Abstract
The capacity of Streptococcus pneumoniae to produce capsular polysaccharide (CPS) is essential for virulence. The CPS biosynthesis proteins CpsB, CpsC, and CpsD function to regulate CPS production via tyrosine phosphorylation of CpsD. This mechanism of regulating CPS production is important for enabling S. pneumoniae to cause invasive disease. Here, we identify mutations affecting the attachment of CPS to the cell wall. These mutations were located in cpsC, such that CpsC functioned independently from CpsD tyrosine phosphorylation. These mutants produced WT levels of CPS, but were unable to cause bacteremia in mice after intranasal challenge. This finding suggests that cell-wall attachment of CPS is essential for invasive pneumococcal disease; production of WT levels of CPS alone is not sufficient. We also show that cpsB mutants, which lack the phosphotyrosine-protein phosphatase, produced less CPS than the WT strain, but attached substantially more CPS to their cell wall. Thus, the phosphorylated form of CpsD promotes attachment of CPS to the cell wall.
Keywords: pneumococcal pathogenesis, tyrosine phosphorylation
The pneumococcus Streptococcus pneumoniae is an important cause of invasive disease in human populations throughout the world, resulting in high morbidity and mortality. An important feature of S. pneumoniae is its capacity to produce capsular polysaccharide (CPS), which is structurally distinct for each of 90 known serotypes, and is essential for pneumococcal virulence (1, 2). Additionally, the ability to regulate the amount of CPS produced is very important for the survival of the pneumococcus in different host environments. Maximal expression of CPS is essential for systemic virulence because of its antiphagocytic properties (1). However, invasive disease is invariably preceded by asymptomatic colonization of the nasopharynx, and the thickness of the capsule influences the degree of exposure of other important pneumococcal surface structures, such as adhesins, which are required during this initial colonization phase. Magee and Yother (3) reported that although expression of CPS is required for colonization of the nasopharynx, substantially reduced levels of CPS expression appeared to be sufficient. Reduced levels of CPS expression have also been shown to increase the ability of pneumococci to adhere to epithelial cells in vitro (4–7).
The mechanisms involved in migration of pneumococci from the nasopharynx into the lungs and subsequently into the bloodstream are still unclear. It is thought that pneumococci are aspirated into the lungs and then adhere to bronchio-epithelial cells (8). This process is followed by cytokine-induced cell activation and inflammation and subsequent invasion of the bronchio-epithelial cells (9, 10). Pneumococci have been shown to accumulate within these cells and translocate throughout the lung tissue in mice. Pneumococci then migrate into the bloodstream and can be isolated from the blood 12 h postinfection (10). Whereas the antiphagocytic role of CPS has been clearly defined in systemic infection, the role of CPS in colonization of the nasopharynx and transition to invasive disease has not been well defined.
The first four genes in the pneumococcal CPS biosynthesis (cps) loci (cpsA–cpsD) are common to all serotypes studied, except types 3 and 37 (11, 12). These four genes all have been implicated in CPS regulation. A CpsA homologue has been shown to be a transcriptional activator of the cps locus in Streptococcus agalactiae (13). CpsC and CpsD are predicted to function together in polymerization and export of CPS, in a fashion similar to ExoP in exopolysaccharide production in Sino-rhizobium meliloti (14, 15) and Wzc from Escherichia coli K12 and K30 (16, 17). We have shown that CpsD, like Wzc, is an autophosphorylating protein-tyrosine kinase and demonstrated that CpsC is required for CpsD Tyr phosphorylation (18). We have also shown that CpsB is a manganese-dependent phosphotyrosine-protein phosphatase required to dephosphorylate CpsD (19). Furthermore, autophosphorylation of CpsD at Tyr in its carboxyl-terminal [YGX] repeat domain attenuates its activity, reduces the level of encapsulation, and negatively regulates CPS production (18, 20).
We have previously shown that regulation of CPS production via CpsD Tyr phosphorylation is required to facilitate migration of pneumococci into the bloodstream (21). Defined cpsD point mutants were unable to cause bacteremia after intranasal challenge of CD1 mice, even though they were capable of killing BALB/c mice after i.p. challenge (21). Understanding the role of CpsB in CpsD phosphorylation is critical for understanding this mechanism of regulating CPS production. In this study, we show that cpsB and cpsC mutants have altered ability to attach CPS to the cell wall and are impaired in their ability to cause bacteremia in mice after intranasal challenge. These findings indicate that regulation of CPS attachment to the cell wall via CpsD Tyr phosphorylation is important for invasive pneumococcal disease. Thus, we have identified an additional role for CpsB, CpsC, and CpsD in CPS attachment in S. pneumoniae.
Results
Construction of Erythromycin-Resistant (EmR) and Tetracycline-Resistant (TcR) Deletion–Insertion cpsB Mutants.
