Abstract
The recent establishment of a robust hepatitis C virus (HCV) cell culture system permits analysis of virus-host interactions during HCV infection. Here, we report that HCV genotype 2a (JFH-1) infection fails to induce IFN-β or IFN-stimulated gene expression in Huh-7 cells, and that it blocks IFN-β and IFN-stimulated gene production after transfection of synthetic dsRNA. Overexpression of individual components of the dsRNA-signaling pathway in HCV-infected and uninfected cells indicates that HCV inhibits IFN-β promoter activity by inactivating the mitochondrial antiviral signaling protein/IFN-β promoter stimulator 1 (MAVS/IPS-1), while leaving the IFN-induced Janus kinases-signal transducers and activators of transcription (JAK-STAT) signaling pathway intact. We also show that MAVS/IPS-1-dependent IFN-β promoter activity in HCV-infected cells is fully restored by the nonstructural protein 3 (NS3) protease inhibitor BILN2061. In contrast, synthetic dsRNA-induced IFN-β promoter activity is not restored by BILN2061, although it is partially restored by overexpression of RIG-I. These results support recently reported evidence that the HCV NS3 protease blunts the ability of HCV to induce IFN-β promoter activity by proteolytically cleaving MAVS/IPS-1. The results also suggest that HCV blocks the synthetic dsRNA-induced signaling pathway at a point upstream of MAVS/IPS-1, and that it does so by an NS3-independent mechanism.
Keywords: IFN-stimulated genes, nonstructural protein 3/4A, retinoic acid-induced gene I
Hepatitis C virus (HCV) is a single-stranded RNA virus in the Flaviviridae family that infects >170 million people worldwide and causes acute and chronic hepatitis and hepatocellular carcinoma (1, 2). The recent establishment of a robust cell culture model of HCV infection (3–5) allows analysis of each step in the HCV life cycle and virus-host interactions that determine the outcome of infection. Viral infections often trigger an immediate cellular response that results in the establishment of an antiviral state (6). A central event in the innate immune response is the secretion of type I IFNs (IFN-α and IFN-β) that stimulate the production of a family of IFN-stimulated genes (ISGs) that have pleotropic inhibitory effects on viral replication in infected and neighboring uninfected cells (6).
IFN-β expression is triggered by double-stranded RNA (dsRNA), a common intermediate in many viral infections including HCV (7). The pathogen-associated molecular pattern in dsRNA is recognized by Toll-like receptor 3 (TLR3), either on the cell surface or in endosome vesicles (8), or by retinoic acid-induced gene I (RIG-I) (9) or melanoma differentiation-associated gene 5 (MDA5) (10). Both RIG-I and MDA5 contain DexD/H-box helicase domains that serve as intracellular dsRNA receptors and caspase recruitment domains (CARDs) that transmit signals to downstream CARD-containing elements (9). After dsRNA binding occurs, the cytoplasmic domain of TLR3 recruits the adapter molecule TICAM-1/TRIF through a MyD88-independent pathway (11). In contrast, RIG-I and MDA5 use their tandem CARD domains to interact with the CARD domains of mitochondrial antiviral signaling protein (MAVS), which is also known as IFN-β promoter stimulator 1 (IPS-1), Cardif, and virus-induced signaling adaptor (VISA) (12–15). Despite these differences, both pathways rapidly induce IFN-β through the posttranslational modification of multiple cellular factors, including the transcription factor IFN regulatory factor 3 (IRF-3) and the kinases, Tank-binding kinase 1 (TBK-1) and inhibitor of κB kinase ε (IKK-ε) (16, 17). IRF-3 is constitutively expressed in the cytoplasm in an inactive state (18). Upon viral infection, phosphorylation of IRF-3 by TBK-1 and IKK-ε induces a conformational change leading to its dimerization, nuclear translocation, and, eventually, transcriptional activation of IFN-β (16–18).
Many human pathogenic viruses have evolved distinct strategies to inhibit the early signaling events leading to IFN production (19, 20). For example, the influenza virus nonstructural protein 1 (NS1) inhibits IRF-3 activation (19), and the HCV NS3 protease has been shown to inhibit IRF-3 phosphorylation (20). Additional studies in HCV replicon-containing cell lines have shown that the HCV genotype 1 NS3 protein blocks the RIG-I-mediated signaling pathway by cleaving the MAVS/IPS-1 protein and blocking downstream IFN-β activation (14, 21, 22). It has not been determined, however, whether the NS3 protein cleaves MAVS/IPS-1 in HCV-infected cells.
In this study, we report that infection of Huh-7 cells with the HCV genotype 2a clone (JFH-1) prevents IFN-β and ISG expression because of its ability to block the dsRNA signaling pathway by proteolytic cleavage of MAVS/IPS-1, thereby preventing the nuclear translocation of IRF-3 and the induction of IFN-β promoter activity. In addition, we show that HCV blocks a second cellular target upstream of MAVS/IPS-1 by an NS3-independent mechanism.
Results
HCV Does Not Induce IFN-β or ISGs When It Infects Huh-7 Cells.
