Abstract
The maintenance of synaptic functions is essential for neuronal information processing, but cellular mechanisms that maintain synapses in the adult brain are not well understood. Here, we report an activity-dependent maintenance mechanism of parallel fiber (PF)–Purkinje cell (PC) synapses in the cerebellum. When postsynaptic metabotropic glutamate receptor (mGluR) or inositol 1,4,5-trisphosphate (IP3) signaling was chronically inhibited in vivo, PF–PC synaptic strength decreased because of a decreased transmitter release probability. The same effects were observed when PF activity was inhibited in vivo by the suppression of NMDA receptor-mediated inputs to granule cells. PF–PC synaptic strength similarly decreased after the in vivo application of an antibody against brain-derived neurotrophic factor (BDNF). Furthermore, the weakening of synaptic connection caused by the blockade of mGluR–IP3 signaling was reversed by the in vivo application of BDNF. These results indicate that a signaling cascade comprising PF activity, postsynaptic mGluR–IP3 signaling and subsequent BDNF signaling maintains presynaptic functions in the mature cerebellum.
Keywords: adult mouse, cerebellum, metabotropic glutamate receptor, neuronal activity, retrograde signaling
Activity-dependent changes in synaptic functions are essential in both developing and adult brains. Certain patterns of conjunctive presynaptic and postsynaptic activities result in plastic changes in synaptic functions, which are thought to underlie learning and memory (1–3). On the other hand, the synaptic functions of remaining sites should be maintained at a basal level for the maintenance of neural circuits in the adult brain. Because synaptic activity is required for the stabilization of synapses at adult neuromuscular junctions (4), it is possible that an ongoing synaptic activity is also required for the maintenance of synaptic functions in the central nervous system. However, cellular mechanisms that underlie the maintenance of synaptic functions in the adult brain are not well understood.
To determine whether ongoing synaptic activity is required for synaptic maintenance in the adult brain, we examined synaptic functions after the chronic inhibition of the activity-dependent signaling cascade in the mouse cerebellum. At cerebellar parallel fiber (PF)–Purkinje cell (PC) synapses, two types of glutamate receptor are present on the postsynaptic membrane: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and type 1 metabotropic glutamate receptors (mGluR1s) (5–7). Whereas AMPA receptors mediate rapid excitatory postsynaptic currents (EPSCs), mGluR1 activation generates inositol 1,4,5-trisphosphate (IP3) and diacylglycerol via the hydrolysis of phosphatidylinositol 4,5-bisphosphate by phospholipase C-β. IP3 then releases Ca2+ from the endoplasmic reticulum via IP3 receptors (8, 9). Because mGluR1 is located in the peripheral region of the postsynaptic densities (10), a repetitive stimulation of PFs at high frequencies is required for mGluR1 activation, leading to the generation of intracellular IP3 signalings (11–13). Thus, it is unlikely that postsynaptic mGluR1 is sufficiently activated by the spontaneous activity of PFs, because of the low spontaneous firing rate (0.5 Hz) of granule cells that extend PFs (14). On the other hand, mossy fiber inputs to granule cells generate high-frequency bursts (three to four pulses at >70 Hz) of PF action potentials (14) sufficient for generating mGluR1–IP3 signaling in PCs. That is, mGluR1–IP3 signaling in PCs may function as a neuronal activity detector in vivo, responding to sensory inputs from mossy fibers.
Thus, we first examined the changes in synaptic functions after a chronic in vivo suppression of IP3 production in PCs by expressing exogenous IP3 5-phosphatase (5-Ppase), which selectively hydrolyzes IP3 (15, 16). Interestingly, the suppression of IP3 production in PCs decreased the probability of transmitter release from PFs but not from climbing fibers (CFs). We then examined both upstream and downstream IP3 signaling in PCs. Our results indicated that the blockade of an ongoing PF activity-dependent postsynaptic mGluR1 activation also decreased the release probability of transmitters from PFs. Moreover, we found that the application of exogenous brain-derived neurotrophic factor (BDNF) rescued the attenuation of synaptic functions in IP3 5-Ppase-expressing PCs. Consistent with this observation, the chronic neutralization of endogenous BDNF with function-blocking antibodies not only inhibited transmitter release from PFs but also occluded the effect of the chronic suppression of IP3 production in PCs. These results demonstrate that neuronal activity-dependent postsynaptic mGluR1–IP3 signaling is crucial for the maintenance of the presynaptic function at PF–PC synapses. Furthermore, our results suggest that BDNF carries a retrograde message downstream of the postsynaptic mGluR1–IP3 signaling to maintain the presynaptic function.
Results
Effect of Suppression of IP3 Signaling on Synaptic Functions.