Derivatives of the type 2 S. pneumoniae strain D39 in which cpsB was replaced by either an erythromycin resistance gene (erm) or a tetracycline resistance gene (tetM), as shown in Fig. 1, and were constructed as described in Materials and Methods. When the EmR or TcR deletion–insertion transformants obtained were examined, two different phenotypes were observed. Whereas the majority of the transformants exhibited a small colony (SC) size, ≈1% exhibited a mucoid colony (MC) phenotype. The transformants showed stable expression of either the MC or the SC phenotype and expressed type 2 CPS as measured by quellung reaction. Two EmR and two TcR transformants expressing the MC phenotype were designated D39-BΔe1, D39-BΔe2 and D39-BΔt1, D39-BΔt2, respectively, and two each expressing the SC phenotype were designated D39-BΔe5, D39-BΔe6 and D39-BΔt5, D39-BΔt6, respectively.
Fig. 1.
Schematic representation of the cpsB mutants. The mutations introduced into various strains are shown schematically as follows: BΔe, cpsB replaced by erm; BΔt, cpsB replaced by tetM; BΔe:DY→F, cpsB replaced by erm and mutation of Y215F, Y218F, Y221F in the [YGX] repeat domain of cpsD; ABΔe, cpsA, and cpsB replaced by erm; AΔe:BNQ, cpsA replaced by erm and mutation of D119N; H201Q in CpsB; BCDΔe1, cpsB, cpsC, and cpsD replaced by erm.
Construction of EmR cpsA, cpsAB, and cpsBCD Mutants.
To determine whether the mutants expressing the MC phenotype produced CPS via the regular pathway involving CpsC and CpsD, EmR cpsBCD deletion–insertion mutants, as shown in Fig. 1, were constructed as described in Materials and Methods. The transformants obtained were all of a uniform rough appearance (colonies appeared small and dull), which was confirmed by quellung reaction. These results show that in the absence of CpsC and CpsD cpsB deletion transformants exhibiting the MC phenotype could not be isolated, confirming that CPS is produced via the normal pathway in the MC mutants. One cpsBCD deletion–insertion mutant, designated D39-BCDΔe1, was sequenced to verify the mutation.
We have previously shown that colonies of D39-AΔ, in which the cpsA gene had been deleted, were smooth but only half the size of D39 when grown on blood agar (BA) plates (21). To determine whether CpsA plays a role in the expression of the mucoid phenotype, EmR cpsA and cpsAB deletion–insertion mutants, as shown in Fig. 1, were constructed as described in Materials and Methods. When the EmR transformants in which only the cpsA gene had been deleted were examined, they exhibited the same colony morphology as the previous D39-AΔ mutant (21). However, the EmR transformants in which both cpsA and cpsB had been deleted exhibited two phenotypes as seen for the cpsB deletion transformants described above. Most EmR transformants exhibited the SC phenotype but ≈1% of colonies exhibited the MC phenotype. These results show that CpsA does not play a role in the expression of the MC and SC phenotypes. One EmR transformant expressing the MC phenotype was designated D39-ABΔe1, and one expressing the SC phenotype was designated D39-ABΔe5.
To determine whether the SC and MC phenotypes are affected by either the absence of the CpsB protein or the loss of its phosphatase activity, a D39 double mutant in which the cpsA gene was replaced by erm and defined point mutations were introduced into cpsB (D119N, H201Q; ref. 19) was constructed as described in Materials and Methods. The resultant EmR transformants again displayed the two distinct phenotypes, confirming that the SC and MC phenotypes result from the loss of CpsB phosphatase activity. One transformant expressing the MC phenotype was designated D39-AΔe:BNQ2, and one expressing the SC phenotype was designated D39-AΔe:BNQ6.
Characterization of a cpsBΔ/cpsDY→F Double Mutant.
We have previously reported two S. pneumoniae mucoid mutants, D39-DY→F and Rx1–19F-DY→F (20, 21). In these mutants the cpsD gene was altered so that the Tyr residues in the C-terminal [YGX] repeat of CpsD were replaced by Phe, thereby preventing CpsD from becoming phosphorylated; the strains exhibited a mucoid phenotype when grown on BA (20, 21). It is unclear whether this mucoid phenotype is the same as the MC phenotype arising in D39 when cpsB mutants are constructed. Rx1–19F-BΔ:DY→F, a double mutant in which the cpsB gene was also deleted, also exhibited a mucoid phenotype and produced similar amounts of CPS compared with Rx1–19F-DY→F (20). These results suggested that in the presence of CpsDY→F, which cannot be phosphorylated, CpsB has no effect on CPS production. To determine whether deletion of cpsB also has no effect on CPS production in a D39-DY→F background, a double mutant in which the cpsB gene was replaced by erm and defined point mutations were also introduced into cpsD (Y215F; Y218F; Y221F) was constructed as described in Materials and Methods (Fig. 1). As expected, all of the EmR transformants obtained had a mucoid phenotype, and no SC EmR transformants were detected. These results suggest that if CpsD cannot be phosphorylated, as in CpsDY→F, the absence of CpsB has no additional effect on CPS production. One such transformant was designated D39-BΔe:DY→F.
Analysis of cpsB Mutants.
