119.51 ± 20.54 | 95.91 ± 12.18 | 0.07 |
| Peak width | 50.15 ± 3.98 | 91.20 ± 28.61 | 66.96 ± 11.09 | 0.35 |
| Peak height | 13.31 ± 3.21 | 10.44 ± 1.00 | 15.00 ± 3.48 | 0.82 |
| Valley frequency | 8.00 ± 0.31 | 5.50 ± 1.06 | 7.14 ± 0.59 | 0.047† |
| Valley mean level | 9.37 ± 2.24 | 6.28 ± 0.84 | 10.30 ± 2.69 | 0.31 |
| Integrated area | 7,462 ± 1830 | 5,358 ± 428 | 8,101 ± 1940 | 0.31 |
| Pulse mass | 1.35 ± 0.37 | 4.01 ± 2.15 | 2.35 ± 0.49 | 0.07 |
| TSH, μIU/ml | ||||
| Mean level | 1.42 ± 0.20 | 0.35 ± 0.08 | 0.51 ± 0.07 | 0.006*†‡ |
| Peak frequency | 8.29 ± 0.68 | 11.33 ± 0.92 | 9.57 ± 0.87 | 0.048† |
| Peak interval | 151.1 ± 11.4 | 114.8 ± 10.8 | 141.4 ± 12.6 | 0.006† |
| Peak width | 105.25 ± 11.5 | 68.18 ± 4.16 | 86.49 ± 11.26 | 0.042† |
| Peak height | 1.75 ± 0.28 | 0.41 ± 0.08 | 0.59 ± 0.08 | 0.006*†‡ |
| Valley frequency | 9.14 ± 0.70 | 11.67 ± 0.88 | 9.71 ± 0.87 | 0.013† |
| Valley mean level | 1.25 ± 0.20 | 0.32 ± 0.07 | 0.46 ± 0.06 | 0.006*†‡ |
| Integrated area | 2,005 ± 283 | 495 ± 109 | 728 ± 98 | 0.006*†‡ |
| Pulse mass | 0.65 ± 0.14 | 0.04 ± 0.02 | 0.13 ± 0.04 | 0.050*†‡ |
| ACTH, pg/ml | ||||
| Mean level | 10.48 ± 1.28 | 9.89 ± 2.01 | 9.33 ± 1.23 | 0.31 |
| Peak frequency | 9.57 ± 1.07 | 12.17 ± 0.87 | 10.14 ± 0.70 | 0.17 |
| Peak interval | 139.6 ± 15.2 | 105.3 ± 5.4 | 123.7 ± 5.6 | 0.51 |
| Peak width | 88.87 ± 11.13 | 67.63 ± 4.92 | 83.39 ± 6.35 | 0.61 |
| Peak height | 13.52 ± 1.74 | 13.51 ± 3.09 | 12.70 ± 1.92 | 0.14 |
| Valley frequency | 10.14 ± 1.06 | 12.50 ± 0.67 | 10.57 ± 0.75 | 0.54 |
| Valley mean level | 8.71 ± 1.12 | 8.74 ± 1.75 | 7.79 ± 0.93 | 0.07 |
| Integrated area | 14,703 ± 1856 | 14,029 ± 2846 | 12,915 ± 1,760 | 0.07 |
| Pulse mass | 6.17 ± 0.86 | 4.22 ± 2.82 | 2.85 ± 0.76 | 0.25 |
P value for nonparametric ANOVA across the three conditions.
*, P < 0.05 by one-way ANOVA;
†, P < 0.05 for fed vs. fasting + placebo;
‡, P < 0.05 for fed vs. fasting + r-metHuLeptin.
Thyroid hormones were stable at baseline (Table 1), and frequently sampled TSH levels showed typical diurnal variation and pulsatility (Fig. 3, which is published as supporting information on the PNAS web site). Seventy-two-hour fasting significantly decreased free triiodothyronine (T3), increased reverse T3, and markedly suppressed several parameters of TSH pulsatility, whereas free thyroxine remained stable. r-metHuLeptin did not alter these fasting-induced changes of the thyroid axis (Fig. 3 and Tables 1 and 2).
IGF-1 decreased by 40% after 72-h fasting alone and with r-metHuLeptin, with similar findings in IGF-binding protein 3 (IGF-BP3) (Table 1). Neither serum cortisol levels nor adrenocorticotropic hormone (ACTH) pulsatility were altered by fasting or r-metHuLeptin (Tables 1 and 2). However, there was an overall significant difference in 24-h urine cortisol, with higher levels in both fasting states vs. the fed state (P = 0.03 for fed vs. fasting). Plasma renin activity (PRA) and aldosterone tended to increase with fasting but with no effect of r-metHuLeptin (Table 1). There was an overall significant change for PRA and aldosterone but no significance by post hoc tests or change from days 1 to 4. Twenty-four-hour urine sodium (Table 1), nitrogen, and volume (data not shown) were not affected by fasting or r-metHuLeptin.
