Abstract
Self-assembled capsules are hosts that recognize and surround smaller molecule guests of appropriate size, shape, and chemical surfaces. The space available inside is a cage of fixed solvent molecules, many of which are aromatic. These aromatics provide anisotropic shielding to guests, and a map of induced magnetic shielding for the inner space can be obtained through nucleus-independent chemical shift calculations. Experimental values of the magnetic environment can be determined by NMR spectra of the guests inside. We describe here the environment in a cylindrical capsule with tapered ends. A series of terminal acetylenes—the narrowest of organic structures—was synthesized and used to probe the magnetic shielding of the capsule’s ends. Their NMR spectra showed that the acetylenic hydrogen experiences deshielding as it is forced deeper into the tapered end of the capsule where four benzene rings converge. Modeling and density functional theory calculations provided excellent agreement with the experimental values and established a molecular ruler to explore steric and magnetic environments inside the capsule.
Keywords: encapsulation, nanochemistry, self-assembly
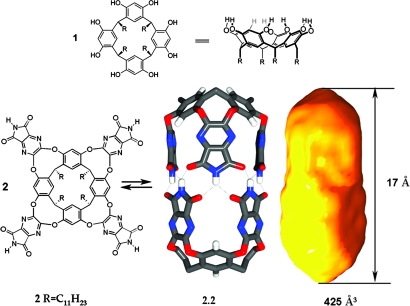
Resorcinarenes 1 (1) feature a shallow, bowl-like shape that has intrinsic capabilities in molecular recognition (2–5). Their gentle curvature and electronic properties attract cationic, convex molecules, but when the resorcinarene is deepened through synthesis, size selectivity can be seen and the molecules are regarded as cavitands (6–7). Such cavitands can maintain their conformations through intramolecular hydrogen bonds along the upper rim (8–9), or, when intermolecular hydrogen bonding is possible, the formation of capsules 2.2 can occur (10–12). This capsule presents guests with an electronic environment dominated by the eight benzene rings at each end (13–15). The four aryls at each of the resorcinarene ends impart an intense magnetic anisotropy: proton nuclei held near these ends show upfield shifts of up to 5 ppm in their NMR signals. Here we plot the details of these shifts within 2.2 using special probes (terminal acetylenes) capable of deep insertion. The results provide a test of predictions made by computational methods.
Results and Discussion
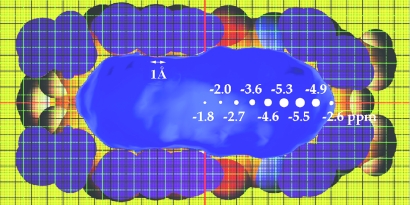
The shape and dimensions of the space inside the capsule are shown in Figs. 1and 2. The calculated values are, inevitably, a function of the graphics software used, and we show results obtained by using grasp (16). A cross section of the capsule appears in Fig. 2, and the section goes through the para position of two of the benzene rings that make up the tapered ends at the right side of the figure. These ends are hollow square pyramids, and the standard probe indicates a space ≈17 Å long from the centroids of the benzenes from one end to the other. But what can fit into these tapered ends? A hydrogen atom of a terminal acetylene is the smallest and narrowest organic substructure we could imagine, so it was selected as a slim probe for the steric and electronic properties of this space.
Fig. 2.
Cross section of the capsule, and B3LYP/6–31G*-calculated NICS values along the center of the cavitand ends.
We explored the inner space of the cavity both experimentally and computationally. The nucleus-independent chemical shifts (NICS) (17) were calculated for the magnetically shielded regions of 2.2 at the B3LYP/6–31G* level of density functional theory (18). These values are shown in Fig. 2 for coordinates along the central axis of 2.2 with a spacing distance of 1 Å. Along this axis the maximum effect is at the uppermost carbon of the resorcinarene. From there a steep drop of the NICS occurs as the cavity narrows and the “hole” in the end of the capsule is reached. This region is not generally accessible to groups the size of methyl and larger but can accommodate a terminal acetylene.