The effect of cpsB mutations on CpsD Tyr phosphorylation was investigated by Western immunoblotting (see Fig. 5, which is published as supporting information on the PNAS web site). As expected, the amount of phosphorylated CpsD (CpsD∼P) detected in all cpsB mutants was greater than in the D39 WT strain except when either CpsD was not expressed (D39-BCDΔe) or the mutant CpsD protein could not be phosphorylated (D39-BΔe:DY→F), indicating that they all lack phosphotyrosine phosphatase activity. All cpsB deletion mutants produced similar amounts of CpsC and CpsD protein when compared with D39 (Fig. 5).
The cpsA–cpsD regions of the cpsB deletion–insertion mutants described above were subjected to DNA sequencing to identify any changes that may be responsible for the SC and/or MC phenotypes. Analysis of the DNA sequences revealed a few seemingly minor changes affecting the amino acid sequences of the encoded proteins. However, when the amino acid sequences of CpsC were aligned for all of the cpsB mutants sequenced, it was apparent that all of the MC cpsB mutants except the double mutant D39-BΔe:DY→F had one or more amino acid changes in CpsC, mostly in the amino-terminal half, as shown in Table 1. In contrast, most of the SC cpsB mutants showed no amino acid changes, and only a single amino acid change was present in both D39-BΔt5 and D39-BΔt6 (Table 1). By comparison, no changes were present in the amino acid sequence of CpsD (except the defined mutations in D39-BΔe:DY→F) and only a few changes affecting CpsA were found (Table 1). Thus, the SC phenotype is the true phenotype of cpsB mutants in D39. The sequence data suggest that the MC phenotype is probably caused by mutations in cpsC that alter the function of CpsC such that it acts independently of CpsB. The CpsC proteins containing the random amino acid changes will be collectively defined as CpsCMC to distinguish them from the WT CpsC protein.
Table 1.
Amino acid changes in CpsB, CpsC, and CpsD for various mutants
| Strain | Colony morphology | Amino acid changes in CpsA | Amino acid changes in CpsC | Amino acid changes in CpsD |
|---|---|---|---|---|
| D39-BΔe1 | MC | — | E135G | — |
| D39-BΔe2 | MC | T410A, P480K | V57G | — |
| D39-BΔt1 | MC | E334V | T52P, I87T, I212M | — |
| D39-BΔt2 | MC | — | T7P, V117I | — |
| D39-ABΔe1 | MC | Δ | Y40C, N61S | — |
| D39-AΔe:BNQ1 | MC | Δ | Y83F | — |
| D39-AΔe:BNQ2 | MC | Δ | N61S | — |
| D39-BΔe:DY→F | MC | A356V, A367V | — | Y214F, Y217F, Y220F |
| D39-BΔe5 | SC | S407P, G440V | — | — |
| D39-BΔe6 | SC | — | — | — |
| D39-BΔt5 | SC | S172G, Y449C | N6T | — |
| D39-BΔt6 | SC | — | N6T | — |
| D39-ABΔe5 | SC | Δ | — | — |
| D39-AΔe:BNQ6 | SC | Δ | — | — |
| 4832-BΔe1 | MC | T131M, G138E | V57A | — |
| 4832-BΔe2 | MC | — | L88P | — |
| 4832-BΔe3 | SC | N256D | I10V | D72V |
| 4832-BΔe4 | SC | — | E4G, A37T | E46G |
| 4861-BΔe1 | MC | M452I | V57A | — |
| 4861-BΔe2 | MC | — | V57A | — |
| 4861-BΔe3 | SC | — | — | — |
| 4861-BΔe4 | SC | — | A37T | D144E |
Characterization of the cpsC Mutations Present in D39 cpsB MC Mutants.
To determine whether the random amino acid changes in CpsCMC were indeed responsible for the MC phenotype, the cpsA–cpsD regions of D39-BΔe1 and D39-BΔe2 were amplified by PCR and transformed into D39. The EmR transformants still exhibited the two distinct phenotypes. However, approximately equal numbers of MC and SC transformants were obtained. Two MC and two SC EmR transformants were subjected to DNA sequencing. Whereas the MC transformants had the same amino acid changes in CpsCMC seen in the original MC mutants, the SC mutants had WT CpsC amino acid sequences. These data suggest that the amino acid changes in CpsCMC are indeed responsible for the MC phenotype observed in some D39 cpsB mutants.
To determine whether the MC phenotype of D39-BΔe1 was affected by the presence of CpsB, the cpsB gene was reintroduced into D39-BΔe1 by back transformation as described in Materials and Methods. The resultant erythromycin-sensitive transformants still exhibited the MC phenotype, indicating that the activity of CpsCMC had altered such that it was no longer regulated by CpsB, and hence, CpsD Tyr phosphorylation/dephosphorylation. Similar back transformants, in which the cpsB gene was restored into the SC mutant D39-BΔe5, resulted in a WT phenotype, indicating that the SC phenotype was caused by the loss of CpsB phosphatase activity.