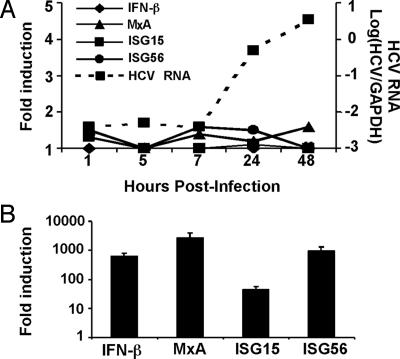
To examine whether HCV infection induces IFN-β and ISG expression, we infected Huh-7 cells with JFH-1 virus at a high (3.0) multiplicity of infection (moi) and monitored the expression of IFN-β, ISGs (MxA, ISG15 and ISG56), and viral RNA expansion by reverse transcription–quantitative PCR (RT-qPCR) at various times thereafter. As shown in Fig. 1A, after an initial lag period, intracellular HCV RNA content increased exponentially, expanding >2 orders of magnitude between 7–48 h postinoculation, at which time >90% of the cells were infected by intracellular NS5A staining, and the intracellular content of HCV RNA reached a plateau (data not shown). In contrast, there was no change in the level of IFN-β or ISG expression during the course of the experiment. Similar results were obtained when the cells were infected at a low (0.01) moi (data not shown).
Fig. 1.
HCV infection does not induce IFN-β or ISG expression. (A) RT-qPCR analysis of cellular HCV RNA, IFN-β, and ISG expression. Huh-7 cells were infected with JFH-1 at a high multiplicity (moi = 3). At the indicated times, total cellular RNA was harvested and extracted. The intracellular gene expression of IFN-β, ISGs (MxA, ISG15 and ISG56), and HCV viral RNA was then measured by RT-qPCR. The expression of IFN-β or ISGs is presented as fold-induction relative to basal levels in uninfected cells. (B) Induction of ISGs in Huh-7 cells after transfection with poly(IC). Huh-7 cells were transfected with poly(IC) by using Lipofectamine 2000. Sixteen hours later, total cellular RNA was harvested. Quantification of the intracellular gene expression of ISGs was performed the same as in A.
To rule out the possibility that Huh-7 cells are intrinsically unable to respond to dsRNA, they were tested for the ability to express IFN-β and ISGs after transfection by a synthetic form of dsRNA, poly(I)·poly(C) [poly(IC)]. As shown in Fig. 1B, IFN-β and ISG expression were highly induced in the Huh-7 cells 16 h posttransfection with a maximally stimulatory dose (0.3 μg) of poly(IC). This response was specific to dsRNA as neither poly(I) nor poly(C) induced IFN-β or ISG expression in the cells (Fig. 7, which is published as supporting information on the PNAS web site). These results indicate that HCV either fails to trigger or efficiently suppresses the intracellular dsRNA signaling pathway upon infection of Huh-7 cells in vitro. It is noteworthy that poly(IC) does not induce IFN-β or ISG expression in Huh-7 cells when it is simply added to the culture medium (data not shown), indicating that the Toll-like receptor 3 signaling pathway is not functional in these cells and, therefore, doesn’t contribute to the results presented herein.
HCV Infection Blocks dsRNA-Induced IRF-3 Nuclear Translocation in Huh-7 Cells.
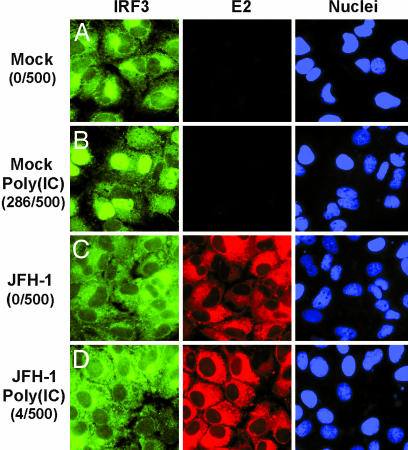
Transcriptional up-regulation of IFN-β by dsRNA requires nuclear translocation of the transcription factor IRF-3. As shown in Fig. 2A Left, IRF-3 is located exclusively in the cytoplasm in mock-infected Huh-7 cells, but it rapidly translocates to the nucleus when those cells are transfected with poly(IC) (Fig. 2B Left). In contrast, nuclear IRF-3 translocation does not occur in HCV-infected Huh-7 cells (Fig. 2C Left), even though virtually all of the cells display immunofluorescence staining for the HCV glycoprotein E2 (Fig. 2C Center). The foregoing suggests either that HCV does not trigger the dsRNA signaling pathway or it blocks activation after it starts. We favor the second alternative because, in contrast to uninfected Huh-7 cells (Fig. 2B Left and Center), poly(IC) transfection failed to induce nuclear IRF-3 translocation in the HCV-infected cells (Fig. 2D Left and Center), suggesting that HCV actively blocks the activation of IRF-3 triggered by dsRNA. A diagram summarizing the steps in the dsRNA signaling pathway is provided for reference in Fig. 8, which is published as supporting information on the PNAS web site.
Fig. 2.
HCV infection prevents dsRNA-induced IRF-3 nuclear translocation. Huh-7 cells were either mock-infected (A and B) or infected with JFH-1 at moi = 0.2 (C and D). On day 4 postinfection, cells were either transfected with poly(IC) by using Lipofectamine 2000 (B and D) or left untransfected (A and C). Sixteen hours later, cells were fixed and stained with antibodies against IRF-3 (green) and HCV E2 (red). Cell nuclei were stained by Hoechst dye (blue). To measure the nuclear accumulation of IRF-3, 500 cells from ten independent fields were examined on a fluorescent microscope, and the results are shown on the left in parentheses as the number of cells with nuclear IRF-3 per 500 cells.