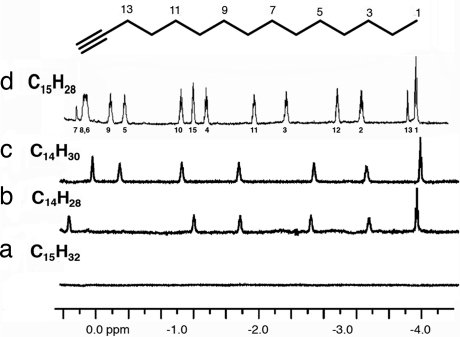
We previously reported that n-tetradecane fits well within the capsule, and Fig. 3c shows the NMR spectrum of encapsulated n-tetradecane (19–21). The methyl groups are positioned in the area of the capsule that produces the highest upfield shifts; Δδ is approximately −4.8 ppm. The alkene 7-trans-tetradecene is also encapsulated with comparable chemical shifts for the nonallylic methylenes (Fig. 3b), but n-pentadecane is not encapsulated at all (Fig. 3a). However, the terminal alkyne 1-pentadecyne is encapsulated (Fig. 3d), and each of the proton resonances can be assigned; the methyne hydrogen is found at −1.2 ppm (Δδ = 2.9 ppm), a value unexpectedly small for a nucleus so deep in the cavity’s end. By way of contrast, the methyl at the other end shows Δδ = −4.7 ppm and even the C3 methylene shows Δδ > 5 ppm (the highest upfield shift ever observed in this capsule).
Fig. 3.
Upfield regions of 1H NMR spectra (600 MHz, mesitylene-d12) of 2 (2 mM) with pentadecane (10 mM) (a), 7-trans-tetradecene (10 mM) (b), tetradecane (10 mM) (c), and 1-pentadecyne (10 mM) (d). The acetylenic hydrogen appears under the multiplet for the hydrogens on C10.
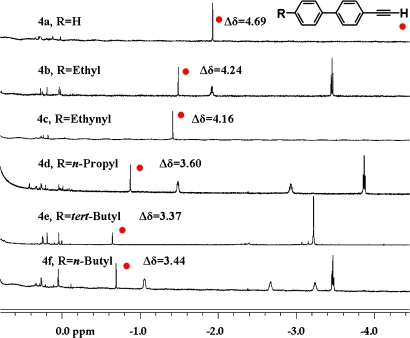
A series of aromatic alkynes 4a–4f were prepared as relatively rigid “rulers.” All were encapsulated, and the relevant parts of the NMR spectra are shown in Fig. 4. The shortest guest is 4a, and it can move to its optimum position in the capsule most freely. It showed the methyne signal the furthest upfield, at Δδ = 4.7 ppm! As the guest lengthens, the narrow, terminal acetylene is forced deeper into the cavitand, but the methyne hydrogen signal moves downfield: the magnetic environment it experiences is less shielding.
Fig. 4.
Upfield regions of 1H NMR spectra (600 MHz, mesitylene-d12) of 2 (2 mM) with 4a–4f (10 mM).
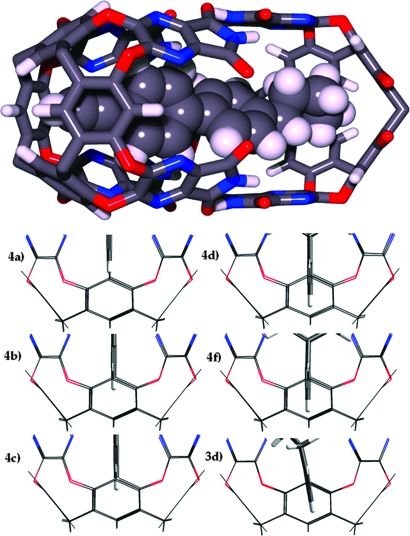
Semiempirical energy minimized (AM1) structures of the encapsulation complexes showed the variation of the positioning of the acetylenic tips given in Fig. 5. The 1-pentadecyne 3d and the p-butylbiphenyl acetylene 4f are seen to be deepest within the cavitand with the hydrogen nearly protruding from the small opening, like the tip of a retracted ballpoint pen. For 1-pentadecyne, this places the C3 methylene in the highest shift region with Δδ = 5.6 ppm. These positions are in accordance with the calculated NICS Δδ values described above. The NICS-calculated shifts are absolute values whereas those of Fig. 4 represent the difference between the chemical shifts in aromatic solvents and inside the capsule. The general agreement with experiment indicates a less shielding environment at the deepest part of the capsule. The aromatic rings of the guests have fewest steric clashes with the pyrazine walls if they are placed diagonally in the box defined by the pyrazines. With such a placement the ortho hydrogens of the proximal phenyl of the n-Bu derivative are also well shielded; the calculated value is 4.4–3.5 ppm, and the observed value is 3.3 ppm.
