Abstract
Although it is now more than two decades since it was first reported that the imidazobenzodiazepine Ro15-4513 reverses behavioral alcohol effects, the molecular target(s) of Ro15-4513 and the mechanism of alcohol antagonism remain elusive. Here, we show that Ro15-4513 blocks the alcohol enhancement on recombinant “extrasynaptic” α4/6β3δ GABAA receptors at doses that do not reduce the GABA-induced Cl− current. At low ethanol concentrations (≤30 mM), the Ro15-4513 antagonism is complete. However, at higher ethanol concentrations (≥100 mM), there is a Ro15-4513-insensitive ethanol enhancement that is abolished in receptors containing a point mutation in the second transmembrane region of the β3 subunit (β3N265M). Therefore, α4/6β3δ GABA receptors have two distinct alcohol modulation sites: (i) a low-dose ethanol site present in α4/6β3δ receptors that is antagonized by the behavioral alcohol antagonist Ro15-4513 and (ii) a site activated at high (anesthetic) alcohol doses, defined by mutations in membrane-spanning regions. Receptors composed of α4β3N265Mδ subunits that lack the high-dose alcohol site show a saturable ethanol dose-response curve with a half-maximal enhancement at 16 mM, close to the legal blood alcohol driving limit in most U.S. states (17.4 mM). Like in behavioral experiments, the alcohol antagonist effect of Ro15-4513 on recombinant α4β3δ receptors is blocked by flumazenil and β-carboline-ethyl ester (β-CCE). Our findings suggest that ethanol/Ro15-4513-sensitive GABAA receptors are important mediators of behavioral alcohol effects.
Keywords: alcohol intoxication, alcohol receptor, anesthetics
Although alcohol is one of the most widely used and abused drugs, the molecular targets that mediate alcohol effects at concentrations relevant for mild social intoxication are only beginning to be revealed. Neurotransmitter receptors for GABA, NMDA and glycine, and G protein-gated K+ channels have been identified as potential alcohol targets that are sensitive to intoxicating alcohol concentrations (1–4). GABAA receptors (GABAARs) have long been suspected to be important mediators of alcohol effects (5, 6) because benzodiazepines (BZs) and barbiturates, classic GABAAR agonists, share common pharmacological properties with ethanol, such as sedative-hypnotic, anti-anxiety, and motor in-coordinating and anticonvulsant effects and have additive, possibly even synergistic effects, when taken together with ethanol (7). In addition, BZs, barbiturates, and ethanol produce tolerance and cross-tolerance to each other (8), consistent with GABAARs as targets of action.
We have recently identified subtypes of GABAARs, those containing the δ and the β3 subunit, that are uniquely sensitive to low alcohol concentrations (9). Consistent with the view that δ subunit-containing receptors are important mediators of alcohol actions is the finding that GABAAR δ-subunit knockout mice show multiple defects in behavioral responses to ethanol (10). Receptors containing the δ subunit have an exclusively nonsynaptic distribution, are sensitive to low ambient extrasynaptic GABA concentrations, and display slow desensitization, properties that enable them to mediate a persistent (or tonic) form of inhibition (11). GABAAR δ subunits seem to almost exclusively associate with the GABAAR α4 and α6 subunits, two closely related and somewhat specialized α subunits that confer insensitivity to classical BZ agonists (but not the imidazobenzodiazepines flumazenil and Ro15-4513), because they carry the amino acid arginine (α6R100, α4R100 instead of histidine present in α1, -2, -3, and -5) at a site critical for BZ binding (12). We showed that the α6-R100Q “BZ-site” mutation, previously identified in alcohol-nontolerant rats (13), is sufficient for behavioral alcohol hypersensitivity, confers alcohol supersensitivity to tonic currents in rat cerebellar granule cells, and dramatically increases the alcohol sensitivity of recombinantly expressed receptors, if the α6R100Q subunit is expressed together with the β3 and the δ subunit (14). Therefore, an amino acid residue important for BZ sensitivity is also critical for low-dose alcohol sensitivity of α6-containing GABAAR.
Two decades ago it was reported that the imidazobenzodiazepine Ro15-4513, a structural analog of the clinically used general BZ antagonist flumazenil, is an alcohol antagonist in mammals (15, 16). The reversal of motor-impairing, sedative, anxiolytic, amnestic, and rewarding alcohol actions by Ro15-4513 at low to moderate alcohol doses were confirmed by various groups in different species (17–24). However, the debate whether Ro15-4513’s alcohol antagonism is due to a specific alcohol counteracting action, or due to nonspecific functional antagonism, due to the weak inverse agonist activity of Ro15-4513 on certain GABAAR subtypes (12), so far has not been resolved (18, 19, 25–28). Three observations argue against a nonspecific, inverse agonist action of Ro15-4513: (i) other inverse agonists are not alcohol antagonists (17, 18); (ii) GABAAR-mediated Cl− flux in synaptoneurosomes is enhanced by ethanol (17, 29–31) and this enhancement can be blocked by Ro15-4513 at concentrations that do not inhibit the GABA current (17); and (iii) in experiments where Ro15-4513 leads to a partial reversal of sedative-hypnotic alcohol actions, it did not reduce the hypothermic actions (32). Hypothermic and analgesic alcohol actions are at least partially mediated by G protein-gated potassium channels (3, 4, 33, 34).