HCV Infection Blocks the dsRNA-Signaling Pathway in Huh-7 Cells but Leaves the JAK-STAT Signaling Pathway Intact.
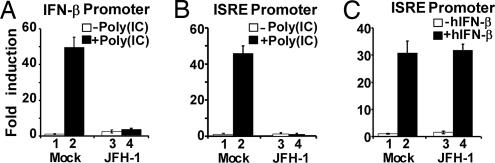
To confirm and quantify the ability of HCV to block dsRNA signaling, we transfected an IFN-β promoter luciferase reporter plasmid into mock-infected and HCV-infected Huh-7 cells, either with or without subsequent poly(IC) transfection. Consistent with the observation that HCV infection does not induce IFN-β gene expression in Huh-7 cells (Fig. 1A), similar basal IFN-β promoter activity was detected in mock-infected and HCV-infected cells (Fig. 3A, lanes 1 and 3). In contrast, the IFN-β promoter was activated ≈50-fold when mock-infected cells were transfected with poly(IC) (Fig. 3A, lanes 1 and 2), whereas it was not activated by poly(IC) transfection in HCV-infected cells (Fig. 3A, lanes 3 and 4).
Fig. 3.
Blockade of dsRNA-induced IFN-β expression in HCV-infected cells. (A) HCV blocks dsRNA-induced IFN-β promoter activation. Huh-7 cells were infected with JFH-1 at moi = 0.2. On day 4 postinfection, cells were cotransfected with the IFN-β promoter luciferase reporter plasmid, an internal control plasmid pRL-TK, and a carrier plasmid pcDNA3.1. Thirty-six hours later, cells were transfected with or without poly(IC). Cells were subjected to dual luciferase assay 16 h after transfection. The results are expressed as fold induction of IFN-β promoter activity relative to the basal level. (B) HCV blocks dsRNA-induced ISRE promoter activation. The experiment was performed the same as in A, except that the ISRE promoter luciferase reporter plasmid was used. (C) HCV does not block IFN-induced JAK-STAT signaling. Similar to B, cells were transfected with the ISRE promoter reporter plasmid and then incubated with 100 units per ml of human IFN-β for 16 h before dual luciferase assay.
Although the foregoing results indicate that HCV blocks the dsRNA-signaling pathway in infected cells, they do not examine the possibility that HCV may also suppress the IFN-β-activated JAK-STAT signaling pathway, which would provide a downstream block to the induction of ISGs. To address this possibility, we examined the transcriptional activation of a consensus ISG promoter [IFN-stimulated response element (ISRE)] luciferase reporter construct in mock-infected and HCV-infected Huh-7 cells by both poly(IC) transfection, which activates the upstream dsRNA-signaling pathway (Fig. 3B), and by administration of recombinant human IFN-β, which activates downstream JAK-STAT signaling (Fig. 3C). As expected, dsRNA-induced activation of the ISRE promoter was completely blocked in the HCV-infected cells (Fig. 3B). In contrast, however, the ISRE promoter was fully activated in the HCV-infected cells by recombinant IFN (Fig. 3C). These results suggest that HCV infection blocks the intracellular dsRNA-mediated signaling pathway and, therefore, IFN-β production, but it leaves the downstream JAK-STAT signaling pathway intact.
HCV Blocks the dsRNA-Signaling Pathway Upstream of IRF-3.
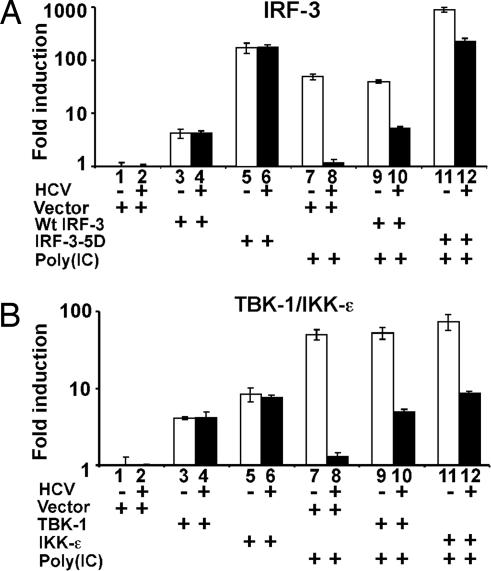
To determine the level at which HCV infection blocks the intracellular dsRNA-mediated signaling pathway, we cotransfected mock-infected and JFH-1-infected Huh-7 cells with a construct that expresses IRF-3 together with the IFN-β promoter luciferase reporter construct described in Fig. 3, and we examined them for IFN-β promoter activity with and without transfection of poly(IC).