Quantitation of CPS.
CPS was extracted from various MC and SC D39 mutants and D39, D39-AΔ, D39-BΔ, D39-DΔ, and D39-DY→F, after growth either on BA plates (CPS isolated from pneumococci grown on BA, total-CPS) or in Todd-Hewitt broth with 0.5% yeast extract (THY) [CPS isolated from pneumococci grown in THY (THY-CPS)]. The cell wall-associated CPS (CW-CPS) was also extracted from these strains after growth on BA. The CPS preparations were quantitated by using the uronic acid assay, as described in Materials and Methods.
Approximately 60% of the total-CPS produced by D39 when grown on BA was found to be cell wall-associated (Fig. 2A and B). Similarly, the amount of THY-CPS extracted from D39 was ≈50% of the total CPS extracted from the same number of D39 grown on BA (Fig. 2C). These results are not surprising as when D39 is grown in liquid culture some CPS is released into the growth media (22, 23). Much of this unattached CPS presumably remains loosely associated with the cell surface when grown on BA plates.
Fig. 2.
Comparison of the total amount of CPS produced by various mutants to the amount associated with the cell wall. The amount of total-CPS (A), CW-CPS (B), and THY-CPS (C) was measured, using the uronic acid assay, from (Left to Right) D39, D39-BΔe1, D39-BΔe2, BΔt1, D39-BΔe5, D39-D39-BΔt5, D39-BCDΔe1, D39-AΔ, D39-ABΔe1, D39-AΔe:BNQ2, D39-ABΔe5, D39-AΔe:BNQ6, D39-BΔe:DY→F, D39-DY→F, and D39-DΔ. The results were graphed as percentage compared with D39 total-CPS.
The MC cpsB deletion–insertion mutants produced WT levels of total-CPS when grown on BA, whereas the SC cpsB mutants produced only ≈60% of WT CPS levels (Fig. 2A). However, this result is reversed when the amount of CW-CPS is examined; whereas the MC cpsB mutants had <50% of WT CW-CPS levels, the SC cpsB mutants had WT levels (Fig. 2B). The amount of THY-CPS extracted from the MC and SC cpsB mutants was similar to the amount of CW-CPS (Fig. 2B). These results indicate that even though the absence of CpsB in the SC cpsB mutants led to an overall reduction in the amount of CPS produced, the proportion of the total CPS produced that was attached to the cell wall compared with D39 increased. Additionally, these results show that the CpsCMC protein present in the MC cpsB mutants was unable to support WT levels of CPS attachment to the pneumococcal cell wall even though these mutants produced WT CPS levels.
The amount of total-CPS produced by the cpsA-cpsB double mutants was similar to that produced by D39-AΔ (Fig. 2A). The amounts of CW-CPS produced by these mutants showed the same trends as described above. The MC cpsAB mutants had approximately half as much CW-CPS as D39-AΔ and the SC cpsAB mutants had similar levels to D39-AΔ (Fig. 2B). The results when grown in THY showed a similar trend to that seen for the SC cpsB mutants described above (Fig. 2C).
The amount of CPS produced by the double mutant D39-BΔe:DY→F was similar to that produced by D39-DY→F under all conditions tested (Fig. 2). They produced approximately half as much total-CPS compared with D39 and had about a third as much CW-CPS, indicating that attachment of CPS to the cell wall was slightly affected by the DY→F mutations in cpsD (Fig. 2 A and B). This finding suggests that the mucoid phenotype seen in D39-DY→F, D39-BΔe:DY→F, and the MC cpsB mutants may be caused by defects in the attachment of CPS to the cell wall.
Construction of EmR cpsB Mutants in Different Pneumococcal Serotypes.
To determine whether the generation of cpsCMC mutants expressing the MC phenotype was specific to D39, or occurred in other serotypes, EmR cpsB deletion–insertion transformants (as for BΔe in Fig. 1) were constructed in two clinical isolates WCH4861 (type 6A) and WCH4832 (type 17). Both SC and MC EmR transformants were isolated from the two clinical isolates, demonstrating that the phenomenon does occur in other pneumococcal serotypes. For each clinical isolate, two EmR SC transformants, designated 4861-BΔe3, 4861-BΔe4 and 4832-BΔe3, 4832-BΔe4, and two EmR MC transformants, designated 4861-BΔe1, 4861-BΔe2 and 4832-BΔe1, 4832-BΔe2, were selected and their cpsA–cpsD regions were subjected to DNA sequencing. Interestingly, three of the four MC mutants had the same amino acid change in CpsCMC (V57A, Table 1). This amino acid was also mutated to glycine in D39-BΔe2, suggesting that this amino acid is important for CpsC function. Three of the four SC mutants also had amino acid changes in CpsC and CpsD but those changes did not lead to the MC phenotype and presumably did not affect the function of CpsC and/or CpsD (Table 1).
Intranasal Challenge Studies.