Here, we show that the enhancement of δ subunit-containing recombinant GABAAR at low to moderately intoxicating ethanol doses (3–30 mM) is antagonized by the behavioral alcohol antagonist Ro15-4513, suggesting that these subtypes of GABAARs, previously thought to be completely insensitive to BZs, have a high-affinity Ro15-4513-binding site. At anesthetic and potentially lethal alcohol concentrations (≥100 mM), there is an additional Ro15-4513-insensitive component of ethanol enhancement. We show that this component disappears in α4β3δ receptors that contain a mutation in the β3 subunit (β3N265M). This mutation has been previously shown to essentially abolish (in β3N265M knockin mice) the in vivo anesthetic actions of etomidate and propofol (35), consistent with the identification of this residue in determining the β subunit selectivity for enhancement of recombinant GABAARs by loreclezole and etomidate (36, 37). Homologous residues in GABAAR subunits have been identified as important for high-dose alcohol and volatile anesthetic actions on recombinant GABAARs (38). As expected for a “true” receptor, α4β3N265Mδ GABAARs that lack the Ro15-4513-insensitive high-dose ethanol enhancement now show a saturable alcohol dose-response curve with a half-maximal effect at 16 mM. As previously shown in behavioral assays, Ro15-4513’s alcohol antagonism on recombinant receptors can be abolished by flumazenil. Most importantly, the fact that low-dose alcohol, as well as the BZ alcohol antagonist Ro15-4513, can exert its effects on common GABAAR subtypes identifies ethanol/Ro15-4513-sensitive GABAA receptors as important mediators of alcohol intoxication at low to moderate alcohol concentrations.
Results
Low-Dose Ethanol Enhancement on α4/6β3δ GABAARs Is Antagonized by Ro15-4513.
Inspired by reports that Ro15-4513 antagonizes most low-dose ethanol actions in animals (17, 19, 32) and that 100 nM Ro15-4513 blocks the low-dose (30 mM) alcohol enhancement in GABA-induced 36Cl− flux in brain homogenate assays (17, 39), we decided to investigate the effect of the BZ alcohol antagonist Ro15-4513 on the alcohol-induced current enhancement in α4/6β3δ receptors expressed in oocytes. Fig. 1 shows that 300 nM Ro15-4513 completely reversed the ethanol enhancement of α4β3δ GABAARs for ethanol concentrations up to 30 mM. This “anti-alcohol effect” of Ro15-4513 is surprisingly specific because, at concentrations where it abolished the alcohol-induced current increase (up to 300 nM), Ro15-4513 did not lead to a reduction in the “basal” GABA-induced current on these receptors; i.e., it is not an inverse agonist in this assay at concentrations that inhibit the ethanol-augmentation of GABA currents (Fig. 1c). The dose dependence of this effect revealed that the concentration of Ro15-4513 required to inhibit 50% of the 30 mM ethanol enhancement (IC50) was ≈10 nM (Fig. 1b). The alcohol antagonist action of low-dose Ro15-4513 suggests that, against common knowledge, α4/6β3δ GABAAR have a high-affinity Ro15-4513-binding site, with a Kd of ≈10 nM.
Fig. 1.
Ro15-4513 antagonizes ethanol effects on recombinant α4β3δ receptor currents. (a and b) To mimic tonic GABA current, 300 nM GABA was perfused onto Xenopus oocytes expressing rat α4β3δ receptors that were held at −80 mV, and currents were measured with a two-electrode voltage clamp system. The indicated doses of ethanol and drugs were applied. (a) Ro15-4513 (300 nM) completely antagonized ethanol enhancement up to an ethanol concentration of 30 mM. (b) Cumulative Ro15-4513 dose-response curve shows a dose-dependent inhibition of ethanol effects. GABA-evoked currents were blocked by 30 μM bicuculline. (c) GABA peak currents with and without ethanol and the indicated concentrations of Ro15-4513. Ro15-4513 led to a dose-dependent inhibition of (10, 30, and 50 mM) ethanol enhancement on α4β3δ receptors. At concentrations up to 300 nM, Ro15-4513 did not block the basal current on α4β3δ receptors (evoked by 300 nM GABA). (a and b) Representative recordings of six and five experiments, respectively.
At higher alcohol concentrations (≥100 mM), a fraction of the alcohol-induced enhancement was not blocked by 300 nM Ro15-4513 (Fig. 1a). This high-dose ethanol enhancement was not surmountable by increasing the Ro15-4513 concentrations (data not shown). Therefore, α4β3δ GABAARs have a distinct Ro15-4513-insensitive component of alcohol enhancement. To demonstrate the dose dependence, we applied increasing concentrations of Ro15-4513 to currents evoked by 300 nM GABA plus 10, 30, or 50 mM ethanol. Ro15-4513 led to a dose-dependent block that was complete for the 10- and 30-mM dose (with 300 nM Ro15-4513) (Fig. 1 b and c). Again, at the 50-mM ethanol dose, a small fraction of the alcohol enhancement was not blocked by Ro15-4513 (Fig. 1c). The complete and specific antagonism of low-dose alcohol effects on these receptors by Ro15-4513 suggests the intriguing possibility that Ro15-4513 might work by competitively displacing EtOH from its binding site.
Antagonizing Ro15-4513’s Alcohol Antagonism by Flumazenil and β-Carboline-Ethyl Ester (β-CCE).