As shown in Fig. 4A, expression of wild-type IRF-3 activated the IFN-β promoter by 4.2- and 4.5-fold in both the mock- and HCV-infected Huh-7 cells, respectively (compare lane 1 with lane 3, and lane 2 with lane 4), implying that the signaling block is upstream of IRF-3. Similarly, transfection of a constitutively active mutant of IRF-3 [IRF-3(5D)] also stimulated IFN-β promoter activity comparably in both cells (Fig. 4A, compare lane 1 with lane 5, and lane 2 with lane 6), although to a much higher level, i.e., 175- and 184-fold, respectively, again suggesting that signaling is intact downstream of IRF-3 in the HCV-infected cells. In contrast to the IRF-3 results, the strong induction of IFN-β promoter activity by poly(IC) in the uninfected cells was greatly reduced in the HCV-infected cells (Fig. 4A, compare lane 1 with lane 7, and lane 2 with lane 8). Importantly, although poly(IC) could further increase IFN-β promoter activity in the mock-infected cells when they were cotransfected with wild-type or constitutively active IRF-3 (Fig. 4A, compare lane 3 with lane 9, and lane 5 with lane 11), it failed to do so in the HCV-infected cells (Fig. 4A, compare lane 4 with lane 10, and lane 6 with lane 12). These observations support the results shown in Fig. 2, implying the existence of a signaling block upstream of IRF-3.
Fig. 4.
HCV blocks the dsRNA-signaling pathway upstream of the IRF-3 kinases TBK-1/IKK-ε. Huh-7 cells were infected with JFH-1 at moi = 0.2. On day 4 postinfection, cells were cotransfected with the reporter plasmid pIFΔ(−125) lucter and plasmid pRL-TK in the presence of an empty vector, or a plasmid expressing wild-type IRF-3 or its active mutant IRF-3–5D (A), or with plasmids expressing the TBK-1 or IKK-ε (B) as indicated. Thirty-six hours later, cells were transfected with or without poly(IC). Luciferase activities were measured 16 h after transfection, and the results are expressed as fold induction of IFN-β promoter activity.
HCV Blocks the dsRNA-Signaling Pathway Upstream of TBK-1 and IKK-ε.
We next examined whether either of the IRF-3 upstream kinases, TBK-1 or IKK-ε, are targeted by HCV. Similar to IRF-3, expression of either TBK-1 or IKK-ε resulted in activation of the IFN-β promoter in both the mock- and HCV-infected Huh-7 cells (Fig. 4B, compare lane 1 with lanes 3 and 5, and compare lane 2 with lanes 4 and 6), suggesting that the signaling pathway downstream of TBK-1/IKK-ε remains active in the HCV-infected cells. When those cells were transfected with poly(IC), further IFN-β promoter activation was only seen in the mock-infected cells (Fig. 4B, compare lanes 3 and 4 with lanes 9 and 10, and compare lanes 5 and 6 with lanes 11 and 12). Taken together, the results suggest that HCV targets the double-stranded RNA signaling pathway upstream of the IRF-3 kinases TBK-1/IKK-ε to block IFN-β induction.
HCV Blocks MAVS/IPS-1-Mediated IFN-β Induction.
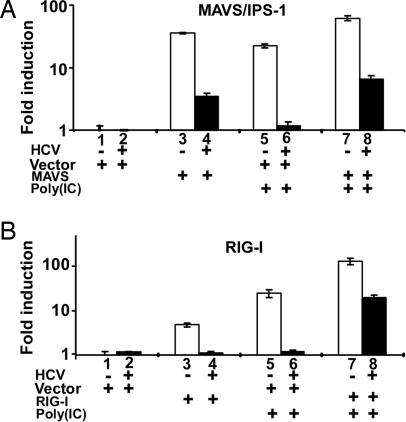
Recent studies using genotype 1b (Con1) HCV subgenomic replicons have shown that the HCV NS3 protease cleaves the mitochondrial antiviral signaling protein MAVS/IPS-1 (14, 22). To determine whether MAVS/IPS-1-cleavage occurs during an HCV genotype 2a infection, we transfected a MAVS/IPS-1 expression plasmid into mock-infected or JFH-1-infected Huh-7 cells. In striking contrast to the effects of IRF-3, TBK-1, and IKK-ε transfection (Fig. 4), the MAVS/IPS-1 expression vector induced IFN-β promoter activity to a much lower degree (3.9-fold) in HCV-infected cells (Fig. 5A, compare lane 2 with lane 4) compared with mock-infected cells (46.2-fold) (Fig. 5A, compare lane 1 with lane 3). This lower induction was not because of decreased transfection efficiency of the HCV-infected cells because they displayed comparable or higher expression levels of the Renilla luciferase-expressing control plasmid (pRL-TK) compared with mock-infected cells (data not shown). Therefore, it is likely that the results reflect the inactivation of MAVS/IPS-1 by HCV, as recently described in HCV replicon cells (22) and in JFH-1-infected Huh-7 cells (23).
Fig. 5.
HCV blocks MAVS/IPS-1- and RIG-I-induced IFN-β expression. HCV blocks MAVS/IPS-1-mediated (A) or RIG-I-mediated (B) IFN-β induction. The experimental conditions were the same as in Fig. 4, except that MAVS/IPS-1 or RIG-I expression plasmids were cotransfected with the IFN-β promoter reporter plasmid.
HCV Blocks RIG-I-Mediated IFN-β Induction.