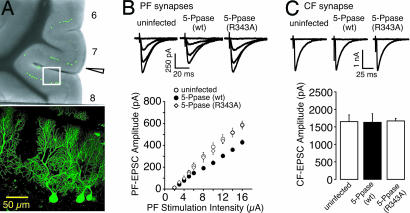
To study the roles of postsynaptic IP3 signaling in the maintenance of synaptic functions in vivo, we examined the effect of postsynaptic IP3 5-Ppase expression on synaptic transmission at PF–PC and CF–PC synapses. IP3 5-Ppase was expressed in PCs by using Sindbis virus carrying the gene encoding the enzyme. The virus also directed the expression of EGFP as the infection marker by internal ribosomal entry site-dependent translation. Twenty-four hours after the injection of the Sindbis virus into the cerebellum of postnatal day 23 (P23) to P29 mice, acute cerebellar parasagittal slices were prepared. Fluorescence signals of EGFP were observed in the cerebellar cortex near the injection site, and most of the fluorescence signals were detected in PCs (Fig. 1A). The apparent specificity for PCs is caused by the higher affinity of this virus to neurons than to glial cells and the much larger surface area of PCs than that of other neurons in the cerebellar cortex. We performed whole-cell recordings from infected or nearby uninfected PCs, and the stimulus strength-dependent graded responses of PF-mediated EPSCs (PF–EPSCs) and all-or-none CF-mediated EPSC (CF–EPSC) were recorded (17). Interestingly, we found that the input–output relationship of the amplitude of PF–EPSCs shifted downward in PCs expressing IP3 5-Ppase as compared with that in uninfected PCs (Fig. 1B). For control experiments, we generated a similar virus in which Arg-343 of IP3 5-Ppase was replaced by alanine. The mutation (R343A) results in the loss of enzyme activity (13, 18–20). We have already shown that IP3 5-Ppase, but not R343A–IP3 5-Ppase, blocks the production of IP3 in PCs under the same conditions by using an IP3 indicator, GFP-PHD (13). The expression of R343A–IP3 5-Ppase did not change the input–output relationship, showing that infection with the Sindbis virus alone did not influence the synaptic transmission (Fig. 1B). Thus, the observed decrease in the synaptic efficacy of PF inputs depended primarily on the suppression of IP3 production in PCs.
Fig. 1.
In vivo expression of IP3 5-Ppase in PCs decreases the PF–EPSC. (A) (Upper) Low-magnification image of cerebellar slice injected with virus carrying gene encoding IP3 5-Ppase (wt) and EGFP in vivo. Arrowhead indicates the site of the insertion of the viral injection pipette. (Lower) High-magnification image of region in white rectangle in Upper. GFP-expressing PCs were clearly imaged. (B) Representative traces of PF–EPSCs for indicated conditions of increasing stimulus intensities of 2, 8, 12, and 16 μA (holding potential = −80 mV). The amplitudes of PF–EPSC in uninfected PCs (○, n = 12), IP3 5-Ppase (wt)-expressing PCs (●, n = 11), and IP3 5-Ppase (R343A)-expressing PCs (◇, n = 12) are plotted as a function of stimulus intensity, indicating a decreased synaptic efficacy at PF synapses by in vivo expression of IP3 5-Ppase in PCs. (C) Representative traces of CF–EPSC for indicated conditions (holding potential = −10 mV). The amplitudes of CF–EPSC recorded from uninfected PCs (n = 10), IP3 5-Ppase (wt)-expressing PCs (n = 11), and IP3 5-Ppase (R343A)-expressing PCs (n = 11) indicate no significant decrease in CF–EPSC.
In contrast, there was no significant decrease in CF–EPSCs in terms of the amplitude (Fig. 1C), rise time (0.74 ± 0.04 ms for uninfected PCs, 0.77 ± 0.03 ms for IP3 5-Ppase-expressing PCs, and 0.78 ± 0.03 ms for R343A–IP3 5-Ppase-expressing PCs, n = 10–11), and decay time constant (13.1 ± 0.8 ms for uninfected PCs, 13.8 ± 0.9 ms for IP3 5-Ppase-expressing PCs, and 13.5 ± 0.9 ms for R343A–IP3 5-Ppase-expressing PCs). Therefore, the effect of IP3 5-Ppase on the synaptic strength was selectively observed in PF–PC synapses.
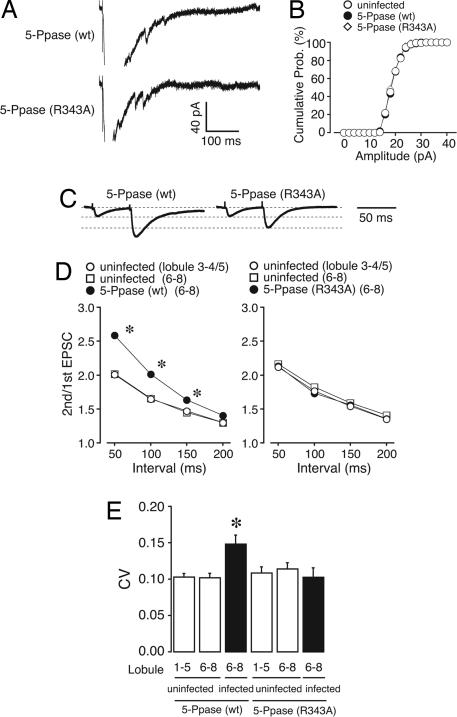
To study the mechanism underlying the decrease in PF–PC synaptic strength, we examined the changes in postsynaptic glutamate sensitivity. Quantal PF–EPSCs were analyzed after replacing extracellular Ca2+ with Sr2+ to induce asynchronous vesicle release (Fig. 2A) (21, 22). The frequency histogram of quantal PF–EPSC amplitudes in IP3 5-Ppase-expressing PCs showed an almost identical distribution to that in R343A–IP3 5-Ppase-expressing PCs (Fig. 2B), indicating that the postsynaptic glutamate sensitivity was not changed.
Fig. 2.