Certain BZ site ligands, like the general BZ antagonist flumazenil (Ro15-1788) and the structurally unrelated BZ-site ligands β-CCE and FG7142, were shown to prevent the alcohol antagonist effects of Ro15-4513 in behavioral assays (17, 39). We reasoned that this result could be due to displacement of Ro15-4513 from its binding site by these compounds, which do not show alcohol antagonism by themselves (17, 39). We therefore tested four selected BZ site ligands for their ability to reverse or mimic Ro15-4513 antagonism of ethanol effects. We applied these compounds to α4β3δ receptors (expressed in oocytes) in the presence of 300 nM (≈EC20) GABA (to mimic tonic GABA current), 30 mM ethanol (to increase the GABA current), and 100 nM Ro15-4513 (to reverse the enhancement by 30 mM ethanol). Flumazenil (Ro15-1788) and β-CCE at 300 nM reversed the Ro15-4513-induced alcohol antagonism. However, the classical BZ agonist flunitrazepam, as well as DMCM (methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate) (a β-carboline with pronounced inverse agonist efficacy on γ2 subunit-containing receptors), each at 1 μM, did not reverse the effects of Ro15-4513 (Fig. 2a), indicating that the Ro15-4513/BZ-binding site on δ subunit-containing receptors is unique and binds only certain BZs and BZ site ligands with high affinity. None of the four compounds tested blocked ethanol enhancement on their own (data not shown). These data are consistent with previous findings that Ro15-4513’s alcohol antagonism can be antagonized by certain BZ-site ligands in 36Cl− flux assays in synaptoneurosomes and provide an in vitro correlate to the behavioral data that show that flumazenil and β-CCE can reverse the alcohol antagonist effects of Ro15-4513 (17, 39). A comparison of the structures of Ro15-4513 and flumazenil shows that these two molecules are identical, except for one moiety, which is an azido group in Ro15-4513 and a fluorine in flumazenil (Fig. 2b).
Fig. 2.
Ro15-4513 alcohol antagonism is antagonized by flumazenil and β-CCE, but not flunitrazepam and DMCM. (a) Currents were evoked by 300 nM GABA and potentiated by 30 mM ethanol, and this potentiation was reversed by 100 nM Ro15-4513. In constant presence of 300 nM GABA, 30 mM ethanol, and 100 nM Ro15-4513, the BZ site ligands Ro15-1788 (300 nM), β-CCE (300 nM), flunitrazepam, and DMCM (each 1 μM) were sequentially applied to test whether they reverse Ro15-4513’s ethanol antagonist effects. At the end of the recording, 30 μM bicuculline was used to block the GABA-induced current. Shown is a representative recording of a total of three experiments. (b) Chemical structures of the imidazobenzodiazepines Ro15-4513 and Ro15-1788 show that they differ only at one single position in the molecule. The clinically used BZ antagonist flumazenil (Ro15-1788) carries a fluorine at the C7 position of the BZ ring, whereas Ro15-4513 carries the larger azido group.
β-CCE Is a Positive GABA Modulator on α4β3δ Receptors.
We consistently observed that β-CCE led to an “overshoot” when we used it to antagonize the alcohol antagonist effects of Ro15-4513 on GABA/ethanol-induced currents (see Fig. 2), suggesting that β-CCE might potentiate alcohol effects on α4/6β3δ GABAAR. We therefore tested β-CCE alone and in combination with 3 mM ethanol for their functional effects on α4β3δ receptors. Fig. 3a shows that β-CCE not only enhances alcohol actions, but also increases the activity of α4β3δ GABAARs in the absence of alcohol. In the same way as alcohol effects on α4β3δ GABAAR, the β-CCE-induced enhancement of GABA currents is reversed by Ro15-4513 (Fig. 3b). A likely explanation for these findings is that β-CCE, as well as Ro15-4513, occupies an overlapping and mutually exclusive binding site, whereas β-CCE and ethanol might be able to bind next to each other in a side-by-side binding pocket, both microdomains blocked by Ro15-4513 (see Fig. 2b).
Fig. 3.
β-CCE enhances ethanol effects and is an agonist on α4β3δ receptors. (a) The β-carboline β-CCE at the indicated concentrations was applied alone or together with ethanol (always in the presence of 300 nM GABA) to oocytes expressing α4β3δ receptors, and peak GABA/Cl− currents were measured. (b) Dose-dependent reversal of 300 nM β-CCE enhancement of α4β3δ currents by Ro15-4513.
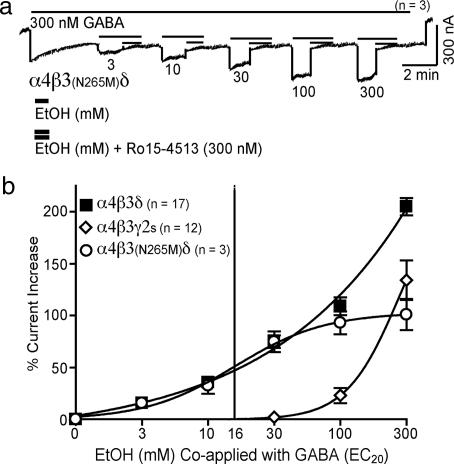
Loss of Ro15-4513-Insensitive Ethanol Actions in α4β3N265Mδ GABAAR.
The Ro15-4513-insensitive component of ethanol enhancement is observed at high alcohol concentrations (>30 mM), where most recombinant and native GABAAR show ethanol enhancement that is likely due to alcohol sites determined by mutations in the second and third transmembrane region of GABAARs (38). We show here (Fig. 4) that α4β3N265Mδ receptors, where the β3 WT subunit is replaced with the mutated β3N265M subunit, retain the Ro15-4513-sensitive alcohol enhancement. However, the β3N265M mutation completely abolished the Ro15-4513-insensitive ethanol enhancement observed at 100 and 300 mM ethanol (Fig. 4a), and even at 1 M ethanol (data not shown). GABAAR composed of α4β3N265Mδ and α4β3δ subunits show identical ethanol enhancement at alcohol concentrations up to 30 mM and differ only at the 100- and 300-mM dose (Fig. 4b). As a consequence of this loss of Ro15-4513-insensitive, high-concentration alcohol effects, recombinant α4β3N265Mδ receptors now have a saturable ethanol response curve with a half-maximal response of 16 mM [at EC20 (300 nM) GABA, Fig. 2b], a concentration close to the legal blood alcohol limit (17 mM) for driving in most US states.