If HCV inactivates MAVS/IPS-1, the ability of dsRNA signaling elements upstream of MAVS/IPS-1 to induce IFN-β promoter activity in HCV-infected cells should also be reduced. To test that hypothesis, we monitored the impact of a wild-type RIG-I expression vector on IFN-β promoter activity in mock-infected and HCV-infected Huh-7 cells. As shown in Fig. 5B, the RIG-I expression vector did not induce IFN-β promoter activity in HCV-infected cells (Fig. 5B, compare lane 2 with lane 4), whereas it did induce a 4.8-fold increase in mock-infected cells (Fig. 5B, compare lane 1 with lane 3). This lack of induction was not due to decreased transfection efficiency of the HCV-infected cells because the HCV-infected cells displayed comparable expression levels of the pRL-TK control plasmid compared with mock-infected cells (data not shown). The most likely interpretation of these results is that HCV blocks RIG-I induced signaling by inactivating its downstream substrate, MAVS/IPS-1, as shown in Fig. 5A.
However, the foregoing results may also suggest that HCV can block dsRNA signaling by other mechanisms as well. For example, RIG-I transfection sensitized the HCV-infected cells to the stimulatory effects of poly(IC) to a much greater degree (18.1-fold) (Fig. 5B, compare lane 4 with lane 8) than HCV-infected cells transfected with vector (1.2-fold) (Fig. 5B, compare lane 2 with lane 6). The ability of RIG-I overexpression to overcome the HCV-induced block in dsRNA signaling suggests that high levels of RIG-I can activate residual intact MAVS/IPS-1 molecules that aren’t inactivated by HCV or that high levels of RIG-I can activate the IFN-β promoter by a MAVS/IPS-1-independent process. The ability of RIG-I to sensitize HCV-infected cells to respond to poly(IC) as strongly as mock-infected cells (Fig. 5B, compare lane 5 with lane 8) argues in favor of the existence of a second mechanism, whereby HCV inhibits the dsRNA-induced signal transduction pathway which is MAVS/IPS-1-independent and upstream of RIG-I.
Reversal of HCV-Induced MAVS/IPS-1-Inactivation by an NS3 Protease Inhibitor.
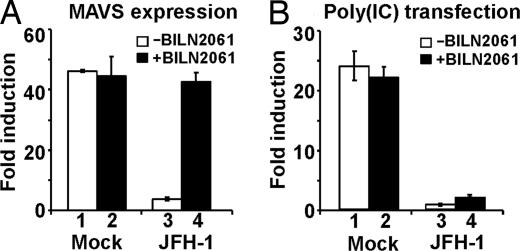
To test the hypothesis that MAVS/IPS-1 inactivation is because of a cleavage by the HCV NS3 protease, mock-infected and HCV-infected Huh-7 cells were treated with the NS3 protease inhibitor BILN2061 before transfection of the MAVS/IPS-1 expression plasmid, and IFN-β promoter activity was monitored 36 h later. As shown in Fig. 6A, HCV-infected cells that were virtually unresponsive to MAVS/IPS-1 transfection under baseline conditions (Fig. 6A, compare lane 1 with lane 3) were as responsive to MAVS/IPS-1 as the mock-infected cells when they were treated with the protease inhibitor (Fig. 6A, compare lane 2 with lane 4). These results suggest that, as recently reported in HCV genotype 1b replicon experiments (22) and in osteosarcoma cells that express genotype 1a NS3/4A (23), during HCV genotype 2a infection, the HCV NS3 protease cleaves MAVS/IPS-1 and, thereby, blocks dsRNA signaling and downstream IFN-β induction.
Fig. 6.
The NS3 protease inhibitor BILN2061 restores MAVS/IPS-1-mediated, but not dsRNA-mediated, IFN-β induction in cells infected with HCV. (A) NS3 protease inhibitor restores the MAVS/IPS-1-mediated IFN-β induction. Twenty-four hours before cotransfection with the MAVS/IPS-1 expression vector and the IFN-β promoter reporter plasmid, the NS3 protease inhibitor BILN2061 was added to mock infected and JFH-1 infected cells at a final concentration of 10 μM. Luciferase activities were analyzed 36 h after transfection in the absence of poly(IC). (B) NS3 protease inhibitor fails to restore the dsRNA-mediated IFN-β induction. Similar to A, cells were pretreated with the protease inhibitor BILN2061 before plasmid transfection. Cells were further transfected with or without poly(IC) 36 h after transfection of the IFN-β promoter reporter plasmid. Sixteen hours later, luciferase activities were analyzed, and the results are expressed as fold induction.
HCV Blockade of dsRNA-Induced IFN-β Production Is NS3 Protease-Independent.
In contrast to the ability of the NS3 protease inhibitor to restore MAVS/IPS-1-mediated IFN-β induction (Fig. 6A, compare lane 3 with lane 4), the protease inhibitor could not restore dsRNA-mediated IFN-β induction in poly(IC)-transfected HCV-infected cells (Fig. 6B, compare lane 3 with lane 4). These results suggest that HCV blocks dsRNA signaling not only by inducing NS3 protease-dependent MAVS/IPS-1 cleavage but also by an NS3-independent step upstream of MAVS/IPS-1, perhaps at the level of RIG-I or higher in the dsRNA signaling cascade. The ability of overexpressed RIG-I to substantially overcome the inhibitory effect of HCV on poly(IC)-induced IFN-β promoter activation is compatible with this notion.