Seventy-Two-Hour Fasting Reduces the Number of Cells of the Adaptive Immune Response, but r-metHuLeptin Has Minimal Effects to Restore Their Number with No Effect on Innate Immunity or in Vitro T Cell Function.
Acute starvation resulted in a decline of total CD3+ T lymphocytes by 838 ± 268 cells per mm3 (461 ± 344 after adjustment for controls) (P = 0.04 for days 1 vs. 4). r-metHuLeptin during fasting blunted this decline to 302 ± 185 cells per mm3 (201 ± 350 adjusted) (P = 0.14 for days 1 vs. 4). The decline in CD3+ T lymphocytes was greater during 72-h fasting alone vs. with r-metHuLeptin (P = 0.04) although not different after adjustment for controls (P = 0.50). We found similar findings in CD4+ and CD8+ T lymphocytes and CD19+ B lymphocytes, with greater declines during 72-h fasting alone vs. with r-metHuLeptin, although the differences were not statistically significant (Table 3 and Table 5, which is published as supporting information on the PNAS web site). Naïve and memory CD3+, CD4+, and CD8+ subpopulations decreased more with fasting than with r-metHuLeptin during fasting, although these differences were not significant after adjustment for controls, except an increase in naïve CD8+/CD45RA+ cells with r-metHuLeptin vs. decrease during fasting (Tables 3 and 5). Decreases in PBMCS were not due to nonspecific factors, because CD3−/CD16+CD56+ natural killer (NK) cells, representing innate immunity, increased slightly with fasting. The proliferative response of T cells to several polyclonal stimuli decreased after 72-h fasting but was not significantly different from fasting with r-metHuLeptin (OKT3, −19,246 ± 5,251 vs. −8,217 ± 14,773 cpm, P = 0.47; PHA, −36,214 ± 11,808 vs. −25,823 ± 10,362 cpm, P = 0.72; PMA/Iono, −31,545 ± 20,328 vs. +4,829 ± 18,050 cpm, P = 0.47; PPD, −445 ± 4,620 vs. −4,125 ± 4,843 cpm, P = 0.72). Stimulated cytokine (IFN-γ, IL-10, and IL-4) production by PBMCs did not change with 72-h fasting or r-metHuLeptin, nor did serum glucose levels (data not shown).
Table 3.
Change in PBMC subpopulations (mean ± SE) from days 1 to 4 of 72-h fasting with placebo or replacement-dose r-metHuLeptin (n = 5)
| PBMC subpopulation | Actual |
Adjusted for controls |
||
|---|---|---|---|---|
| Fasting + placebo | Fasting + leptin | Fasting + placebo | Fasting + leptin | |
| CD3+ | −838 ± 268† | −302 ± 185* | −461 ± 344 | −201 ± 350 |
| CD4+ | −534 ± 184† | −247 ± 138 | −327 ± 246 | −139 ± 273 |
| CD8+ | −257 ± 77† | −57 ± 52 | −128 ± 93 | −43 ± 88 |
| CD3+/CD45RA+ | −443 ± 124† | −134 ± 134 | −197 ± 188 | +14 ± 243 |
| CD3+/CD45RO+ | −396 ± 177 | −168 ± 85 | −274 ± 175 | −215 ± 152 |
| CD4+/CD45RA+ | −234 ± 76† | −149 ± 72 | −126 ± 104 | −71 ± 151 |
| CD4+/CD45RO+ | −300 ± 119† | −98 ± 74* | −201 ± 143 | −68 ± 129 |
| CD8+/CD45RA+ | −209 ± 78† | +15 ± 72 | −71 ± 98 | +85 ± 104* |
| CD8+/CD45RO+ | −96 ± 111 | −70 ± 6 | −73 ± 74 | −147 ± 67 |
| CD19+ | −152 ± 51† | −82 ± 24 | −86 ± 69 | −71 ± 70 |
| CD3−/CD16+CD56+ | +80 ± 27 | +91 ± 34† | +27 ± 26 | +56 ± 53 |
*, P < 0.05 vs. fasting alone;
†, P < 0.05 vs. day 1.
Changes in IGF-1 Correlate with Changes in Several PBMC Subpopulations in Response to 72-h Fasting Alone and with r-metHuLeptin.