Fig. 4.
A point mutation eliminates Ro15-4513-insensitive alcohol effects at high alcohol concentrations. (a) A single point mutation (β3N265M in membrane-spanning segment TM2 of the β3 subunit) abolishes the Ro15-4513-resistant alcohol enhancement observed at high ethanol concentrations in α4β3N265Mδ receptors. (b) Alcohol dose-response curve, determined by brief coapplications of ethanol and GABA EC20 (300 nM for α4β3δ and α4β3N265Mδ, and 30 μM GABA for α4β3γ2 GABAAR). Currents through α4β3N265Mδ GABAAR show a saturable alcohol enhancement and an EC50 of around 16 mM. The complete reversal of even very high dose alcohol effects by 300 nM Ro15-4513 in these experiments is surprising, because this behavior is not expected from an ideal competitive relationship of ligands with apparent dissociation constants (10 nM for Ro15-4513 and 16 mM for ethanol). The reasons why Ro15-4513 is so potent in antagonizing high-dose ethanol actions on functional receptors remain to be clarified.
Discussion
Extrasynaptic δ Subunit-Containing Receptors as Targets for Ethanol Action.
GABAARs containing the δ subunit have been shown to have an extra- or perisynaptic localization (40, 41) and give rise to tonic (sustained) GABA currents in neurons that express these GABAAR subunits (11). Recombinant GABAARs (α4/6βδ) known to mediate tonic currents, as well as δ subunit-containing receptor-mediated tonic currents in neurons, are augmented by low alcohol concentrations reached during social alcohol consumption (9, 14, 42–44). Whereas δ subunit-containing GABAARs comprise only between 5 and 10% of all of GABAARs in the mammalian brain (45), their constant or tonic activity (in marked contrast to synaptic receptors that open only for fractions of a second after synaptic GABA release) more than compensates in total charge transfer for the low abundance (46), and, therefore, extrasynaptic receptors are important regulators of neuronal excitability. The alcohol-induced augmentation of tonic currents in neurons and δ subunit-containing GABAARs at relevant physiologic alcohol doses is expected to lead to a decrease in neuronal excitability in neurons expressing these subunits and make these receptors excellent candidates for mediating acute alcohol effects at intoxicating concentrations. Our demonstration that the behavioral alcohol antagonist Ro15-4513 leads to a dose-dependent inhibition of ethanol-induced current enhancement in recombinant α4β3δ GABAARs provides strong support for this notion.
α4β3δ GABAAR Are Sensitive to Certain BZs and BZ-Site Ligands.
The discovery that the BZ Ro15-4513 reduces alcohol actions on α4/6β3δ receptors is unexpected because it has been thought that the γ2 subunit is required for high-affinity binding of BZs and most BZ-site ligands (47). In recombinantly expressed functional receptors, δ subunit-containing receptors have been shown to be insensitive to classical BZ agonists like diazepam and flunitrazepam (48, 49). Here, we reveal the activity of Ro15-4513 on α4/6β3δ receptors by their ability to act as an alcohol antagonist (Figs. 1, 2, and 4) whereas the binding of flumazenil and the BZ site ligand β-CCE is inferred from their ability to reverse Ro15-4513’s alcohol antagonism (Fig. 2a). Consistent with the view that α4/6β3δ GABAAR are insensitive to classical BZ agonists, we show that flunitrazepam does not antagonize the alcohol antagonistic actions of Ro15-4513 on recombinant α4β3δ receptors (Fig. 2).
Ethanol/Ro15-4531-Sensitive GABAAR in 36Cl− Flux Assays Show Striking Similarities with Recombinantly Expressed δ Subunit-Containing GABAAR.
Our data on the Ro15-4513 reversal are in agreement with previous work that showed that Ro15-4513 can abolish ethanol augmentation of Cl− flux in cerebral cortex synaptoneurosomal preparations and that the Ro15-4513’s alcohol antagonism is reversed by flumazenil and β-CCE (17, 39). Several lines of evidence support the view that the ethanol-sensitive Cl− flux through GABAAR in synaptosomal preparations may be mediated by alcohol-sensitive extrasynaptic receptors: (i) like δ subunit-containing receptors, this Cl− flux seems highly sensitive to GABA and muscimol (17, 29), and the ability to carry sustained 36Cl− flux suggests that they show slow desensitization; (ii) both δ subunit-containing receptors, as well as the 36Cl− flux, are strikingly similar in their low-dose response to physiologically relevant (3–30 mM) ethanol concentrations; (iii) we show here with recombinant receptors that, as previously observed in the 36Cl− flux assays (17), 30 mM ethanol augmentation is completely reversed by 300 nM of the behavioral alcohol antagonist Ro15-4513.
Why Are GABAAR as Alcohol Targets Controversial?