In vivo expression of IP3 5-Ppase in PCs decreases release probability at PF synapses. (A and B) Induced quantal EPSCs at PF synapses were recorded in a buffer solution containing Sr2+ (5 mM). (B) The histogram shows the distribution of amplitude recorded from uninfected PCs (○, 340 events from six cells), IP3 5-Ppase (wt)-expressing PCs (●, 364 events from eight cells), and IP3 5-Ppase (R343A)-expressing PCs (◇, 375 events from nine cells). There was no significant difference among the three groups (Kolmogorov–Smirnov test), indicating no change in postsynaptic glutamate sensitivity. (C and D) Paired-pulse plasticity of PF–EPSC. (C) Representative EPSCs were elicited by pairs of PF stimulations separated by 50 ms. The first EPSC is scaled to the amplitude of the first EPSC in PCs expressing IP3 5-Ppase (R343A). (D) PPRs were plotted as a function of the interval (n = 8). There was a significant increase in PPR in IP3 5-Ppase-expressing PCs, suggesting that the postsynaptic expression of IP3 5-Ppase decreases the release probability of neurotransmitters at PF synapses. (E) Average CV of PF–EPSC amplitude in PCs expressing IP3 5-Ppase (wt) or IP3 5-Ppase (R343A) (n = 8) was compared with that in uninfected PCs. Open and filled bars represent results from uninfected and infected PCs, respectively. There was no difference between lobules 1–5 and 6–8, showing that damage or other artifacts resulting from the viral injection procedure had no effect on neurotransmitter release. Only IP3 5-Ppase-expressing PCs show a significant increase in CV, which is consistent with the results in D.
Next, we examined the changes in presynaptic release probability. An inverse relationship between release probability and paired-pulse ratio (PPR) has been observed in various synapses (23), including PF–PC synapses (22). Whereas expression of R343A–IP3 5-Ppase did not significantly change the PPR of PF-EPSC as compared with that of uninfected PCs (Fig. 2D Right), there was a significant increase in PPR in IP3 5-Ppase-expressing PCs (Fig. 2D Left). This finding suggests that the postsynaptic expression of IP3 5-Ppase decreases the release probability of neurotransmitters from PF terminals. Consistent with these results, the coefficient of variation (CV) of the amplitude of PF–EPSCs, another indicator for the assessment of release probability, also markedly increased in IP3 5-Ppase-expressing PCs (Fig. 2E), showing the decrease in release probability. Moreover, there was no significant difference in either PPR or CV in CF–EPSCs with or without IP3 5-Ppase expression (Table 1, which is published as supporting information on the PNAS web site), consistent with the absence of the effect of IP3 5-Ppase expression on the amplitude of CF–EPSCs (Fig. 1C). Taken together, the synaptic function at PF–PC synapses was attenuated because of the decrease in presynaptic function when postsynaptic IP3 signaling was chronically blocked.
Effects of in Vivo Chronic Modulation of mGluR Activity on Presynaptic Function.
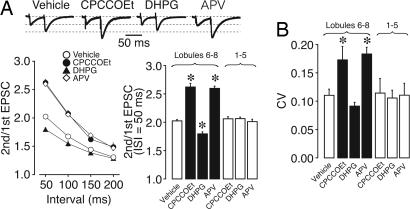
We then examined the effect of the pharmacological inhibition of upstream IP3 signaling by using a mGluR1 antagonist, 7-(hydroxyimino)cyclopropachromen-1a-carboxylate ethyl ester (CPCCOEt), to the mouse cerebellum by continuous infusion from ethylene-vinyl acetate copolymer (Elvax) implants (24–27). A small piece of Elvax was implanted on the surface of the cerebellum (lobules 6–8 of the vermis) on P23. Three to seven days after Elvax implantation, cerebellar slices were prepared and whole-cell recordings were obtained from PCs close to the Elvax implant (lobules 6–8). The chronic blockade of mGluR1 significantly increased the PPR and CV of PF–EPSCs compared with vehicle-treated PCs (Fig. 3). However, when measurements were obtained from PCs in the remaining regions (lobules 3–4/5, distant from the implant) as an internal control, no significant effect on PPR and CV was observed (Fig. 3 A Lower Right and B). This result indicates that the effects of CPCCOEt are confined to cerebellar lobules near the implant, and the local action of the mGluR1 antagonist is sufficient to attenuate presynaptic functions at PF synapses. The effect on PPR and CV was nearly constant 3–7 days after the Elvax implantation. When CPCCOEt-containing Elvax was implanted in the cerebellum for only 1 day, the change in PPR was ≈65% of that obtained 3 days after the implantation (n = 6). On the other hand, CPCCOEt did not decrease release probability at CF synapses 3–7 days after Elvax implantation (Table 1). These effects of the mGluR1 antagonist are the same as those of IP3 5-Ppase expression in PCs.
Fig. 3.