To evaluate whether changes in PBMC subpopulations are related to changes in hormones known to be affected by fasting and/or leptin, we analyzed the correlation between changes in PBMC subpopulations with changes in hormones relevant to immune function, including leptin, insulin, FFA, estradiol, FSH, testosterone, free T3, free thyroxine, IGF-1, IGF-BP3, and cortisol. The most significant correlations were observed for IGF-1, and borderline significant correlations were noted for free T3 (see Table 6, which is published as supporting information on the PNAS web site). Correlations with other hormones were not significant after adjustment for controls (data not shown).
Complete Leptin Depletion from Human Serum or Neutralization in Culture Medium Significantly Impairs in Vitro T Cell Proliferation and Is Reversible with Leptin Replacement.
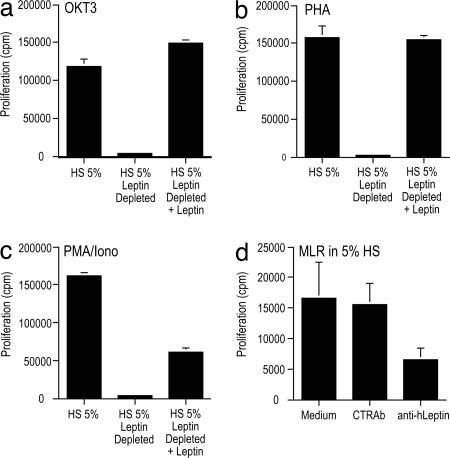
We then investigated whether the lack of major changes in PBMC proliferation and cytokine production may have been due to the continued presence of leptin at physiological levels despite being suppressed to ≈20% of baseline (2.8 ± 0.3 ng/ml), i.e., above a putative threshold level for immune function. T cells were first stimulated with OKT3, PHA, or PMA/Iono in 5% human serum, which corresponds to a leptin level of ≈1 ng/ml. In contrast to the lack of effect of 72-h fasting (with leptin level ≈2.8 ng/ml) on T cell function, complete removal of leptin from serum with anti-human leptin Ab dramatically suppressed the proliferative response to essentially no proliferation (Fig. 2a–c). Specificity of the system was confirmed by adding recombinant human leptin back to leptin-depleted serum, which restored proliferation to a level comparable with that in the basal state. The restoration of proliferation was less efficient with PMA/Iono compared with OKT3 and PHA and was reachable in all conditions only with high doses of leptin (ranging from 50 to 100 ng/ml). To further dissect the role of leptin present in culture medium on T cell proliferation, we added anti-leptin neutralizing Abs to culture medium during mixed lymphocyte reactions, which inhibited T cell proliferation between 45% and 60% compared with medium alone or control Ab, suggesting that leptin levels above a certain threshold are necessary for sustaining in vitro T cell proliferation (Fig. 2d).
Fig. 2.
Leptin depletion or neutralization inhibits polyclonal T cell proliferation and mixed lymphocyte reactions (MLR), respectively. (a–c)The proliferative response of T lymphocytes to polyclonal stimuli (OKT3, PHA, and PMA/Iono) from controls is completely inhibited in medium with human serum depleted of leptin. Addition of recombinant human leptin (at 100 ng/ml final concentration) completely reverses this phenomenon. (d) Anti-leptin blocking Abs partially inhibit the antigen-specific proliferative response of T cells during MLR. HS, human serum.
Discussion
Maintenance of normal neuroendocrine and immune function depends critically on the availability of adequate energy stores. Evidence from animal and human models supports a central role for leptin as a signal of energy sufficiency and mediator of the adaptation to starvation (9, 10, 14). Congenital complete leptin deficiency (3–5) or acute hypoleptinemia to <1 ng/ml in leptin-replete humans induces changes in reproductive, thyroid, and IGF axes (10) and immune function (12), whereas increasing serum leptin levels to >2–3 ng/ml with r-metHuLeptin corrects, fully or in part, these abnormalities (4, 10). However, whether a critical leptin threshold of ≈3 ng/ml exists, above which leptin has no major additional physiological effect on neuroendocrine and/or immune function, has been proposed based on observational studies (13) but not yet tested in an interventional fashion.
We studied women who have substantially higher baseline leptin levels than lean men. Although 72-h fasting results in a similar percent decline of leptin levels as in men (≈15–20% of baseline), the absolute levels achieved are ≈10-fold higher (2.8 ng/ml) (13). Leptin levels after r-metHuLeptin (28.8 ng/ml) were higher than baseline, but remained well within the physiologic range and below levels (≈400–4,000 ng/ml) achieved with pharmacologic dosing (15, 16). Decreasing serum leptin levels to 2.8 ng/ml and increasing leptin back to a high physiologic range had only modest effects on LH pulsatility and did not substantially alter any other neuroendocrine or immune parameter. This alteration in levels also had no statistically significant effect on metabolic variables. Larger, longer-term studies are needed to more fully elucidate this finding.