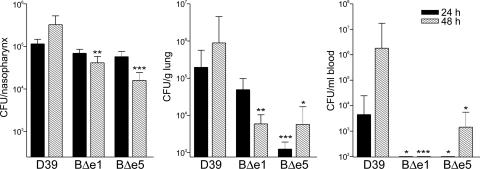
The abilities of D39, D39-BΔe1 (MC), and D39-BΔe5 (SC) to colonize the mouse nasopharynx, survive in the lungs, and migrate to the bloodstream were compared. CD1 mice were used for these experiments because they are susceptible to invasive pneumococcal disease via the intranasal route. CD1 mice were inoculated intranasally with 107 colony-forming units (CFU) for each strain. The mice were killed after 24 and 48 h, and the CFU in the nasopharynx, lungs, and blood were determined as described in Materials and Methods. The numbers of pneumococci recovered per nasopharynx, per g of lung tissue, and per ml of blood are shown in Fig. 3.
Fig. 3.
The number of pneumococci recovered from the nasopharynx, the lung, and the blood after intranasal infection of CD1 mice. The graphs show log10 CFUs (±SE) recovered from 10 CD1 mice for D39, D39-BΔe1 (BΔe1), and BΔe5 (BΔe5) per nasopharynx (total CFUs from nasal wash plus excised nasopharynx, Left), per g of lung tissue (Center), and per ml of blood (Right) after 24 and 48 h. Significant differences when compared with D39, using Student’s unpaired t test (two-tailed), are indicated as follows: ∗∗∗, P < 0.005; ∗∗, P < 0.01; ∗, P < 0.05.
As expected, in all mice tested pneumococci colonized the nasopharynx. However, by 48 h the number of CFU recovered from the nasopharynx of mice infected with both D39-BΔe1 and D39-BΔe5 had decreased significantly when compared with D39 (P < 0.0025 and P < 0.0002, respectively; Fig. 3). There were no significant differences in the in vitro growth rates (in serum broth) of these strains over 5 h, as measured by either absorbance at 600 nm or viable count (unpublished data), suggesting that D39-BΔe1 and D39-BΔe5 may be cleared more efficiently than D39 in vivo.
Pneumococci could be recovered only from the lungs in some of the mice tested, as shown in Table 2, which is published as supporting information on the PNAS web site. At 24 h postinfection D39-BΔe5 was significantly impaired in its ability to infect the lungs when compared with D39 (P < 0.0003; Fig. 3). More bacteria could be recovered after 48 h, but the numbers were still significantly less than for D39 (P < 0.0184). D39-BΔe1 also showed reduced ability to survive in the lungs compared with D39 but the difference was only significant at 48 h (P < 0.008; Fig. 3).
A published study (10) suggested that pneumococci translocate from the lungs into the bloodstream, and as expected, all mice from which pneumococci were recovered in the bloodstream had infected lungs. Interestingly, pneumococci were not recovered from the bloodstream of any mice infected with either D39-BΔe1 or D39-BΔe5 after 24 h and from only three mice infected with D39-BΔe5 after 48 h compared with seven for D39 (P < 0.0143; Fig. 3 and Table 3, which is published as supporting information on the PNAS web site). This finding suggests that the deletion of the cpsB gene in D39-BΔe5 and the presence of cpsCMC in D39-BΔe1 greatly affected the ability of the pneumococcus to translocate into the bloodstream. In fact, the data indicate that the cpsCMC mutation that led to the CPS-cell wall attachment defect appeared to be more detrimental to the translocation process in D39 than loss of CpsB and its effect on regulation of CPS production via CpsD Tyr phosphorylation.
Adherence Studies.
The abilities of D39, D39-BΔe1 (MC), D39-BΔe5 (SC), and the rough strain D39-DΔ to adhere to A549 human type II pneumocytes were compared as described in Materials and Methods. As expected, the rough strain D39-DΔ was able to adhere ≈100-fold more efficiently than the WT D39 strain (P < 0.0003; Fig. 4). The SC mutant D39-BΔe5 was also able to adhere to A549 cells ≈2-fold more efficiently than D39 (P < 0.0326), but the MC mutant D39-BΔe1 was 4-fold less efficient (P < 0.0193; Fig. 4). These data correlate with the observed phenotypes of these mutants. The smaller colony size and the decreased amount of CPS produced by D39-BΔe5 compared with D39, even though they have the same amount of CPS attached to their cell walls, suggests it has a more compact CPS zone surrounding the cell, allowing D39-BΔe5 to adhere to A549 cells more efficiently than D39. Conversely, the MC morphology and the potentially large amount of unattached CPS produced by D39-BΔe1 (behaving similar to exopolysaccharide) could prevent close contact with the A549 cell surface, resulting in a decrease in adherence compared with D39.
Fig. 4.
The effect of cpsB and cpsC mutations on adherence of D39 to A549 cells. The graphs show log10 CFUs (±SE) recovered from each well containing A549 cells infected with D39, D39-BΔe1 (BΔe1), BΔe5 (BΔe5), or D39-DΔ (DΔ). Significant differences when compared with D39, using Student’s unpaired t test (two-tailed), are indicated as follows: ∗∗∗, P < 0.005; ∗∗, P < 0.01; ∗, P < 0.05.