Our findings also provide an explanation for the controversial findings concerning the alcohol sensitivity of GABAARs in native neurons. Whereas most synaptic physiologists failed to find evidence for low-dose alcohol effects on GABAergic synaptic transmission (42, 50), there is abundant evidence that many neurons can respond to physiological (3–30 mM) ethanol concentrations at conditions that favor the detection of highly GABA-sensitive nondesensitizing extrasynaptic GABAARs (6, 51). Such conditions are, for example, the prolonged application of relatively low GABA and ethanol concentrations, like those resulting from local application of GABA/ethanol in the vicinity of neurons, conditions that synaptic physiologists might have considered nonphysiological. Consistent with this view and the results presented here, it has been shown that such low-dose alcohol enhancement of GABA currents in neurons (51) and the (presumably) resulting changes in firing frequency (52) can be reversed by the alcohol antagonist Ro15-4513 (51, 52). The fact that not all neurons express these alcohol/Ro15-4513-sensitive δ subunit-containing GABAARs is consistent with the observation that not all neurons are sensitive to pharmacologically relevant ethanol concentrations (6, 42, 51). Another complicating factor is that electrophysiological studies are often performed on neurons and slices harvested from immature brains that do not yet express δ subunit-containing extrasynaptic GABAAR (53). Consistent with a slow onset of expression of δ subunit-containing receptors during development and adolescence in rodents, reaching mature levels at around sexual maturation (54, 55), is the finding that younger animals are less sensitive to the sedative and motor-impairing effects of ethanol (56).
Whereas our experimental data explain and extend >20 yr of often puzzling observations concerning ethanol actions on GABAARs, a recent report that fails to reproduce low-dose ethanol enhancement on α4β3δ receptors, in particular negative results from the attempted expression of human α4β3δ receptors in oocytes and mammalian cell lines (57), likely ensures that our findings and related work (e.g., refs. 14, 17, 29, and 39) will remain controversial. We are puzzled by the differences, not only in the apparent lack of alcohol sensitivity, but also in the peak currents published by Borghese et al. (57), which are five times higher than we see with the (rat) clones that we provided to them. In marked contrast, the GABA responses from the human clones expressed in oocytes reported by Borghese et al. (57) are marginal, indicating a possible lack of expression of one or more of their human clones.
In our hands, variable ethanol responses in α4β3δ are likely due to the tendency of oocytes to express receptors that are composed of αβ subunits alone. GABAARs composed of αβ alone are insensitive to relevant alcohol concentrations and to classical BZs but show high sensitivity to etomidate, propofol, and steroid anesthetics (58, 59). The phenomenon of αβ GABAAR expression in oocytes has been well documented for γ2 subunit-containing receptors and explains the observed variability in BZ effects in α1β2γ2 receptors expressed in oocytes (60). The expression of αβ receptors in oocytes, highly sensitive to GABAAR anesthetics, is also the likely explanation for a previous challenge (58) to the finding that ε subunit-containing GABAARs lack anesthetic enhancement (59).
Behavioral Alcohol Effects Antagonized by Ro15-4513.
The alcohol antagonist Ro15-4513 has been reported to prevent many acute alcohol effects. These effects include increased exploration and locomotion at very low doses (0.25, 0.5, and 0.75 g/kg in rats) (61), the anxiolytic effects at low doses (1 g/kg) (17, 39), and sedative, motor-impairing as well as the amnestic effects at moderate alcohol doses (2 g/kg) (17, 19, 21, 62). In addition, the observation that Ro15-4513 reduces alcohol self-administration (23, 24, 63) suggests that the rewarding effects of ethanol might be mediated by ethanol/Ro15-4513-sensitive GABAARs. However, Ro15-4513 does not prevent all ethanol effects: at higher alcohol doses (≥2 g/kg in rats), Ro15-4513 significantly reduces, but does not prevent the anesthetic (“sleep”-inducing) effects of ethanol, and Ro15-4513 does not prevent the hypothermic effects of ethanol (32, 64). In addition, Ro15-4513 does not prevent lethal effects at massive alcohol doses (20, 27). This finding suggests that, at high alcohol doses, Ro15-4513-insensitive ethanol targets mediate the anesthetic ethanol effects. Our data on recombinant α4β3δ GABAARs suggest the possibility that, at such high alcohol concentrations (blood alcohol levels >30 mM), Ro15-4513-sensitive recombinant α4/6β3δ receptors have a high ethanol (>80%) occupancy and are therefore close to saturated.
Established targets of Ro15-4513-insensitive alcohol actions are alcohol-sensitive G protein-gated (GIRK) potassium channels (33) that likely contribute to the analgesic (4) and hypothermic effects of ethanol (34). Other potential alcohol targets that might contribute to Ro15-4513-insensitive acute alcohol actions are NMDA (1), glycine (2), and adenosine receptors (65), and voltage- and Ca2+-activated (BK) potassium channels (66, 67). In addition, we show here that the Ro15-4513-insensitive alcohol action on α4β3δ GABAAR is abolished by a mutation (β3N265M) in the β3 subunit. It is therefore possible that GABAARs (including the abundant synaptic γ2-containing GABAARs) may contribute to high-dose (≥30 mM) Ro15-4513-resistant, anesthetic ethanol actions.
Potential Mechanisms of Alcohol Antagonism by Ro15-4513.
A comparison of the structure of the alcohol and BZ antagonist Ro15-4513 and flumazenil, which differ only at a single moiety, suggests a possible mechanism of alcohol antagonism. The larger azido group (at the C7 position of the BZ ring) might be the group that occupies the alcohol-binding site on the receptor. Flumazenil and the β-carbolines β-CCE and FG7142 likely antagonize Ro15-4513 alcohol antagonist actions by displacing Ro15-4513 from its binding site. Flumazenil, β-CCE, and FG7142 do not act as alcohol antagonists by themselves, because they might fit together with ethanol in the Ro15-4513-binding pocket. Therefore, we think that the most parsimonious explanation for Ro15-4513’s alcohol antagonism is that the unique azido group in Ro15-4513 occupies the alcohol-binding site. However, there could be other possible mechanisms: e.g., the azido group in Ro15-4513 may cause allosteric changes in these receptors. We have used native immunopurified and recombinant expressed δ subunit-containing receptors in [3H]Ro15-4513-binding assays to show further evidence that Ro15-4513 and ethanol have a competitive relationship on α4/6βδ receptors (68).