Discussion
We have previously demonstrated (24) that many IFN-stimulated genes are strongly induced in the liver during HCV infection, that the strength of their induction is directly related to the level of intrahepatic and circulating HCV RNA, and that the virus appears to be resistant to their antiviral effects because it can persist indefinitely despite their induction. Insight into the mechanistic basis for these observations was provided by elegant studies from Gale and colleagues (20, 23), demonstrating that the HCV NS3 protein can block the dsRNA signaling pathway in Huh-7 cells bearing HCV genotype 1 replicons and JFH-1-infected Huh-7 cells by preventing IRF-3 translocation, and the recent demonstration by Li et al. (22) that NS3 blocks the induction of IFN-β by cleaving MAVS/IPS-1. These studies suggest two alternative hypotheses for the in vivo observations that HCV induces, yet apparently ignores, the dsRNA-signaling pathway.
First, as suggested by Loo et al. (23), HCV could sequentially induce the pathway in the infected cells by production of a dsRNA replication intermediate, and then quickly suppress the pathway when the intracellular NS3 protein content reaches inhibitory levels. This hypothesis implies that the ISGs observed in the HCV-infected liver are produced only by newly infected cells and reflect the continuous spreading of HCV to uninfected hepatocytes. Arguing against this hypothesis, however, is the observation (Fig. 1) that IFN-β and ISG gene expression was not detectable at several early time points of the JFH-1 infection. The second hypothesis implies that the inhibitory effect of the NS3 protein occurs sufficiently early and is sufficiently strong to preclude the production of IFN-β by infected cells. Instead, it postulates that HCV triggers IFN-β production by uninfected cells in the liver, perhaps by stimulating plasmacytoid dendritic cells and other nonparenchymal cells that have strong IFN-β producing potential (25).
Although both of these alternatives could be operative in vivo, our results illustrate that HCV does not induce IFN-β or ISGs in infected Huh-7 cells (Fig. 1). Instead, it strongly suppresses IRF-3 translocation (Fig. 2) and the induction of IFN-β by transfected dsRNA (Fig. 3), suggesting that the ISGs expressed in the HCV-infected liver are likely produced by uninfected cells. Because our results demonstrate that HCV-infected hepatocytes are fully able to express ISGs in response to IFN-β stimulation (Fig. 3), infected cells could contribute to the ISG profile in the infected liver by their intact JAK-STAT signaling pathway, even if they are not the originating source of the IFN-β. Further studies will be necessary to confirm this hypothesis and to determine whether the current observations extend beyond the specific HCV genotype 2a JFH-1 molecular clone we used in our experiments.
Our results demonstrate that, as previously shown in HCV genotype 1 replicon cells (20, 21), dsRNA-mediated IFN-β induction is blocked at a point upstream of the IRF-3 kinases TBK-1/IKK-ε in HCV-infected Huh-7 cells. Indeed, overexpression of each of these signaling components induced comparable levels of IFN-β in both mock-infected and HCV-infected cells (Fig. 4). However, when these cells were subsequently transfected with poly(IC), a further induction of IFN-β was observed only in the mock-infected cells. In contrast, overexpression of the dsRNA-sensing protein, RIG-I, induced IFN-β production in mock-infected but not HCV-infected cells (Fig. 5), suggesting that HCV either targets RIG-I itself or a component of the signaling pathway downstream of RIG-I and upstream of TBK-1/IKK-ε. In this regard, we showed that MAVS/IPS-1-induced IFN-β production was also blocked in HCV-infected cells (Fig. 5), strongly suggesting that MAVS/IPS-1 itself is targeted by HCV, because all known signaling elements downstream of MAVS/IPS-1 are intact. Importantly, the inability of overexpressed MAVS/IPS-1 to induce IFN-β promoter activity in HCV-infected cells was fully corrected by pretreating the cells with the HCV NS3 protease inhibitor BILN2061 (Fig. 6), strongly supporting the notion that NS3 blocks IFN-β induction by cleaving MAVS/IPS-1, as has been recently reported in HCV genotype 1 replicon cells (22) and by Loo et al. (23) using JFH-1 infected Huh-7 cells.
We are particularly intrigued by the inability of BILN2061 to restore dsRNA-induced IFN-β production in HCV-infected cells (Fig. 6), and the failure of overexpressed MAVS/IPS-1 to restore dsRNA-induced IFN-β promoter activation in infected cells (Fig. 5, compare lane 8 with lane 5). These results suggest that HCV may block IFN-β production at an NS3-independent step in the dsRNA signaling pathway that is upstream of MAVS/IPS-1. In keeping with this hypothesis, we found that the overexpression of RIG-I partially restored the dsRNA-signaling response and IFN-β promoter activation in HCV-infected cells (Fig. 5). A similar result was also seen in an HCV replicon cell line that overexpresses RIG-I (Fig. 9, which is published as supporting information on the PNAS web site). This restoration is intriguing because the downstream adaptor protein, MAVS/IPS-1, is targeted for cleavage by HCV (14, 22) in both of these systems. Several explanations are possible for this phenotype. One is that excess RIG-I could protect MAVS/IPS-1 from protelytic cleavage by sequestration through interaction of their CARD domains, or by activating any MAVS/IPS-1 that is not cleaved by NS3. Alternatively, RIG-I overexpression could activate a currently undefined MAVS/IPS-1-independent dsRNA-signaling pathway leading to the induction IFN-β.