Discussion
In this study we have shown that deletion of a single gene (cpsB) leads to two distinct phenotypes. Transformants in which the cpsB gene had been replaced by either the erm or tetM gene exhibited either a SC or a MC phenotype. Direct transformation of PCR products with antibiotic selection is an efficient way to generate a large number of transformants. In contrast, isolating the unmarked deletion mutants published previously (18–21, 24) requires screening of a large number of colonies to identify only a few mutants per transformation. Thus, the mucoid transformants that appear at relatively low frequency (1%) were not identified previously.
The MC phenotype observed in 1% of D39 cpsB mutants was caused by secondary mutations in cpsC. These mutations appear to be random, affecting primarily the amino-terminal half of CpsC (the first 135 aa of 231; Table 1) but all have similar affects on CPS biosynthesis. Only a few amino acid changes (all in the first 37 aa; Table 1) were identified in several SC cpsB mutants; these did not detectably affect the function of CpsC. This study identifies a role for CpsC in cell wall attachment of CPS; the MC mutants, containing CpsCMC, attached only about half as much CPS to the cell wall compared with D39 (Fig. 2). The amino acid changes in CpsCMC probably affected the interactions between CpsCMC and other proteins involved in CPS biosynthesis such as the hypothetical cell wall-CPS ligase. They also resulted in a loss of requirement for CpsB, and therefore, a loss of regulation of CPS biosynthesis via CpsD Tyr phosphorylation, which was demonstrated by reinstating the cpsB gene in the MC mutant D39-BΔe1, which failed to restore the WT phenotype.
We have previously hypothesized that CPS biosynthesis requires the switching of CpsD between its active (nonphosphorylated) and its inactive (phosphorylated) state. In its active state we hypothesize that CpsC and CpsD interact, allowing ATP to bind to CpsD, promoting interaction between CpsC and other proteins such as the polysaccharide polymerase, thereby enabling CPS biosynthesis/polymerization to proceed at a maximal level. When CpsD becomes phosphorylated, the interaction between CpsD∼P, CpsC, and other proteins changes such that CPS polymerization slows, promoting transfer of the CPS polymer to the undefined cell wall-CPS ligase. Dephosphorylation of CpsD∼P by CpsB, a manganese-dependent phosphotyrosine-protein phosphatase, then allows the cycle to be repeated (20, 21). In fact, both the MC and SC cpsB mutants support this model. CpsCMC, present in the MC mutants, functions independently of CpsB, and therefore CpsD Tyr phosphorylation, and these mutants show a defect in CPS-cell wall attachment. The absence of CpsB, and the consequential increase in the amount of CpsD∼P in the SC cpsB mutants, leads to a reduction in the rate of CPS biosynthesis and a substantial increase in the proportion of total CPS produced that is attached to the cell wall relative to D39 (Fig. 2). The factors that regulate the rates of CpsD Tyr phosphorylation and/or CpsB phosphatase activity have yet to be determined, although environmental oxygen concentration may be one of them (23).
The results reported in this article clarify the conflicting data that had been reported previously. Whereas we have reported that high levels of CpsD∼P resulted in decreased levels of CPS biosynthesis (18, 20, 21), Bender et al. (24) reported that cpsB deletion mutants, containing increased levels of CpsD∼P, produced increased levels of CPS compared with the WT strain. However, they only analyzed the amount of CW-CPS produced by their mutants (24). We have shown that SC cpsB deletion mutants produce only ≈60% of D39 WT CPS levels when grown on BA, even though they appear to produce the same amount of CW-CPS as D39 and even up to ≈130% of D39 CPS levels when grown in THY (Fig. 2 B and C). Weiser et al. (23) reported that decreased environmental oxygen levels increased the amount of both CPS and CpsD∼P detected in opaque variants of S. pneumoniae. In their study, they measured the levels of CPS produced by pneumococci grown in liquid culture. We have shown that the amount of CPS obtained from pneumococci grown in liquid culture (THY) is very similar to the amount of CW-CPS and does not reflect the total amount of CPS produced (Fig. 2). Based on our findings, one would predict that if the amount of CpsD∼P has been up-regulated by decreased environmental oxygen levels, it follows that the amount of CW-CPS should increase. High cellular levels of CpsD∼P reduces the overall rate of CPS biosynthesis and increases the rate of CPS attachment to the cell wall.