Alcohol Receptors as Potential Drug Targets.
Our finding that alcohol effects on α4β3δ receptors can be reversed by the BZ Ro15-4513 and that this action in turn is sensitive to flumazenil and the BZ site ligand β-CCE suggests that these receptors likely contain a modified BZ-binding site. Such previously unrecognized BZ sites, on less abundant but functionally important GABAAR subtypes, provide opportunity for new drug development. For example, it might be advantageous to synthesize alcohol antagonists that lack the inverse agonist effects of Ro15-4513 on certain GABA receptor subtypes. In addition, given Ro15-4513’s structural similarity with the general BZ antagonist flumazenil, it is not surprising that Ro15-4513 is also a general BZ antagonist, and like flumazenil likely has a fairly short half-life (≈30 min) in vivo. Furthermore, the alcohol antagonist Ro15-4513 and the clinically used general BZ antagonist flumazenil are highly hydrophobic with likely poor bioavailability when applied orally or i.p. (flumazenil is administered intravenously in the clinic as a BZ antidote). Given the high incidence of ethanol intoxication cases in emergency rooms and the short half-life of flumazenil, leading to resedation in cases where it is used to antagonize much longer acting BZs, the identification of α4/6β3δ GABAARs as ethanol/Ro15-4513 targets may spur the development of combined BZ/alcohol antagonists with longer half-life. Such combined antagonists, without inverse agonist activity and better solubility, not only might be useful in the clinic, but also may provide research tools for a precise dissection of the contribution of GABAAR subtypes to certain aspects of acute ethanol actions.
We show that the BZ-site ligand β-CCE, a β-carboline, is an agonist on α4β3δ receptors and like EtOH allosterically enhances the GABA response without directly activating these receptors. The activation of α4β3δ GABAARs by β-CCE provides proof of principle that it might be possible to develop specific alcohol receptor agonists. Ideally, such positive modulators (alcohol mimetics) should be specific for the EtOH/Ro15-4513 site and lack activity (like the inverse agonist activity of β-CCE) on classical GABAAR α1, -2, -3, and -5γ2 BZ sites. Such specific alcohol receptor agonists could be useful to harness ethanol receptors for therapeutic purposes by mimicking the anxiolytic, anti-depressive, and mood-enhancing actions of alcohol, without the undesired effects like liver toxicity and other toxic effects of metabolites like acetaldehyde.
Materials and Methods
Electrophysiology.
Clones used were as described previously and were confirmed by sequencing to ensure that they were free of errors and agreed with the consensus sequences for rat α4, α6, β3, and δ subunit proteins (9). Mutagenesis was performed by using the QuikChange site-directed mutagenesis kit (Stratagene). cRNA was transcribed after plasmid linearization by using the mMessage mMachine kit (Ambion, Austin, TX). Transcripts were purified by LiCl precipitation, and RNA concentration was determined on a gel and by photometry. Oocytes were coinjected with a mixture of α, β, and δ (or γ2) subunits in a 1:1:5 (or 1:1:10) subunit molar ratio. Currents were measured at room temperature (22°C–24°C) in the two-electrode voltage clamp configuration at −80 mV holding potential with an Axoclamp 2A Axon Instruments (Union City, CA) amplifier. Two electrode voltage clamp on Xenopus oocytes was performed in ND96 salt solution (96 mM NaCl/2 mM KCl/1.8 mM CaCl2/1 mM MgCl2/5 mM Hepes, pH 7.2). Because of the slow onset in the expression of highly alcohol-sensitive δ subunit-containing receptors, oocytes were measured 7–14 days after injection. Currents were measured either in a tonic current mimicking mode as steady-state current (Figs. 1 and 2), or by brief (<10 s) coapplications of 300 nM GABA with drugs (ethanol, BZ-site ligands), to evoke peak currents, followed by a recovery time of at least 1 min.
Reagents.
Hoffman-LaRoche (Nutley, NJ) kindly provided Ro15-4513, flumazenil or Ro15-1788 (ethyl 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate), diazepam, and flunitrazepam. DMCM was a gift from Ferrosan (Copenhagen), and FG7142 (N-methyl-β-carboline-3-carboxamide) and β-CCE were provided by Schering. Ethanol, GABA, and bicuculline were purchased from Sigma. Compounds were dissolved in DMSO as a 10-mM stock solution and were used at the indicated concentrations. DMSO at final concentrations used did not lead to changes in GABA receptor currents (data not shown).
Acknowledgments
We thank Dr. C. Gundersen [University of California, Los Angeles (UCLA)] and the UCLA Anesthesiology Department for providing Xenopus oocytes and Qui Vu (UCLA) for help with oocyte injections. This work was supported by National Institutes of Health (NIH) Predoctoral Fellowship AA015460 (to H.J.H.), an Alcoholic Beverage Medical Research Foundation grant (to M.W.), NIH grants NS35985 and AA07680, and funds provided by the State of California for medical research on alcohol and substance abuse (to R.W.O.).
Abbreviations
- β-CCE
β-carboline-3-carboxy ethyl ester
- GABAAR
GABAA receptor
- DMCM
methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate
- BZ
benzodiazepine
Conflict of interest statement: M.W., R.W.O. and H.J.H. have filed a U.S. Provisional Patent Application, Serial No. 60/693,844.
This paper was submitted directly (Track II) to the PNAS office.
See Commentary on page 8307.