The unique aspect of this study lies in the in vivo exploration of decreasing leptin from mid- to low-physiologic levels but not to the extremely low levels of severe leptin deficiency (10). Previous leptin administration data in congenital (4) and lipoatrophic (17) leptin-deficient subjects and leptin-replete subjects with short-term relative leptin deficiency (10) or 10% reduced body weight (18) suggest that a leptin level of ≈2–3 ng/ml appears to be necessary for the regulation of neuroendocrine axes (particularly the hypothalamic–pituitary–gonadal axis). Importantly, these data provide insight into the “normal range” of leptin levels, an issue of considerable diagnostic and therapeutic importance when r-metHuLeptin gains a place in the therapeutic armamentarium. Our findings are consistent with the existence of a threshold leptin level for regulation of neuroendocrine and immune function, whereas an upper limit to leptin’s effect on metabolic parameters may exist (i.e., leptin resistance). Given the pleiotropic nature of leptin and the complexity of the leptin system, it remains to be determined whether different thresholds exist for other physiological functions influenced by leptin and/or whether differences in populations with varying degrees or duration of leptin deficiency exist.
We studied women in the midfollicular phase of their menstrual cycles, an important time for development of dominant follicles. Decreasing leptin to low-physiologic levels decreased overnight LH pulse frequency only modestly by 40% but not other pulsatility parameters, FSH, or sex steroid levels. This finding stands in distinct contrast to r-metHuLeptin’s ability to (i) restore several LH pulsatility parameters and the 40% decline in testosterone with the same duration but greater magnitude of fasting-induced hypoleptinemia in lean men (10) and (ii) normalize LH pulsatility and ovulatory cycles in women with hypothalamic amenorrhea and chronic leptin deficiency (14). Although follicle development was not directly evaluated, the maintenance of normal menstrual cycles argues against substantial disruption of follicular growth and ovulation. Interestingly, normal-weight women have decreased LH pulse frequency during short-term starvation despite maintaining ovulatory cycles in several (19, 20) but not all (21–23) studies. Partial restoration of leptin levels with r-metHuLeptin during 4-day fasting was associated with a decrease in overnight LH pulses in normal-weight women, but fed-state leptin levels were not reached (23). Potential effects of r-metHuLeptin may have been obscured by interindividual differences, which our study and others (19, 20, 24, 25) have minimized by studying the same subjects under different conditions at the same times of their menstrual cycles.
It has been suggested that LH pulsatility is disrupted at a threshold of energy availability deep in negative energy balance (26). Low energy availability, but not the stress of exercise alone, suppressed leptin rhythm (27) and altered LH pulsatility (26) in eumenorrheic, sedentary women. Similarly, 72-h fasting disrupted follicle growth and lengthened the follicular phase in lean women with body fat <20% (24), but not in normal-weight women with body fat similar to that of our subjects (≈27%) (25). Taken together, these studies suggest that lean women with lower leptin levels may be more vulnerable to energy deficit than normal-weight women. Thus, evaluation of very lean women during short-term starvation may provide insights into the relative contribution of a critical leptin level vs. duration of hypoleptinemia.
In this study, 72-h fasting caused marked suppression of TSH pulsatility, decrease in free T3, and increase in reverse T3. Humans with complete functional leptin deficiency due to defects in the leptin or leptin-receptor gene have altered TSH pulsatility (28) or central hypothyroidism (6). In small, uncontrolled studies, r-metHuLeptin increased free thyroxine and free T3 levels in leptin-deficient children (4) and reversed the decrease in thyroid hormones in subjects during weight loss (18). Although normalizing leptin from very low levels (≈0.2 ng/ml) blunted the fasting-induced suppression of TSH pulsatility in lean men (10), increasing leptin from ≈2.8 to ≈28.8 ng/ml had no effect on TSH pulsatility in women. We propose that these differences in TSH pulsatility may be related to a threshold leptin level similar to that for the hypothalamic–pituitary–gonadal axis.