We have shown that regulation of CPS production via Tyr phosphorylation of CpsD plays an important role in virulence. Both the MC (D39-BΔe1) and SC (D39-BΔe5) mutants showed signs of early clearance from the nasopharynx; by 48 h the number of pneumococci present had reduced significantly compared with D39 (Fig. 3). They also infected the lungs significantly less efficiently than D39, with D39-BΔe5 recovered from only 3/10 infected mice at 24 and 48 h postinfection. D39-BΔe1 did not enter the bloodstream of mice infected intranasally and D39-BΔe5 could only be recovered after 48 h (Fig. 3 and Table 2). Previous studies using the mucoid D39-DY→F strain have shown that mucoid strains are capable of killing mice if infected i.p (21). These results show that the loss of CpsB and therefore the loss of regulation of CPS production and attachment to the cell wall via phosphorylation of Tyr in CpsD affect the ability of S. pneumoniae to migrate into the bloodstream and cause invasive disease. Furthermore, the amino acid mutations in CpsCMC, leading to decreased attachment of CPS to the cell wall, completely prevent the MC mutants from entering the bloodstream, which indicates that production of WT levels of CPS alone is insufficient for S. pneumoniae to cause invasive disease; the CPS needs to be attached to the pneumococcal cell wall. Further studies investigating the location of the mucoid strains in the lungs of infected mice compared with D39 may provide some insight into the exact nature of this defect.
Materials and Methods
Bacterial Strains.
The S. pneumoniae strains used in this study are listed in Table 3. Pneumococci were grown in serum broth, THY, or on BA as described (25). Where appropriate, either erythromycin or tetracycline was added to the media at concentrations of 0.2 μg/ml or 5 μg/ml, respectively. Production of type 2 capsule by pneumococci was assessed by quellung reaction using antisera obtained from Statens Seruminstitut, Copenhagen.
Construction of Pneumococcal Mutants.
Mutants in which the cpsB gene was deleted and replaced by either the erm or the tetM gene were constructed as described (21). Mutants in which cpsA, cpsAB, or cpsBCD were replaced by the erm gene were also constructed as described above. A double mutant in which the cpsB gene was deleted and the cpsD gene had defined mutations altering the three Tyr residues in the [YGX] repeat domain of CpsD to Phe (Y215F; Y218F; Y221F) was constructed as described above by using chromosomal DNA from the strain D39-DY→F (21) as template for the PCRs. A double mutant in which the erm gene replaced the cpsA gene and defined point mutations were introduced into cpsB was also constructed as described above. Transformants, in which the cpsB gene was restored into D39-BΔe1 and D39-BΔe1, were constructed by PCR amplifying the cpsAB region and the cpsCD region from D39 and either D39-BΔe1 or D39-BΔe5 chromosomal DNA, respectively. The two individual PCR products were then amplified together to obtain an overlap-extension PCR product that was transformed into either D39-BΔe1 or D39-BΔe5, as described above. Erythromycin-sensitive back transformants were selected as described (18).
Preparation of CPS from Pneumococci.
CPS samples were prepared by resuspending pneumococci grown either in THY or on BA plates in PBS, such that A600 = 0.5. An aliquot of 10 ml was pelleted in a bench centrifuge at 3,000 × g for 20 min and resuspended in 500 μl of 150 mM Tris·HCl (pH 7.0) and 1 mM MgSO4. The samples were then treated with 0.1% (wt/vol) deoxycholate (Sigma), 100 units of mutanolysin (Sigma), 50 μg DNase I (Roche Applied Science), 50 μg RNaseA (Roche Diagnostics), and 50 μg Proteinase K (Roche Diagnostics) as described (20). To determine the amount of CPS attached to the cell wall, pneumococci grown on BA plates were resuspended in PBS, as described above, pelleted, and then resuspended in 10 ml of PBS with 2% (wt/vol) SDS preheated to 100°C. The cells were heated at 100°C for 30 min, cooled to room temperature, and then centrifuged at 3,000 × g for 30 min. The cell walls were washed three times in PBS and then resuspended in 500 μl of 150 mM Tris·HCl (pH 7.0) and 1 mM MgSO4. The samples were then treated with mutanolysin and proteinase K, as described above.
Analysis of Pneumococcal CPS Samples.
The amount of CPS present in the samples was determined by using an assay for quantitative determination of uronic acids as described by Blumenkrantz and Asboe-Hansen (26).
Intranasal Colonization Model.
Animal experimentation was conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and was approved by the Animal Ethics Committee of the University of Adelaide. The method published previously (21) was modified as follows. The mice were perfused with sterile PBS before removal of the lungs and nasopharynx. Blood samples were collected from the heart of each mouse before perfusion. The experiment was performed twice, and the data were pooled. The number of CFU per nasopharynx was calculated by combining the nasal wash and the excised nasopharynx data.
Adherence Assay.
Adherence of pneumococci to A549 cells (human type II pneumocytes) was determined essentially as described (5). Assays were carried out in triplicate. The experiment was performed twice, and the data were pooled. The differences in adherence were analyzed by using prism 3.0 (GraphPad, San Diego).
Supplementary Material
Acknowledgments
We thank David Ogunniyi, Kim LeMessurier, Jan Cook, and Uwe Stroeher for their expert assistance with the intranasal challenge experiments. This research was supported by a program grant from the National Health and Medical Research Council of Australia.