Local mGluR- or NMDAR-mediated activity regulates release probability at PF synapses. (A) Paired-pulse plasticity of PF–EPSC in vehicle-, CPCCOEt-, DHPG-, or APV-treated PCs. (Upper) Representative EPSCs elicited by pairs of PF stimulations separated by 50 ms. The first EPSC is scaled to the amplitude of first EPSC in control. (Lower Left) PPRs recorded from PCs at lobules 6–8 were plotted as a function of the interval (n = 7 for vehicle, n = 5 for CPCCOEt, n = 6 for DHPG, and n = 7 for APV). The application of CPCCOEt or APV increases PPR, whereas that of DHPG decreases PPR. (Lower Right) Summary bars of PPR show that CPCCOEt, DHPG, and APV were effective in lobules 6–8 (closest to the implants), but not in lobules 3–4/5 (distant from the implants), showing the local effect of drugs from Elvax. These results suggest that presynaptic function at PF synapses correlates with NMDAR and mGluR activity. (B) Average CV of PF–EPSC amplitude in CPCCOEt-, DHPG-, or APV-treated PCs (n = 5–7). ∗, Differences were considered significant when P < 0.05.
These results suggest that release probability at PF terminals correlates with mGluR1-dependent IP3 signaling in PCs. We further tested this hypothesis by the chronic application of the mGluR1 agonist (S)-3,5-dihydroxyphenylglycine (DHPG) from the Elvax implant. Fig. 3 shows that DHPG significantly decreased the PPR and CV of PF–EPSCs. The effect was observed only in the vicinity of the Elvax implant and was not observed in lobules 3–4/5 (distant from the implant). On the other hand, DHPG had no effect on the release probability at CF synapses (Table 1). Thus, sustained mGluR1 activation promoted release probability at PF terminals but not at CF terminals.
Effects of in Vivo Chronic Suppression of PF Inputs on Presynaptic Function.
The above results show that the chronic inhibition of mGluR1–IP3 signaling in PCs in vivo decreased transmitter release probability at PF–PC synapses. To assess whether the activation of mGluR1 is induced by ongoing PF activity, we suppressed the activity of granule cells that extend PFs. Granule cells receive NMDA receptor-mediated excitatory inputs from mossy fibers and generate bursts of action potentials (14, 28). Therefore, PF activity can be suppressed by an NMDA receptor antagonist, d-2-amino-5-phosphonovaleric acid (APV). On the other hand, CF–EPSCs recorded from PCs are insensitive to APV (5). We found that the chronic application of APV by continuous infusion from the Elvax implant significantly increased the PPR and CV of PF–EPSCs (Fig. 3), whereas there was no effect in distant lobules 3–4/5. In contrast, the chronic APV application had no effect on release probability at CF synapses (Table 1).
Because the chronic suppression of PF activity by APV had the same effects as those of the chronic inhibition of mGluR1–IP3 receptor signaling in PCs, ongoing PF activity, mGluR1 activation, and IP3 production may constitute a sequence of signaling events leading to the maintenance of presynaptic function at PF–PC synapses. However, the chronic suppression of PF activity may have an additional effect at presynaptic terminals, that is, transmitters released from terminals may have a direct effect on PF functions via a possible autocrine mechanism. If this is indeed the case, then APV application should have an additional effect on PF–PC synapses in IP3 5-Ppase-expressing PCs. However, there was no additional effect of APV on the PPR and CV of PF–EPSCs in IP3 5-Ppase-expressing PCs (Fig. 6, which is published as supporting information on the PNAS web site), indicating that the effects of APV and IP3 5-Ppase targeted the same mechanism underlying presynaptic maintenance.
We tested whether the effect of APV on presynaptic function is reversible. Mice that received chronic cerebellar application of APV from the Elvax implant exhibited impairment in motor coordination, and we estimated the duration of APV effect on the basis of their performance on a rotating rod (Fig. 7A, which is published as supporting information on the PNAS web site). Motor discoordination was observed for >7 days after Elvax implantation, whereas no significant difference was observed on day 14 caused likely by APV washout. An inverse time course of PPR at PF–PC synapses was observed in slices taken 1–14 days after the Elvax implantation (Fig. 7B). These results suggest that the effect of APV on release probability is reversible, and that the restoration of neuronal activity results in the recovery of presynaptic function.
Endogenous BDNF Maintains Presynaptic Function.
The results so far indicate that the signaling cascade leading to IP3 signaling in postsynaptic PCs regulates presynaptic PF release probability. This finding strongly suggests that there is a retrograde messenger generated in PCs and modulates transmitter release at PF terminals. Endocannabinoids suppress PF inputs to PCs through presynaptic CB1 receptor activation (29). We, therefore, examined the possibility that the activation of CB1 receptor is involved in the decreased release probability when IP3 5-Ppase is expressed in PCs. However, a CB1 receptor antagonist, AM251, had no effect on the PPR of PF–EPSC in IP3 5-Ppase-expressing PCs (Fig. 8, which is published as supporting information on the PNAS web site), indicating that endocannabinoids are not involved in the decrease in presynaptic function. On the other hand, recent studies suggest that BDNF has a neuronal activity-dependent effect on presynaptic functions at excitatory synapses (30–33). In the adult cerebellum, BDNF is highly expressed in PCs (34–36), and granule cells express the BDNF receptor TrkB (37, 38). In addition, BDNF–knockout mice show an impaired short-term plasticity at PF–PC synapses (39), suggesting the involvement of BDNF-mediated signaling in the regulation of presynaptic functions at PF–PC synapses. However, it has not been examined whether BDNF–TrkB signaling is involved in the maintenance of neurotransmission in the mature cerebellum. Thus, we assessed whether endogenous BDNF is involved in the maintenance of presynaptic functions.