Short-term fasting decreased IGF-1 levels with no effect of r-metHuLeptin and no major alterations in IGF-BP3. We have shown that r-metHuLeptin in fasting men modestly blunted the starvation-induced decrease in IGF-1 levels (10) and increased IGF-1 and IGF-BP3 levels over 2–3 mo in leptin-deficient women with hypothalamic amenorrhea (14). In congenital leptin-deficient humans, r-metHuLeptin for 18 mo increased IGF-BP1 and IGF-BP2 but not IGF-1 or IGF-BP3 (5). Thus, although the role of leptin in regulating IGF-1 requires further study, the above observations suggest that leptin may need to decrease below a certain level for IGF-1 to decrease because of leptin deficiency.
We found mild activation of the HPA axis with 72-h fasting but no effect of r-metHuLeptin. Leptin-deficient humans have elevated basal cortisol and ACTH levels and disturbed diurnal rhythm (3), and long-term r-metHuLeptin in three leptin-deficient adults increased cortisol levels, but these studies were uncontrolled (5). In healthy humans, regulation of the HPA axis appears to be independent of leptin (10) in contrast to leptin’s reversal of starvation-induced HPA activation in mice (9). We report herein a lack of change in ACTH pulsatility in response to r-metHuLeptin. Although observational human studies have shown an inverse relationship between pulsatility of ACTH and cortisol with that of leptin (29), our interventional studies do not support a direct role for leptin in regulating the HPA axis; whether pulsatile or longer duration of r-metHuLeptin is required remains to be studied. Consistent with our prior findings (10), fasting-induced changes in PRA and aldosterone appear to be independent of leptin.
In this study, acute starvation in leptin-replete humans determines a specific change in peripheral lymphocyte distribution that differentially affects the number of circulating cells of the adaptive and innate immune response. Restoration of leptin levels had no major effect on fasting-induced changes in immunophenotypes, except for naïve CD8+CD45RA+ cells, indicating that decreasing leptin levels to ≈2.8 ng/ml has a minor role in mediating the effects of short-term starvation on PBMCs. Similarly, the in vitro proliferative and cytokine-producing capacity of T cells was not affected, suggesting that this degree of short-term leptin deficiency disrupts immune function only minimally, i.e., induces partial changes in immune cell distribution but no impairment of T cell proliferation against classical polyclonal and recall antigens.
Leptin reverses the immunosuppression associated with 48-h starvation in normal mice (8, 11), increases thymic or splenic cellularity in leptin-deficient ob/ob mice (8), and, by using a similar protocol for acquiring PBMCs and analyzing immune function, improves severely impaired T cell function in children with congenital leptin deficiency (4). Thus, our findings highlight the contrast between short-term mild and long-term severe leptin deficiency on immune function. Relative leptin deficiency after leptin withdrawal in mice depleted of fat after high-dose leptin administration reduces thymic and splenic cellularity (30). The bioequivalence of 48-h starvation in mice is likely closer to a few weeks in humans, and, thus, 72-h fasting in humans may be insufficient for substantial alterations in immune function to occur, but further studies are needed to clarify this role of leptin over a wider range of leptin levels. This robustness of the immune system is consistent with evidence that acute starvation does not affect susceptibility to infectious diseases, whereas more long-term starvation profoundly alters inflammatory immune responses and infectious disease susceptibility in mice and humans (31). Indeed, both anorexic and malnourished subjects (particularly those with protein energy malnutrition) have impaired T cell-mediated immune responses and very low leptin levels (31).
We studied T cell function in vitro to differentiate between insufficient duration vs. degree of hypoleptinemia and found a striking reduction of T cell proliferation to polyclonal stimuli when leptin was absent from culture medium that was fully restored when leptin was added back at low doses. Importantly, the use of autologous serum in the in vivo immune studies preserves the existing environment of the cells and avoids introducing exogenous, confounding factors. The lack of change in serum glucose levels, verified viability of immune cells, and observed proliferative response argue against a substantial change in metabolic environment due to shipment, because T cells become anergic in low-glucose settings (32).
The simultaneous assessment of neuroendocrine and immune function in this paradigm provides a unique opportunity to investigate whether leptin regulation of immune function may be mediated in part by leptin-associated changes of hormones that influence immune function. We observed a significant correlation between IGF-1 and PBMC subpopulations. The IGF-1 receptor is expressed on immune cell types including activated T cells, B cells, NK cells, and monocytes and exerts effects on T cells and antigen-presenting cells (33), supporting the notion that IGF-1 may have immunomodulatory effects. Importantly, we did not find any correlation with cortisol, similar to the mouse model of acute leptin deficiency after leptin withdrawal (30). Correlations cannot prove causality and must be interpreted with caution in cross-sectional analysis. Further clinical and mechanistic investigations into the role of these hormones acting alone or in concert with leptin to affect immune function are needed.