Abbreviations
- BA
blood agar
- CFU
colony-forming units
- CPS
capsular polysaccharide
- CpsD∼P
phosphorylated CpsD
- CW-CPS
cell wall-associated CPS
- EmR
erythromycin-resistant
- MC
mucoid colony
- SC
small colony
- THY
Todd-Hewitt broth with 0.5% yeast extract
- THY-CPS
CPS isolated from pneumococci grown in THY
- total-CPS
CPS isolated from pneumococci grown on BA.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Austrian R. Rev. Infect. Dis. 1981;3(Suppl):S1–S17. doi: 10.1093/clinids/3.supplement_1.s1. [DOI] [PubMed] [Google Scholar]
- 2.Henrichsen J. J. Clin. Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magee A. D., Yother J. Infect. Immun. 2001;69:3755–3761. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selinger D. S., Reed W. P. Infect. Immun. 1979;23:455–458. doi: 10.1128/iai.23.2.545-548.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talbot U. M., Paton A. W., Paton J. C. Infect. Immun. 1996;64:3772–3777. doi: 10.1128/iai.64.9.3772-3777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamou J. E., Wizemann T. M., Barren P., Langermann S. Infect. Immun. 1998;66:820–822. doi: 10.1128/iai.66.2.820-822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammerschmidt S. Curr. Opin. Microbiol. 2006;9:12–20. doi: 10.1016/j.mib.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Catterall J. R. Thorax. 1999;54:929–937. doi: 10.1136/thx.54.10.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergeron Y., Ouellet N., Deslauriers A. M., Simard M., Olivier M., Bergeron M. G. Infect. Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadioglu A., Sharpe J. A., Lazou I., Svanborg C., Ockleford C., Mitchell T. J., Andrew P. W. FEMS. Microbiol. Lett. 2001;194:105–110. doi: 10.1111/j.1574-6968.2001.tb09454.x. [DOI] [PubMed] [Google Scholar]
- 11.Paton J. C., Morona J. K. In: Gram-Positive Pathogens. Fischetti V. A., Novick R., Ferretti J., Portnoy D., Rood J., editors. Washington, DC: Am. Soc. Microbiol.; 1999. pp. 201–213. [Google Scholar]
- 12.Llull D., Lopez R., Garcia E. Curr. Mol. Med. 2001;1:475–491. doi: 10.2174/1566524013363618. [DOI] [PubMed] [Google Scholar]
- 13.Cieslewicz M. J., Kasper D. L., Wang Y., Wessels M. R. J. Biol. Chem. 2001;276:139–146. doi: 10.1074/jbc.M005702200. [DOI] [PubMed] [Google Scholar]
- 14.Glucksmann M. A., Reuber T. L., Walker G. C. J. Bacteriol. 1993;175:7045–7055. doi: 10.1128/jb.175.21.7045-7055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidolin A., Morona J. K., Morona R., Hansman D., Paton J. C. Infect. Immun. 1994;62:5384–5396. doi: 10.1128/iai.62.12.5384-5396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent C., Doublet P., Grangeasse C., Vaganay E., Cozzone A. J., Duclos B. J. Bacteriol. 1999;181:3472–3477. doi: 10.1128/jb.181.11.3472-3477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paiment A., Hocking J., Whitfield C. J. Bacteriol. 2002;184:6437–6447. doi: 10.1128/JB.184.23.6437-6447.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morona J. K., Paton J. C., Miller D. C., Morona R. Mol. Microbiol. 2000;35:1431–1442. doi: 10.1046/j.1365-2958.2000.01808.x. [DOI] [PubMed] [Google Scholar]
- 19.Morona J. K., Morona R., Miller D. C., Paton J. C. J. Bacteriol. 2002;184:577–583. doi: 10.1128/JB.184.2.577-583.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morona J. K., Morona R., Miller D. C., Paton J. C. J. Bacteriol. 2003;185:3009–3019. doi: 10.1128/JB.185.10.3009-3019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morona J. K., Miller D. C., Morona R., Paton J. C. J. Infect. Dis. 2004;189:1905–1913. doi: 10.1086/383352. [DOI] [PubMed] [Google Scholar]
- 22.Cruz-Leal Y., Menendez T., Coizeau E., Espinosa R. R., Canaan L., Blanco F., Carmenate T., Chang J., Quinones D., Tamargo I., et al. Biotechnol. Appl. Biochem. 2006;44:101–108. doi: 10.1042/BA20060007. [DOI] [PubMed] [Google Scholar]
- 23.Weiser J. N., Bae D., Epino H., Gordon S. B., Kapoor M., Zenewicz L. A., Shchepetov M. Infect. Immun. 2001;69:5430–5439. doi: 10.1128/IAI.69.9.5430-5439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bender M. H., Cartee R. T., Yother J. J. Bacteriol. 2003;185:6057–6066. doi: 10.1128/JB.185.20.6057-6066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogunniyi A. D., Folland R. L., Briles D. E., Hollingshead S. K., Paton J. C. Infect. Immun. 2000;68:3028–3033. doi: 10.1128/iai.68.5.3028-3033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenkrantz N., Asboe-Hansen G. Anal. Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.