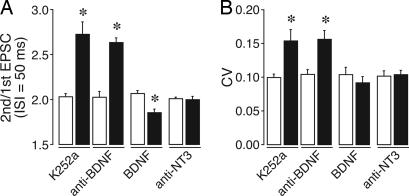
We first chronically inhibited the downstream BDNF–TrkB signaling by applying K252a, a general Trk–receptor blocker, from the Elvax implant. As shown in Fig. 4, the PPR and CV of K252a-treated PCs significantly increased, suggesting that an ongoing in vivo activation of TrkB is necessary for the maintenance of the release probability of PF terminals. On the other hand, K252a did not increase the PPR and CV of CF–EPSCs (Table 1). These results are consistent with those obtained with IP3 5-Ppase-expressing PCs. We also examined the possible effects of K252a on basal transmission at PF synapses. The bath application of K252a (200 nM) on cerebellar slices did not change the PPR of PF–EPSCs (Fig. 9B, which is published as supporting information on the PNAS web site), suggesting that K252a did not have an acute effect on the release probability of PF terminals.
Fig. 4.
BDNF signaling regulates release probability at PF synapses. (A) Average PPR of PF–EPSC (interstimulus interval = 50 ms) in K252a-, anti-BDNF IgG-, BDNF-, or anti-NT3-treated PCs (n = 5–10). Open and solid bars represent results for PCs recorded from lobules 1–4/5 (distant from the implants) and lobules 6–7 (closest to the implants), respectively (A and B). These results suggest that an ongoing activation of TrkB by BDNF in vivo is necessary for the maintenance of the presynaptic function at PF synapses. Representative EPSC data are shown in Fig. 9A. (B) Average CV of PF–EPSC amplitude (n = 5–10) in K252a-, anti-BDNF IgG-, BDNF-, or anti-NT3-treated PCs. ∗, Differences were considered significant when P < 0.05.
We next examined the effect of anti-BDNF IgG, which neutralizes BDNF activity (40, 41). The Elvax-mediated chronic application of anti-BDNF IgG induced a significant increase in the PPR and CV of PF–EPSCs (Fig. 4). To confirm the role of BDNF, we then examined the effect of chronic BDNF treatment and found that exogenous BDNF significantly decreased the PPR and CV of PF–EPSCs (Fig. 4). The magnitudes of changes in PPR and CV caused by BDNF were similar to those induced by the mGluR1 agonist DHPG (Fig. 3). Moreover, neither anti-BDNF IgG nor BDNF had any appreciable effect on the PPR and CV of CF–EPSCs (Table 1). Neurotrophin 3 (NT3) is a neurotrophic factor that is also highly expressed in the cerebellum (34, 42, 43). However, the Elvax-mediated chronic treatment with anti-NT3 IgG (44) had no effect on PF-EPSCs (Fig. 4) or CF–EPSCs (Table 1). We therefore concluded that BDNF is indispensable for the maintenance of transmitter release at PF–PC synapses in the mature cerebellum.
Anti-BDNF IgG had the same inhibitory effect on PF–PC neurotransmission as that of the inhibition of ongoing PF activity, the inhibition of mGluR1 activity, or the inhibition of postsynaptic IP3 signaling. This finding raises the possibility that BDNF is the downstream signal of postsynaptic IP3 signaling. Indeed, in cultured hippocampal neurons and neuronal lines, BDNF is released after the activation of mGluR (45). If BDNF is the retrograde modulator, the inhibitory effect of IP3 5-Ppase on PF–EPSCs should be reversed by the application of exogenous BDNF. We examined this possibility by the chronic application of BDNF to the IP3 5–Ppase-expressing cerebellum. Exogenous BDNF reversed the increase in the PPR or CV of PF–EPSCs in IP3 5-Ppase-expressing PCs (Fig. 5A and B). Similarly, BDNF also reversed the increase in PPR and CV by chronic CPCCOEt treatment (Fig. 5 A and B). On the other hand, there was no additional effect of anti-BDNF IgG on PF–EPSCs in the IP3 5-Ppase-expressing and CPCCOEt-treated PCs (Fig. 5 A and B), suggesting that the blockade of mGluR1–IP3 signaling share the same mechanism, leading to a decrease in release probability at PF terminals with the blockade of BDNF signaling.
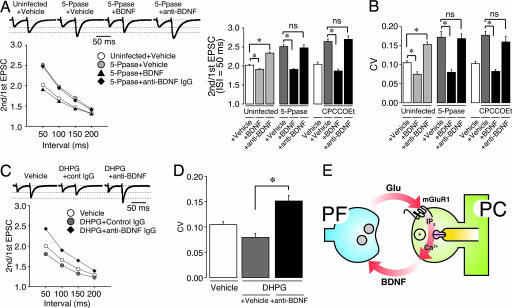
Fig. 5.