In summary, our findings suggest that leptin serves a permissive role in regulating neuroendocrine and immune function. Given the beneficial effect of leptin replacement in ameliorating these defects in more severe and/or chronic leptin deficiency, we propose that, similar to other hormone deficiency syndromes, a leptin-deficiency syndrome exists. The lower limit of normal leptin levels appears to be ≈3 ng/ml (by using the assay reported herein), but the exact normal range remains to be defined precisely. Neuroendocrine alterations during more chronic relative hypoleptinemia in the setting of obesity (e.g., during weight loss) may involve different physiological mechanisms and adaptations. Whether changes of another putative factor are responsible for leptin-independent changes that occur when leptin remains above this threshold, and/or whether duration or an interaction of degree and duration of hypoleptinemia are important remains to be studied.
Methods
Study Design.
This protocol was approved by our Institutional Review Board, and clinical quality r-metHuLeptin (Amgen, Thousand Oaks, CA) was administered under an Investigational New Drug application to the Food and Drug Administration. All subjects were healthy without immunologic or endocrine disease based on examination and routine blood tests. Seven women (age = 22.4 ± 1.2 yr) with body mass index <25 kg/m2 and regular menstrual cycles (length 26–32 days) not on oral contraceptives for at least 6 mo participated in three separate studies in our General Clinical Research Center: a baseline isocaloric fed state as described in ref. 10 and two 72-h fasting studies scheduled in random order in double-blind fashion with administration of r-metHuLeptin (dose 0.08 mg/kg/day on day 1, increased to 0.2 mg/kg/day on days 2–3 to account for declining leptin levels with additional fasting, divided into 4 equal doses given s.c. every 6 h, starting at 8 a.m. on day 1), during one fasting study, or placebo (same schedule and volume as the corresponding r-metHuLeptin dose), during the other fasting study. Each subject completed three studies, separated by at least 8 wk to permit recovery of hematocrit, leptin levels, and weight to baseline, except one subject for the fasting/placebo study. For each subject, the frequent sampling was matched to a cycle day within 2 days of the cycle day of the other two frequent sampling studies and within menstrual cycle days 6–11. During fasting studies, subjects ate a snack the night before day 1 and then had only calorie-free liquids and daily multivitamin, NaCl (500 mg), and KCl (40 meq) until 10 a.m. on day 4. Leptin, insulin, FFA, estradiol, FSH, testosterone, free T3, reverse T3, free thyroxine, IGF-1, IGF-BP3, cortisol, PRA, and aldosterone were measured at 8 a.m. on days 1 and 4 and 24-h urine cortisol, sodium, and urea nitrogen on day 3. Starting at 8 a.m. on day 3, blood samples for leptin, LH, TSH, and ACTH were drawn every 15 min for 24 h through an indwelling peripheral i.v. line. At 8–9 a.m. on days 1 and 4, resting metabolic rate (DeltaTrac II Metabolic Monitor; SensorMedics) and body composition (bioelectric impedance analysis; RJL Systems, Clinton Township, MI) were measured. For five consecutive subjects completing both fasting studies, a blood sample obtained at 8 a.m. on days 1 and 4 of the fasting studies was shipped to Naples, Italy, by express courier to be processed within 48–72 h for immune assays along with a blood sample from a matched healthy, nonsmoker, fed control to account for any potential external effects. Processing of PBMCs within this time frame assures their viability, according to standard immunology procedures (34) and verified by our extensive studies in healthy controls and leptin-deficient subjects using a similar shipment protocol (4). As described in ref. 10, hormone levels were run in duplicate by using standard immunoassays and within the same run for a given subject, and the program cluster 8.0 was used to characterize leptin, LH, TSH, and ACTH pulsatility.
Immunophenotypic Analysis, T Cell Proliferation, and Cytokine Production.
Viability of cells was verified at 85–95% by using trypan blue staining and annexin-5 binding during flow cytometry, and serum glucose levels were measured as a marker of metabolic activity. Immunophenotypic analysis of peripheral blood (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site) and T cell cultures in medium supplemented with 5% (vol/vol) autologous serum were performed as described (4). Cell culture supernatants (100 μl) were removed 48–60 h after stimulation and frozen at −80°C until assayed for IFN-γ, IL-4, and IL-10 (PharMingen).
In Vitro Leptin Neutralization and Depletion of Leptin from Human Serum.