Fig. 5.
Calculated structures of the encapsulated acetylenes. (Upper) Modeled encapsulation of 4-ethynyl-4′-n-butylbiphenyl in 2.2. The butyl adopts a gauche conformation (some groups omitted for clarity). (Lower) Semiempirical calculated (AM1) positions of the acetylenic hydrogens. The C15 3dand t-Bu 4ederivative are seen to penetrate deepest into the tapered ends.
What price is paid for forcing the acetylene deeper into the cavity? Pairwise competition studies between the rigid guests established the affinity order Et (4b) is 10 times greater than Pr (4c) is 9 times greater than H (4a) ∼ t-Bu (4e) is 9 times greater than n-Bu (4f). The range of affinities varies by <1,000 fold, or ≈4 kcal/mol. Each change affects not only the positioning of the guest but also the packing coefficient and the conformation, so interpretations must be made with caution. For example, the worst guest 4f is nearly the deepest but must also adopt a gauche conformation along the butane chain to be encapsulated (Fig. 5; see the supporting information, which is published on the PNAS web site, for NMR evidence). The shortest 4a leaves empty space and is only a fair guest.
In summary, reversible encapsulation provides a means to probe through-space magnetic-shielding effects. The acetylenic hydrogens of 4a–4f explore different magnetic environments produced by the cavitand’s aryls: the hydrogen deepest in the tapered ends of the capsule experiences a less shielding environment than one above the cavitand.
Materials and Methods
General.
All chemicals were used without further purification unless otherwise specified. Cavitand 2 was prepared from corresponding resorcinarene as follows in an improvement on the published procedure (11). The acetylenes 4-ethynyl-4′-ethylbiphenyl (4b), 4,4′-diethynylbiphenyl (4c), and 4-ethynyl-4′-propylbiphenyl (4d) were prepared according to literature procedures (22). 4-ethynyl-4′-tert-butylbiphenyl (4e) and 4-ethynyl-4′-n-butylbiphenyl (4f) were prepared as described below using a modified procedure for the cross-coupling reactions. Proton (1H) and carbon (13C) NMR spectra were recorded on Bruker DRX- 600 (600 MHz) spectrometers. Electrospray ionization time-of-flight reflectron experiments were performed on an Agilent Electrospray ionization time-of-flight mass spectrometer.
Improved Procedure for Synthesis of Cavitand 2.
A mixture of 5,6-dichloropyrazine-2,3-dicarboxylic acid imide (400 mg, 1.84 mmol, recrystallized from ethanol) and C-undecylcalix[4]resorcinarene (485 mg, 0.439 mmol) in dimethylformamide (25 ml) under a nitrogen atmosphere was slightly heated until the mixture turned to a clear yellow solution. After stirring for 10 min at room temperature, Et3N (363 mg, 3.59 mmol) was added dropwise via syringe over 1 min and stirred for 50 min. The reaction mixture was filtered off, and all of the volatiles were removed at reduced pressure. To the residue was added a mixture of methanol (11 ml) and water (29 ml), and this was sonicated for 1 h. The yellow suspension was filtered and then washed with a mixture of methanol (22 ml) and water (59 ml). Further washing with methanol (47 ml) gave the desired product as a pale yellow powder in 72% yield (535 mg).