Application of BDNF or anti-BDNF IgG simultaneously with modulation of postsynaptic IP3 signaling shows predominant effect of BDNF on maintenance of presynaptic function at PF synapses. (A) Effect of application of vehicle, BDNF, or anti-BDNF IgG on paired-pulse plasticity of PF–EPSC in PCs treated with IP3 5-Ppase or CPCCOEt. (Upper) Representative EPSCs were elicited by pairs of PF stimulations separated by 50 ms. The first EPSC is scaled to the amplitude of first EPSC in control. Holding potential was −80 mV. (Lower Left) PPRs were plotted as a function of pulse interval, showing that in vivo application of BDNF rescued attenuated presynaptic function by blockade of IP3 signaling. In contrast, anti-BDNF IgG had no additive effect on PF-PPR in IP3 5-Ppase-expressing PCs. (Lower Right) Average PPR of PF-EPSC (interstimulus interval = 50 ms) in vehicle-, BDNF-, or anti-BDNF IgG-treated PCs with either IP3 5-Ppase expression or CPCCOEt treatment (n = 8–9). (B) Average CV of PF–EPSC amplitude in vehicle-, BDNF-, or anti-BDNF IgG-treated PCs with either IP3 5-Ppase expression or CPCCOEt treatment (n = 8–9). ns, not significant. ∗, Differences were considered significant when P < 0.05. (C) Effect of application of vehicle, or anti-BDNF IgG on paired-pulse plasticity of PF–EPSC in PCs treated with DHPG. PPRs recorded in PCs (n = 6–7) were plotted as a function of interval, suggesting that DHPG-induced PF presynaptic potentiation is mediated by endogenous BDNF. (D) Average CV of PF–EPSC amplitude in vehicle- or DHPG-treated PCs with or without anti-BDNF IgG treatment (n = 6–7). ∗, Differences were considered significant when P < 0.05. (E) Schema showing possible mechanism of maintenance of presynaptic function in PF–PC synapse.
To further examine whether endogenous BDNF functions downstream of mGluR1 activity in maintaining the PF function, we examined the relationship between the effects of the mGluR1 agonist DHPG and BDNF. If the effect of DHPG on PPR and CV of PF–EPSC (Fig. 3) is mediated by BDNF, anti-BDNF IgG should block the effect of DHPG. Indeed, the effect of DHPG was abolished by the simultaneous application of anti-BDNF IgG (Fig. 5 C and D), suggesting that DHPG-induced PF presynaptic potentiation is mediated by endogenous BDNF.
Discussion
The present study showed that release probability at the synaptic terminals of PFs is under the continuous regulation of the signaling cascade comprising an ongoing PF activity, mGluR1 activity, IP3 signaling in PCs, and subsequent BDNF signaling in vivo. The blockade of one of these signaling mechanisms had the same effect on PF–PC synapses, that is, decreased release probability, and masked or occluded the effect of the blockade of another mechanism. Thus, mGluR1–IP3–BDNF signaling mediates synaptic maintenance at PF–PC synapses. On the other hand, the blockade of this signaling cascade had no effect on CF–PC synapses.
Synaptic Maintenance Mediated by Metabotropic Signaling.
Synaptic maintenance has been extensively studied at adult neuromuscular junctions, where nicotinic acetylcholine receptor-mediated postsynaptic electrical activities play the major role in the maintenance of synaptic strength (4). Similarly, CF–PC synapses in the adult cerebellum are maintained by postsynaptic AMPA receptor-mediated depolarization (27). In sharp contrast, the present study shows that mGluR1–IP3 signaling rather than AMPA receptor-mediated depolarization is involved in the maintenance of PF–PC synaptic strength in the adult cerebellum. Indeed, the chronic blockade of AMPA receptors by its inhibitor induces no functional alteration in PF–PC synapses (27).
What may be the advantage of mGluR-mediated maintenance of synaptic functions? Because mGluR1 is located in the peripheral region of each PF–PC synaptic site (10), mGluR1–IP3 signaling in PCs may function as a PF activity detector in vivo, responding only to bursts of action potentials caused by sensory inputs from mossy fibers to granule cells (14). Therefore, mGluR1 activity in PCs provides a good measure of synaptic activities, filtering out spontaneous PF inputs not associated with sensory stimulation. In addition, the mGluR-mediated mechanism is more suited for synapse-specific maintenance. In CF–PC synapses or neuromuscular junctions, each postsynaptic cell receives a strong input from a single nerve fiber, and the synaptic input generates all-or-none postsynaptic action potentials. On the other hand, each PC receives synaptic inputs from numerous PFs at different dendritic spines, and the synaptic site-specificity is critically important in PF–PC synapses. The electrical signaling mediated by AMPA receptors spreads beyond the input sites through the dendritic arbor of PCs blurring the strong input specificity, particularly when high-frequency bursts of PF activity has reached the synapses. In contrast, both mGluR–IP3 signaling and BDNF actions are spatially restricted (11, 12, 46, 47). Therefore, mGluR1–IP3–BDNF signaling is suited for the input-specific regulation of synaptic functions by neuronal activities.
Possible Mechanisms Underlying Presynaptic Actions of Postsynaptic IP3 Signaling and BDNF Signaling.
The relationship between mGluR1–IP3 signaling and BDNF signaling remains to be clarified. One possibility is that BDNF is released postsynaptically downstream of IP3 signaling and acts directly on the presynaptic site as a retrograde messenger (48, 49) (Fig. 5E). It has been shown in cultured hippocampal neurons that BDNF is secreted in a mGluR-, phospholipase C-, and Ca2+ release-dependent manner (45). Although this mechanism has not yet been examined in PCs, it is notable that all of the required molecular components are present in PCs. There are other possible mechanisms. For example, BDNF may act in an autocrine way through TrkB expressed in PCs (50) and activate other mechanisms, which in turn have a retrograde effect on the presynaptic function. Indeed, BDNF leads to an activation of IP3 signaling in neurons via TrkB activation (51). However, BDNF-mediated IP3 signaling in PCs is probably not the major mechanism of presynaptic maintenance, because extrinsic BDNF reversed the inhibitory effect of postsynaptically expressed IP3 5-Ppase (Fig. 5 A and B). Future studies should elucidate the molecular mechanism of the function of BDNF.