Mixed lymphocyte reactions were performed on isolated PBMCs (stimulator cells) and their corresponding HLA-mismatched irradiated (30-Gy) stimulators in the absence and presence of purified polyclonal rabbit anti-human leptin Ab (anti-hOb, 10–20 μg/ml; provided by Radek Sokol, BioVendor, Brno, Czech Republic) with affinity-purified rabbit polyclonal IgG as a control. Responder and stimulator cells were cultured for 5 days in RPMI medium 1640, supplemented with 5% human AB serum (Sigma) at a 1:1 ratio. For leptin depletion from serum, a protein G-Sepharose affinity column (Amersham Pharmacia) was used after adhesion on G protein of a polyclonal rabbit anti-human leptin Ab (BioVendor). Human AB serum was passed through the columns three times and collected. Anti-human leptin-specific ELISA and Western blotting to measure the serum content of leptin indicated that the amount of leptin was below the assay detection limit after depletion. Human recombinant leptin (R & D System) was used to verify specificity.
Statistical Analysis.
Data (mean ± SE) were analyzed by using spss 8.0 (SPSS, Chicago). Changes of variables from days 1 to 4 were compared by using Wilcoxon signed-rank paired tests or with nonparametric ANOVA with post hoc tests by least-significant difference across the three conditions. Immune data were adjusted for controls by subtracting the change in a parameter for the matched control from that for the subject, and data were analyzed by absolute values as well as adjusted for controls. P < 0.05 was considered significant. Bivariate Spearman correlation analyses were performed on changes in hormone levels and PBMC subpopulations from days 1 to 4, and P < 0.01 was considered significant to correct for multiple comparisons.
Supplementary Material
Acknowledgments
We thank the General Clinical Research Center (GCRC) nurses, nutritionists, and core lab and John Bullen, Violeta Stoyneva, and Jennifer Blakeman for assay assistance. This work was supported by National Institutes of Health Grants RR 01032 (to Beth Israel Deaconess Medical Center GCRC), R01-58785 (to C.S.M.), and K23 RR018860 (to J.L.C.); Fondazione Italiana Sclerosi Multipla Grant 2002/R/55 and Juvenile Diabetes Research Foundation-Telethon-Italy Grant GJT04008 (to G.M.); and a grant from Amgen, Inc. (to C.S.M.).
Abbreviations
- ACTH
adrenocorticotropic hormone
- FFA
free fatty acid
- FSH
follicle-stimulating hormone
- T3
triiodothyronine
- IGF-BP
IGF-binding protein
- LH
luteinizing hormone
- PBMC
peripheral blood mononuclear cell
- PRA
plasma renin activity
- TSH
thyroid-stimulating hormone.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Chehab F. F., Lim M. E., Lu R. Nat. Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 3.Ozata M., Ozdemir I. C., Licinio J. J. Clin. Endocrinol. Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 4.Farooqi I. S., Matarese G., Lord G. M., Keogh J. M., Lawrence E., Agwu C., Sanna V., Jebb S. A., Perna F., Fontana S., et al. J. Clin. Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Licinio J., Caglayan S., Ozata M., Yildiz B. O., De Miranda P. B., O’Kirwan F., Whitby R., Liang L., Cohen P., Bhasin S., et al. Proc. Natl. Acad. Sci. USA. 2004;101:4531–4536. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement K., Vaisse C., Lahlou N., Cabrol S., Pelloux V., Cassuto D., Gourmelen M., Dina C., Chambaz J., Lacorte J. M., et al. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 7.Chandra R. K. Am. J. Clin. Nutr. 1980;33:13–16. doi: 10.1093/ajcn/33.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Howard J. K., Lord G. M., Matarese G., Vendetti S., Ghatei M. A., Ritter M. A., Lechler R. I., Bloom S. R. J. Clin. Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahima R. S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E., Flier J. S. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 10.Chan J. L., Heist K., DePaoli A. M., Veldhuis J. D., Mantzoros C. S. J. Clin. Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lord G. M., Matarese G., Howard J. K., Baker R. J., Bloom S. R., Lechler R. I. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 12.Chan J. L., Moschos S. J., Bullen J., Heist K., Li X., Kim Y. B., Kahn B. B., Mantzoros C. S. J. Clin. Endocrinol. Metab. 2005;90:1625–1631. doi: 10.1210/jc.2004-1823. [DOI] [PubMed] [Google Scholar]