References
- 1.Popp R. L., Lickteig R. L., Lovinger D. M. J. Pharmacol. Exp. Ther. 1999;289:1564–1574. [PubMed] [Google Scholar]
- 2.Davies D. L., Trudell J. R., Mihic S. J., Crawford D. K., Alkana R. L. Alcohol Clin. Exp. Res. 2003;27:743–755. doi: 10.1097/01.ALC.0000065722.31109.A1. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K., Kobayashi T., Kumanishi T., Yano R., Sora I., Niki H. Neurosci. Res. 2002;44:121–131. doi: 10.1016/s0168-0102(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 4.Blednov Y. A., Stoffel M., Alva H., Harris R. A. Proc. Natl. Acad. Sci. USA. 2003;100:277–282. doi: 10.1073/pnas.012682399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liljequist S., Engel J. Psychopharmacology. 1982;78:71–75. doi: 10.1007/BF00470592. [DOI] [PubMed] [Google Scholar]
- 6.Aguayo L. G., Peoples R. W., Yeh H. H., Yevenes G. E. Curr. Top. Med. Chem. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- 7.Hu W. Y., Reiffenstein R. J., Wong L. Alcohol Drug Res. 1987;7:107–117. [PubMed] [Google Scholar]
- 8.Khanna J. M., Kalant H., Chau A., Shah G. Pharmacol. Biochem. Behav. 1998;59:511–519. doi: 10.1016/s0091-3057(97)00477-2. [DOI] [PubMed] [Google Scholar]
- 9.Wallner M., Hanchar H. J., Olsen R. W. Proc. Natl. Acad. Sci. USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihalek R. M., Bowers B. J., Wehner J. M., Kralic J. E., VanDoren M. J., Morrow A. L., Homanics G. E. Alcohol Clin. Exp. Res. 2001;25:1708–1718. [PubMed] [Google Scholar]
- 11.Farrant M., Nusser Z. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 12.Benson J. A., Low K., Keist R., Möhler H., Rudolph U. FEBS Lett. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- 13.Korpi E. R., Kleingoor C., Kettenmann H., Seeburg P. H. Nature. 1993;361:356–359. doi: 10.1038/361356a0. [DOI] [PubMed] [Google Scholar]
- 14.Hanchar H. J., Dodson P. D., Olsen R. W., Otis T. S., Wallner M. Nat. Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonetti E. P., Burkhard W. P., Gabl M., Möhler H. Br. J. Pharmacol. 1985;86:463P.. [Google Scholar]
- 16.Polc P. Br. J. Pharmacol. 1985;86:465P. [Google Scholar]
- 17.Suzdak P. D., Glowa J. R., Crawley J. N., Schwartz R. D., Skolnick P., Paul S. M. Science. 1986;234:1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- 18.Syapin P. J., Jones B. L., Kobayashi L. S., Finn D. A., Alkana R. L. Brain Res. Bull. 1990;24:705–709. doi: 10.1016/0361-9230(90)90012-o. [DOI] [PubMed] [Google Scholar]
- 19.Bonetti E. P., Burkard W. P., Gabl M., Hunkeler W., Lorez H. P., Martin J. R., Möhler H., Osterrieder W., Pieri L., Polc P. Pharmacol. Biochem. Behav. 1988;31:733–749. doi: 10.1016/0091-3057(88)90259-6. [DOI] [PubMed] [Google Scholar]
- 20.Kolata G. Science. 1986;234:1198–1199. doi: 10.1126/science.3775379. [DOI] [PubMed] [Google Scholar]
- 21.Nabeshima T., Tohyama K., Kameyama T. Eur. J. Pharmacol. 1988;155:211–217. doi: 10.1016/0014-2999(88)90506-7. [DOI] [PubMed] [Google Scholar]
- 22.June H. L., Hughes R. W., Spurlock H. L., Lewis M. J. Psychopharmacology. 1994;115:332–339. doi: 10.1007/BF02245074. [DOI] [PubMed] [Google Scholar]
- 23.Petry N. M. Psychopharmacology. 1995;121:192–203. doi: 10.1007/BF02245630. [DOI] [PubMed] [Google Scholar]
- 24.Rassnick S., D’Amico E., Riley E., Koob G. F. Alcohol Clin. Exp. Res. 1993;17:124–130. doi: 10.1111/j.1530-0277.1993.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 25.Britton K. T., Ehlers C. L., Koob G. F. Science. 1988;239:648–650. doi: 10.1126/science.3340849. [DOI] [PubMed] [Google Scholar]
- 26.Suzdak P. D., Glowa J. R., Crawley J., Skolnick P., Paul S. M. Science. 1987;239:649–650. doi: 10.1126/science.239.4840.649. [DOI] [PubMed] [Google Scholar]
- 27.Lister R. G., Nutt D. J. Trends Neurosci. 1987;10:223–225. [Google Scholar]
- 28.Lister R. G., Nutt D. J. Pharmacol. Biochem. Behav. 1988;31:731. doi: 10.1016/0091-3057(88)90260-2. [DOI] [PubMed] [Google Scholar]
- 29.Suzdak P. D., Schwartz R. D., Skolnick P., Paul S. M. Proc. Natl. Acad. Sci. USA. 1986;83:4071–4075. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allan A. M., Harris R. A. Life Sci. 1986;39:2005–2015. doi: 10.1016/0024-3205(86)90324-3. [DOI] [PubMed] [Google Scholar]
- 31.Suzdak P. D., Paul S. M., Crawley J. N. J. Pharmacol. Exp. Ther. 1988;245:880–886. [PubMed] [Google Scholar]