Requirement of Neuronal Activity for Maintenance of Synaptic Functions in Mature Brains.
Several studies have indicated that experience-dependent neuronal activity is required for the establishment of precise synaptic connections in developing brains, endowing neural circuits with specific functions (52–56). On the other hand, once established, synaptic functions must be maintained for reliable information processing and storage throughout life. The present study highlights the role of neuronal activity in the mature brain, and we found attenuation of PF–PC synapses after the chronic blockade of ongoing PF activity, postsynaptic mGluR-mediated IP3 production, and subsequent BDNF signaling in vivo. Thus, experience-dependent neuronal activity and subsequent metabotropic signaling cascade are crucial for the maintenance of synaptic functions in mature brains and functional development in immature brains. Our results provide an understanding of the functional significance of an ongoing neuronal activity throughout life.
Materials and Methods
Viral Infection of PCs.
To chronically suppress IP3 production, we expressed IP3 5-Ppase in PCs by using Sindbis virus carrying the gene encoding the enzyme. We delivered virus-containing medium to the cerebella of mice (C57BL/6) at P23 by air pressure applied through the glass pipette. Preparation of Sindbis viruses, their delivery into mouse cerebella, and infection of PCs were performed as detailed in Supporting Text, which is published as supporting information on the PNAS web site. The images shown in Fig. 1A were taken with a custom-made two-photon laser scanning microscope (13).
Implantation of Elvax.
To chronically regulate excitatory postsynaptic activity or neurotrophin-mediated activity in the cerebellum, we applied CPCCOEt, DHPG, APV, K252a, anti-BDNF IgG, anti-NT3 IgG, and BDNF locally to the mouse cerebellum by continuous infusion from Elvax implants. We implanted Elvax to the cerebella of mice at P23. Preparation of Elvax pieces and their implantation into mouse cerebella are detailed in Supporting Text.
Electrophysiology.
Mice were killed by cervical dislocation under deep halothane anesthesia. Parasagittal cerebellar slices (250 μm thickness) were prepared as described (25, 57, 58). Recording, stimulation, and data acquisition were performed as detailed in Supporting Text.
Statistical Analyses.
All data are reported as mean ± SEM. The statistical test was the two-tailed Student’s t test. The Kolmogorov–Smirnov test was used to compare the cumulative probability distributions of quantal EPSC (Fig. 2B). Differences were considered significant when P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, and Technology of Japan.
Abbreviations
- PC
Purkinje cell
- PF
parallel fiber
- CF
climbing fiber
- mGluR
metabotropic glutamate receptor
- mGluR1
type 1 mGluR
- IP3
inositol 1,4,5-trisphosphate
- 5-Ppase
5-phosphatase
- BDNF
brain-derived neurotrophic factor
- NT3
neurotrophin 3
- PPR
paired-pulse ratio
- CV
coefficient of variation
- Elvax
ethylene-vinyl acetate copolymer
- CPCCOEt
7-(hydroxyimino)cyclopropa chromen-1a-carboxylate ethyl ester
- DHPG
(S)-3,5-dihydroxyphenylglycine
- APV
d-2-amino-5-phosphonovaleric acid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- EPSC
excitatory postsynaptic current
- Pn
postnatal day n.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bliss T. V., Collingridge G. L. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Ito M. Annu. Rev. Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 3.Malenka R. C., Bear M. F. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Balice-Gordon R. J., Lichtman J. W. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- 5.Llano I., Marty A., Armstrong C. M., Konnerth A. J. Physiol. (London) 1991;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tempia F., Miniaci M. C., Anchisi D., Strata P. J. Neurophysiol. 1998;80:520–528. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- 7.Shigemoto R., Abe T., Nomura S., Nakanishi S., Hirano T. Neuron. 1994;12:1245–1255. doi: 10.1016/0896-6273(94)90441-3. [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi S. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 9.Berridge M. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 10.Nusser Z., Mulvihill E., Streit P., Somogyi P. Neuroscience. 1994;61:421–427. doi: 10.1016/0306-4522(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 11.Finch E., Augustine G. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 12.Takechi H., Eilers J., Konnerth A. Nature. 1998;396:757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- 13.Okubo Y., Kakizawa S., Hirose K., Iino M. J. Neurosci. 2004;24:9513–9520. doi: 10.1523/JNEUROSCI.1829-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chadderton P., Margrie T., Häusser M. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- 15.Majerus P. W. Annu. Rev. Biochem. 1992;61:225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- 16.Laxminarayan K. M., Chan B. K., Tetaz T., Bird P. I., Mitchell C. A. J. Biol. Chem. 1994;269:17305–17310. [PubMed] [Google Scholar]
- 17.Konnerth A., Llano I., Armstrong C. M. Proc. Natl. Acad. Sci. USA. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Communi D., Lecocq R., Erneux C. J. Biol. Chem. 1996;271:11676–11683. doi: 10.1074/jbc.271.20.11676. [DOI] [PubMed] [Google Scholar]