In conclusion, the current results support, in an HCV genotype 2a infection system, recently reported evidence that the HCV NS3 protease blunts the ability of HCV to induce IFN-β promoter activity by proteolytically cleaving MAVS/IPS-1 (22, 23). We have also identified a heretofore unsuspected NS3-independent mechanism that blunts the dsRNA signaling pathway in HCV-infected cells. The nature of this target is currently unknown, but it probably is not a unique component of the Toll-like receptor 3-signaling pathway because that pathway is not inducible in Huh-7 cells. Finally, the ability of RIG-I overexpression to restore dsRNA signaling in HCV-infected cells in which MAVS/IPS-1 is inactivated, suggests the existence of a RIG-I dependent signaling pathway that could bypass MAVS/IPS-1 to trigger IFN-β expression. This process deserves further investigation because RIG-I is strongly induced in the liver of HCV-infected chimpanzees (26), where it could circumvent the NS3/4A mediated cleavage of MAVS/IPS-1, trigger an innate antiviral response, and influence the outcome of infection.
Materials and Methods
Cell Culture and HCV Stock Preparation.
The cell culture conditions and JFH-1 plasmid construct used for stock virus production and analysis in this study were described (3). Details are provided as Supporting Text, which is published as supporting information on the PNAS web site.
Plasmids.
The IFN-β promoter-containing plasmid, pIFΔ (−125) lucter, was a gift of S. Goodbourn (University of London, London) (27). The plasmid pISRE-Luc was from Clontech. The plasmid pRL-TK expressing Renilla luciferase was purchased from Promega. The plasmids pCMVBL-IRF-3 and pCMVBL-IRF-3(5D) were gifts from J. Hiscott (McGill University, Montreal) (18). The pcDNA3.1-TBK-1 and pcDNA3.1-IKK-ε expression plasmids were gifts of K. Fitzgerald (University of Massachusetts Medical School, Worcester, MA) (17). The expression plasmids for wild-type RIG-I (pEF-flag-RIG-I) and its constitutively active mutant (pEF-flag-RIG-I ΔN) were obtained from T. Fujita (Tokyo Metropolitan Institute, Tokyo) and described in ref. 9. The expression construct pcDNA3-Flag-MAVS was a gift from Z. Chen (University of Texas Southwestern Medical Center, Dallas) (22).
RNA Quantification and Indirect Immunofluorescence.
RNA analysis and protein staining in this study were performed as described in ref. 3. Details are provided in Supporting Text.
Transfection and Reporter Assay.
Sixteen to twenty hours before transfection, Huh-7 cells were seeded in 48-well plates (Corning) at a density of 1.0–1.5 × 104 cells per well. In each well, cells were cotransfected with a total of 240 ng of plasmid DNA by using the Exgen 500 transfection reagent (Fermentas, Hanover, MD), according to the manufacturer’s protocol. In each transfection, the plasmid mixture included 20 ng IFN-β promoter reporter pIF(−125) lucter, 20 ng Renilla luciferase-expressing plasmid pRL-Tk used to normalize transfection efficiency, and 200 ng protein expression plasmid. Cells were harvested 16 h later for luciferase assay. To assay firefly and Renilla luciferase activity, cells were lysed by using passive lysis buffer, and luciferase activities were determined by a dual-luciferase reporter assay system (Promega). The IFN-β promoter firefly luciferase activity was first normalized for the transfection efficiency as measured by Renilla luciferase activity, and expressed as fold induction relative to the basal promoter activity in the mock-infected cells without poly(IC) transfection. In selected experiments, 36 h after transfection of the previously described constructs, cells were either mock transfected or transfected with 0.3 μg of poly(I)·poly(C) [poly(IC)] purchased from Sigma by using the Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s protocol. To examine the effect of the protease inhibitor BILN2061 (28) (Boehringer Ingelheim, Quebec), cells were cultured with antibiotic-free medium containing 10 μM of BILN2061 for 24 h before transfection. All of the experiments were done at least twice, with at least duplicate samples in each study. Results are presented as mean ± SD of a representative experiment.
Supplementary Material
Acknowledgments
We thank Dr. Takaji Wakita (Tokyo Metropolitan Institute, Tokyo) for providing the JFH-1 cDNA plasmid, Dr. S. Goodbourn (University of London, London) for providing the plasmid pIFΔ(−125) lucter, Dr. J. Hiscott (McGill University, Montreal) for the IRF-3 expression constructs, Dr. K. Fitzgerald (University of Massachusetts Medical School, Worcester, MA) for the TBK-1 and IKK-ε expression plasmids, Dr. T. Fujita (Tokyo Metropolitan Institute, Tokyo) for the RIG-I expression plasmids, Dr. Z. Chen (University of Texas Southwestern Medical Center, Dallas) for the MAVS expression construct, and Dr. J. Tschopp (University of Lausanne, Switzerland) for the Cardif expression plasmid. BILN2061 was provided by Dr. R. Schooley (University of California at San Diego). We would also like to thank Dr. H. Maier for critical reading of the manuscript and excellent suggestions and Dr. S. Wieland for help with the manuscript assembly. This work was supported by National Institutes of Health Grant R01-CA108304. This is manuscript 18148-MEM from The Scripps Research Institute.