- 13.Holtkamp K., Mika C., Grzella I., Heer M., Pak H., Hebebrand J., Herpertz-Dahlmann B. J. Neural Transm. 2003;110:427–435. doi: 10.1007/s00702-002-0800-x. [DOI] [PubMed] [Google Scholar]
- 14.Welt C. K., Chan J. L., Bullen J., Murphy R., Smith P., DePaoli A. M., Karalis A., Mantzoros C. S. N. Engl. J. Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 15.Heymsfield S. B., Greenberg A. S., Fujioka K., Dixon R. M., Kushner R., Hunt T., Lubina J. A., Patane J., Self B., Hunt P., et al. J. Am. Med. Assoc. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 16.Hukshorn C. J., Lindeman J. H., Toet K. H., Saris W. H., Eilers P. H., Westerterp-Plantenga M. S., Kooistra T. J. Clin. Endocrinol. Metab. 2004;89:1773–1778. doi: 10.1210/jc.2003-030803. [DOI] [PubMed] [Google Scholar]
- 17.Musso C., Cochran E., Javor E., Young J., DePaoli A. M., Gorden P. Metabolism. 2005;54:255–263. doi: 10.1016/j.metabol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum M., Murphy E. M., Heymsfield S. B., Matthews D. E., Leibel R. L. J. Clin. Endocrinol. Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 19.Loucks A. B., Heath E. M. J. Clin. Endocrinol. Metab. 1994;78:910–915. doi: 10.1210/jcem.78.4.8157720. [DOI] [PubMed] [Google Scholar]
- 20.Bergendahl M., Evans W. S., Pastor C., Patel A., Iranmanesh A., Veldhuis J. D. J. Clin. Endocrinol. Metab. 1999;84:883–894. doi: 10.1210/jcem.84.3.5536. [DOI] [PubMed] [Google Scholar]
- 21.Soules M. R., Merriggiola M. C., Steiner R. A., Clifton D. K., Toivola B., Bremner W. J. Clin. Endocrinol. 1994;40:725–731. doi: 10.1111/j.1365-2265.1994.tb02505.x. [DOI] [PubMed] [Google Scholar]
- 22.Berga S. L., Loucks T. L., Cameron J. L. Fertil. Steril. 2001;75:926–932. doi: 10.1016/s0015-0282(01)01686-7. [DOI] [PubMed] [Google Scholar]
- 23.Schurgin S., Canavan B., Koutkia P., DePaoli A. M., Grinspoon S. J. Clin. Endocrinol. Metab. 2004;89:5402–5409. doi: 10.1210/jc.2004-1102. [DOI] [PubMed] [Google Scholar]
- 24.Alvero R., Kimzey L., Sebring N., Reynolds J., Loughran M., Nieman L., Olson B. R. J. Clin. Endocrinol. Metab. 1998;83:76–80. doi: 10.1210/jcem.83.1.4512. [DOI] [PubMed] [Google Scholar]
- 25.Olson B. R., Cartledge T., Sebring N., Defensor R., Nieman L. J. Clin. Endocrinol. Metab. 1995;80:1187–1193. doi: 10.1210/jcem.80.4.7714088. [DOI] [PubMed] [Google Scholar]
- 26.Loucks A. B., Thuma J. R. J. Clin. Endocrinol. Metab. 2003;88:297–311. doi: 10.1210/jc.2002-020369. [DOI] [PubMed] [Google Scholar]
- 27.Hilton L. K., Loucks A. B. Am. J. Physiol. Endocrinol. Metab. 2000;278:E43–E49. doi: 10.1152/ajpendo.2000.278.1.E43. [DOI] [PubMed] [Google Scholar]
- 28.Mantzoros C. S., Ozata M., Negrao A. B., Suchard M. A., Ziotopoulou M., Caglayan S., Elashoff R. M., Cogswell R. J., Negro P., Liberty V., et al. J. Clin. Endocrinol. Metab. 2001;86:3284–3291. doi: 10.1210/jcem.86.7.7644. [DOI] [PubMed] [Google Scholar]
- 29.Licinio J., Mantzoros C., Negrao A. B., Cizza G., Wong M. L., Bongiorno P. B., Chrousos G. P., Karp B., Allen C., Flier J. S., et al. Nat. Med. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 30.Montez J. M., Soukas A., Asilmaz E., Fayzikhodjaeva G., Fantuzzi G., Friedman J. M. Proc. Natl. Acad. Sci. USA. 2005;102:2537–2542. doi: 10.1073/pnas.0409530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matarese G., La Cava A., Sanna V., Lord G. M., Lechler R. I., Fontana S., Zappacosta S. Trends Immunol. 2002;23:182–187. doi: 10.1016/s1471-4906(02)02188-9. [DOI] [PubMed] [Google Scholar]
- 32.Frauwirth K. A., Riley J. L., Harris M. H., Parry R. V., Rathmell J. C., Plas D. R., Elstrom R. L., June C. H., Thompson C. B. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 33.Sharp L. L., Jameson J. M., Cauvi G., Havran W. L. Nat. Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 34.Lefkovits I. Immunology Methods Manual. San Diego: Academic; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.