- 19.Hirose K., Kadowaki S., Tanabe M., Takeshima H., Iino M. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- 20.Okubo Y., Kakizawa S., Hirose K., Iino M. Neuron. 2001;32:113–122. doi: 10.1016/s0896-6273(01)00464-0. [DOI] [PubMed] [Google Scholar]
- 21.Abdul-Ghani M. A., Valiante T. A., Pennefather P. S. J. Physiol. (London) 1996;495:113–125. doi: 10.1113/jphysiol.1996.sp021578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lévénés C., Daniel H., Soubrié P., Crépel F. J. Physiol. (London) 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zucker R. S., Regehr W. G. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 24.Langer R., Folkman J. Nature. 1976;263:797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 25.Kakizawa S., Yamasaki M., Watanabe M., Kano M. J. Neurosci. 2000;20:4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakizawa S., Yamada K., Iino M., Watanabe M., Kano M. Eur. J. Neurosci. 2003;17:545–554. doi: 10.1046/j.1460-9568.2003.02486.x. [DOI] [PubMed] [Google Scholar]
- 27.Kakizawa S., Miyazaki T., Yanagihara D., Iino M., Watanabe M., Kano M. Proc. Natl. Acad. Sci. USA. 2005;102:19180–19185. doi: 10.1073/pnas.0504359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Angelo E., De Filippi G., Rossi P., Taglietti V. J. Physiol. (London) 1995;484:397–413. doi: 10.1113/jphysiol.1995.sp020673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown S. P., Brenowitz S. D., Regehr W. G. Nat. Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- 30.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 31.Poo M. M. Nat. Rev. Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 32.Lu B. Learn. Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu B., Pang P. T., Woo N. H. Nat. Rev. Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 34.Maisonpierre P. C., Belluscio L., Friedman B., Alderson R. F., Wiegand S. J., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 35.Kawamoto Y., Nakamura S., Nakano S., Oka N., Akiguchi I., Kimura J. Neuroscience. 1996;74:1209–1226. doi: 10.1016/0306-4522(96)00245-x. [DOI] [PubMed] [Google Scholar]
- 36.Neveu I., Arenas E. J. Cell Biol. 1996;133:631–646. doi: 10.1083/jcb.133.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao W. Q., Zheng J. L., Karihaloo M. J. Neurosci. 1995;15:2656–2667. doi: 10.1523/JNEUROSCI.15-04-02656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segal R. A., Pomeroy S. L., Stiles C. D. J. Neurosci. 1995;15:4970–4981. doi: 10.1523/JNEUROSCI.15-07-04970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter A., Chen C., Schwartz P., Segal R. J. Neurosci. 2002;22:1316–1327. doi: 10.1523/JNEUROSCI.22-04-01316.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohn J., Aloyz R. S., Toma J. G., Haak-Frendscho M., Miller F. D. J. Neurosci. 1999;19:5393–5408. doi: 10.1523/JNEUROSCI.19-13-05393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh K. K., Miller F. D. Neuron. 2005;45:837–845. doi: 10.1016/j.neuron.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 42.Rocamora N., Garcia-Ladona F. J., Palacios J. M., Mengod G. Brain Res. Mol. Brain Res. 1993;17:1–8. doi: 10.1016/0169-328x(93)90065-w. [DOI] [PubMed] [Google Scholar]
- 43.Wetmore C., Ernfors P., Persson H., Olson L. Exp. Neurol. 1990;109:141–152. doi: 10.1016/0014-4886(90)90068-4. [DOI] [PubMed] [Google Scholar]
- 44.Jin L., Hu X., Feng L. J. Neurochem. 2005;93:1251–1261. doi: 10.1111/j.1471-4159.2005.03118.x. [DOI] [PubMed] [Google Scholar]
- 45.Canossa M., Gärtner A., Campana G., Inagaki N., Thoenen H. EMBO J. 2001;20:1640–1650. doi: 10.1093/emboj/20.7.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horch H. W., Katz L. C. Nat. Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X., Poo M. M. Neuron. 2002;36:675–688. doi: 10.1016/s0896-6273(02)01023-1. [DOI] [PubMed] [Google Scholar]
- 48.Schinder A. F., Berninger B., Poo M. Neuron. 2000;25:151–163. doi: 10.1016/s0896-6273(00)80879-x. [DOI] [PubMed] [Google Scholar]
- 49.Hartmann M., Heumann R., Lessmann V. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein R., Martin-Zanca D., Barbacid M., Parada L. F. Development (Cambridge, U.K.) 1990;109:845–850. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- 51.Kovalchuk Y., Holthoff K., Konnerth A. Curr. Opin. Neurobiol. 2004;14:558–563. doi: 10.1016/j.conb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Purves D., Lichtman J. W. Science. 1980;210:153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- 53.Lohof A. M., Delhaye-Bouchaud N., Mariani J. Rev. Neurosci. 1996;7:85–101. doi: 10.1515/revneuro.1996.7.2.85. [DOI] [PubMed] [Google Scholar]
- 54.Katz L. C., Shatz C. J. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 55.Zhang L. I., Poo M. M. Nat. Neurosci. 2001;4(Suppl.):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- 56.Hua J. Y., Smith S. J. Nat. Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- 57.Edwards F. A., Konnerth A., Sakmann B., Takahashi T. Pflügers. Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- 58.Kano M., Hashimoto K., Chen C., Abeliovich A., Aiba A., Kurihara H., Watanabe M., Inoue Y., Tonegawa S. Cell. 1995;83:1223–1231. doi: 10.1016/0092-8674(95)90147-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.