Abbreviations
- CARD
caspase recruitment domain
- HCV
hepatitis C virus
- IKK-ε
inhibitor of κB kinase ε
- IPS-1
IFN-β promoter stimulating factor 1
- IRF-3
IFN-regulatory factor 3
- ISG
IFN-stimulated gene
- ISRE
IFN-stimulated response element
- poly(IC)
poly(I)·poly(C)
- MAVS
mitochondrial antiviral signaling protein
- moi
multiplicity of infection
- NS
nonstructural protein
- RIG-I
retinoic acid-induced gene I
- TBK-1
Tank-binding kinase-1.
Footnotes
Conflict of interest statement: F.V.C. consults for several companies that are developing antiviral drugs and/or vaccines for HCV.
References
- 1.Hoofnagle J. H. Hepatology. 2002;36:S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 2.Alter H. J., Seeff L. B. Semin. Liver Dis. 2000;20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 3.Zhong J., Gastaminza P., Cheng G., Kapadia S., Kato T., Burton D. R., Wieland S. F., Uprichard S. L., Wakita T., Chisari F. V. Proc. Natl. Acad. Sci. USA. 2005;102:9294–9499. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindenbach B. D., Evans M. J., Syder A. J., Wolk B., Tellinghuisen T. L., Liu C. C., Maruyama T., Hynes R. O., Burton D. R., McKeating J. A., Rice C. M. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 5.Wakita T., Pietschmann T., Kato T., Date T., Miyamoto M., Zhao Z., Murthy K., Habermann A., Krausslich H. G., Mizokami M., et al. Nat. Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taniguchi T., Ogasawara K., Takaoka A., Tanaka N. Annu. Rev. Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 7.Major M. E., Rehermann B., Feinstone S. M. In: Fields Virology. Knipe D. M., Howley P. M., editors. Vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1127–1161. [Google Scholar]
- 8.Medzhitov R., Janeway C. A., Jr Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 9.Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 10.Andrejeva J., Childs K. S., Young D. F., Carlos T. S., Stock N., Goodbourn S., Randall R. E. Proc. Natl. Acad. Sci. USA. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto M., Sato S., Mori K., Hoshino K., Takeuchi O., Takeda K., Akira S. J. Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 12.Seth R. B., Sun L., Ea C. K., Chen Z. J. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T., Takahashi K., Sato S., Coban C., Kumar H., Kato H., Ishii K. J., Takeuchi O., Akira S. Nat. Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 14.Meylan E., Curran J., Hofmann K., Moradpour D., Binder M., Bartenschlager R., Tschopp J. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 15.Xu L. G., Wang Y. Y., Han K. J., Li L. Y., Zhai Z., Shu H. B. Mol. Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Sharma S., tenOever B. R., Grandvaux N., Zhou G. P., Lin R., Hiscott J. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald K. A., McWhirter S. M., Faia K. L., Rowe D. C., Latz E., Golenbock D. T., Coyle A. J., Liao S. M., Maniatis T. Nat. Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 18.Lin R., Heylbroeck C., Pitha P. M., Hiscott J. Mol. Cell. Biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talon J., Horvath C. M., Polley R., Basler C. F., Muster T., Palese P., Garcia-Sastre A. J. Virol. 2000;74:7989–7996. doi: 10.1128/jvi.74.17.7989-7996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foy E., Li K., Wang C., Sumpter R., Jr, Ikeda M., Lemon S. M., Gale M., Jr Science. 2003;300:1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 21.Foy E., Li K., Sumpter R., Jr, Loo Y. M., Johnson C. L., Wang C., Fish P. M., Yoneyama M., Fujita T., Lemon S. M., Gale M., Jr Proc. Natl. Acad. Sci. USA. 2005;102:2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X. D., Sun L., Seth R. B., Pineda G., Chen Z. J. Proc. Natl. Acad. Sci. USA. 2005;102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loo Y. M., Owen D. M., Li K., Erickson A. K., Johnson C. L., Fish P. M., Carney D. S., Wang T., Ishida H., Yoneyama M., et al. Proc. Natl. Acad. Sci. USA. 2006;103:6001–6006. doi: 10.1073/pnas.0601523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su A. I., Pezacki J. P., Wodicka L., Brideau A. D., Supekova L., Thimme R., Wieland S., Bukh J., Purcell R. H., Schultz P. G., Chisari F. V. Proc. Natl. Acad. Sci. USA. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegal F. P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P. A., Shah K., Ho S., Antonenko S., Liu Y. J. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 26.Bigger C. B., Guerra B., Brasky K. M., Hubbard G., Beard M. R., Luxon B. A., Lemon S. M., Lanford R. E. J. Virol. 2004;78:13779–13792. doi: 10.1128/JVI.78.24.13779-13792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King P., Goodbourn S. J. Biol. Chem. 1994;269:30609–30615. [PubMed] [Google Scholar]
- 28.Thibeault D., Bousquet C., Gingras R., Lagace L., Maurice R., White P. W., Lamarre D. J. Virol. 2004;78:7352–7359. doi: 10.1128/JVI.78.14.7352-7359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.