- 32.Syapin P. J., Gee K. W., Alkana R. L. Brain Res. Bull. 1987;19:603–605. doi: 10.1016/0361-9230(87)90078-5. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi T., Ikeda K., Kojima H., Niki H., Yano R., Yoshioka T., Kumanishi T. Nat. Neurosci. 1999;2:1091–1097. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- 34.Costa A. C., Stasko M. R., Stoffel M., Scott-McKean J. J. J. Neurosci. 2005;25:7801–7804. doi: 10.1523/JNEUROSCI.1699-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurd R., Arras M., Lambert S., Drexler B., Siegwart R., Crestani F., Zaugg M., Vogt K. E., Ledermann B., Antkowiak B., Rudolph U. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 36.Belelli D., Lambert J. J., Peters J. A., Wafford K., Whiting P. J. Proc. Natl. Acad. Sci. USA. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wafford K. A., Bain C. J., Quirk K., McKernan R. M., Wingrove P. B., Whiting P. J., Kemp J. A. Neuron. 1994;12:775–782. doi: 10.1016/0896-6273(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 38.Mihic S. J., Ye Q., Wick M. J., Koltchine V. V., Krasowski M. D., Finn S. E., Mascia M. P., Valenzuela C. F., Hanson K. K., Greenblatt E. P., et al. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 39.Glowa J. R., Crawley J., Suzdak P. D., Paul S. M. Pharmacol. Biochem. Behav. 1988;31:767–772. doi: 10.1016/0091-3057(88)90263-8. [DOI] [PubMed] [Google Scholar]
- 40.Nusser Z., Sieghart W., Somogyi P. J. Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei W., Zhang N., Peng Z., Houser C. R., Mody I. J. Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei W., Faria L. C., Mody I. J. Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carta M., Mameli M., Valenzuela C. F. J. Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang J., Zhang N., Cagetti E., Houser C. R., Olsen R. W., Spigelman I. J. Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whiting P., Wafford K., McKernan R. M. In: GABA in the Nervous System: The View at Fifty Years. Martin D. L., Olsen R. W., editors. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 113–126. [Google Scholar]
- 46.Nusser Z., Mody I. J. Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- 47.Sigel E. Curr. Top. Med. Chem. 2002;2:833–839. doi: 10.2174/1568026023393444. [DOI] [PubMed] [Google Scholar]
- 48.Saxena N. C., Macdonald R. L. Mol. Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- 49.Brown N., Kerby J., Bonnert T. P., Whiting P. J., Wafford K. A. Br. J. Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Criswell H. E., Breese G. R. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- 51.Reynolds J. N., Prasad A., MacDonald J. F. Eur. J. Pharmacol. 1992;224:173–181. doi: 10.1016/0014-2999(92)90802-b. [DOI] [PubMed] [Google Scholar]
- 52.Palmer M. R., van Horne C. G., Harlan J. T., Moore E. A. J. Pharmacol. Exp. Ther. 1988;247:1018–1024. [PubMed] [Google Scholar]
- 53.Ming Z., Knapp D. J., Mueller R. A., Breese G. R., Criswell H. E. Brain Res. 2001;920:117–124. doi: 10.1016/s0006-8993(01)03044-x. [DOI] [PubMed] [Google Scholar]
- 54.Laurie D. J., Wisden W., Seeburg P. H. J. Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brickley S. G., Revilla V., Cull-Candy S. G., Wisden W., Farrant M. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 56.White A. M., Truesdale M. C., Bae J. G., Ahmad S., Wilson W. A., Best P. J., Swartzwelder H. S. Pharmacol. Biochem. Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- 57.Borghese C. M., Storustovu S. I., Ebert B., Herd M. B., Belelli D., Lambert J. J., Marshall G., Wafford K. A., Harris R. A. J. Pharmacol. Exp. Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- 58.Thompson S. A., Bonnert T. P., Cagetti E., Whiting P. J., Wafford K. A. Neuropharmacology. 2002;43:662–668. doi: 10.1016/s0028-3908(02)00162-4. [DOI] [PubMed] [Google Scholar]
- 59.Davies P. A., Hanna M. C., Hales T. G., Kirkness E. F. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 60.Boileau A. J., Baur R., Sharkey L. M., Sigel E., Czajkowski C. Neuropharmacology. 2002;43:695–700. doi: 10.1016/s0028-3908(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 61.June H. L., Lewis M. J. Psychopharmacology. 1994;116:309–316. doi: 10.1007/BF02245334. [DOI] [PubMed] [Google Scholar]
- 62.Dar M. S. Pharmacol. Biochem. Behav. 1995;52:217–223. doi: 10.1016/0091-3057(95)00107-8. [DOI] [PubMed] [Google Scholar]
- 63.June H. L., Lummis G. H., Colker R. E., Moore T. O., Lewis M. J. Alcohol. Clin. Exp. Res. 1991;15:406–411. doi: 10.1111/j.1530-0277.1991.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 64.Hoffman P. L., Tabakoff B., Szabo G., Suzdak P. D., Paul S. M. Life Sci. 1987;41:611–619. doi: 10.1016/0024-3205(87)90415-2. [DOI] [PubMed] [Google Scholar]
- 65.Dar M. S., Mustafa S. J., Wooles W. R. Life Sci. 1983;33:1363–1374. doi: 10.1016/0024-3205(83)90819-6. [DOI] [PubMed] [Google Scholar]
- 66.Davies A. G., Pierce-Shimomura J. T., Kim H., VanHoven M. K., Thiele T. R., Bonci A., Bargmann C. I., McIntire S. L. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- 67.Liu P., Xi Q., Ahmed A., Jaggar J. H., Dopico A. M. Proc. Natl. Acad. Sci. USA. 2004;101:18217–18222. doi: 10.1073/pnas.0406096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanchar H. J., Chutsrinopkun P., Meera P., Supavilai P., Sieghart W., Wallner M., Olsen R. W. Proc. Natl. Acad. Sci. USA. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]