1H NMR (CDCl3, 600 MHz) δ 9.81 (s, 4H), 8.14 (s, 4H), 7.35 (s, 4H), 5.70 (t, J = 7.8 Hz, 4H), 2.31 (m, 8H), 1.49–1.30 (m, 72H), 0.90 (t, J = 6.9 Hz, 12H). 13C NMR (CDCl3, 150 MHz)) δ 163.2, 158.6, 152.8, 142.9, 137.2, 124.6, 119.1, 34.4, 32.5, 32.2, 30.0, 29.9, 29.7, 28.2, 23.0, 14.4. MALDI MS m/z: 1,686 [M+] (calculated for C96H108N12O16, 1,686).
4-Ethynyl-4′-tert-butylbiphenyl (4e).
To predried potassium fluoride (348 mg, 6 mmol) in a 25-ml Schlenk flask were added (4-bromophenylethynyl)trimethylsilane (506 mg, 2 mmol), 4-tert-butylphenylboronic acid (2.4 mmol), palladium acetate (18 mg, 0.08 mmol), and tricyclohexylphosphine (28 mg, 0.1 mmol). The flask was evacuated and backfilled with nitrogen three times before the addition of anhydrous tetrahydrofuran (2 ml). After stirring overnight at 60°C, the reaction mixture was diluted with ethyl acetate (50 ml) and filtered through a Celite pad, and all of the volatiles were evaporated. The residue was dissolved in ethyl acetate (50 ml), washed with water (15 ml) and brine (15 ml), dried over sodium sulfate, filtered, and concentrated in vacuo. Purification of the crude by silica-gel column chromatography afforded a cross-coupling adduct. The following desilylation of the product with n-tetrabutylammonium fluoride (1 M tetrahydrofuran solution, 2.2 ml) were carried out in tetrahydrofuran (2 ml) at room temperature for 30 min. The reaction was quenched with saturated NH4Cl aqueous solution (15 ml) and extracted with ethyl acetate (15 ml three times). The combined organic layer was washed with water (15 ml), brine (15 ml), dried over sodium sulfate, filtered, and concentrated in vacuo. Purification by silica-gel column chromatography with hexane as an eluent afforded a desired compound in 70% overall yield.
1H NMR (600 MHz, C6D6) δ 7.48 (d, J = 8.4 Hz, 2H), 7.35 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H), 7.29 (d, J = 8.4 Hz, 2H), 2.80 (s, 1H), 1.23 (s, 9H). 13C NMR (150 MHz, C6D6) δ 150.7, 141.8, 137.7, 132.9, 127.2, 127.1, 126.0, 121.4, 83.9, 78.3, 34.5, 31.4. High-resolution MS (electrospray ionization) m/z (MH+): calculated and found for C18H19, 235.1481 and 235.1491, respectively.
4-Ethynyl-4′-n-butylbiphenyl (4f).
The same procedure as described above was followed using 4-n-butylphenylboronic acid.1H NMR (600 MHz, C6D6) δ 7.47 (d, J = 8.4 Hz, 2H), 7.32 (d, J = 8.4 Hz, 2H), 7.28 (d, J = 8.4 Hz, 2H), 7.07 (d, J = 8.4 Hz, 2H), 2.80 (1H, s), 2.48 (t, J = 7.8 Hz, 2H), 1.50 (m, 2H), 1.25 (m, 2H), 0.862 (t, J = 7.8 Hz, 3H). 13C NMR (150 MHz, C6D6) δ 142.6, 141.9, 138.0, 132.9, 129.2, 127.3, 127.1, 121.3, 84.0, 78.2, 35.6, 33.9, 22.6, 14.1. High-resolution MS (electrospray ionization) m/z (MH+): calculated and found for C18H19, 235.1481 and 235.1478, respectively.
Supplementary Material
Fig. 1.
Resorcinarene 1, its derived tetraimide cavitand 2, the dimeric, capsule 2.2 (some groups omitted for clarity), and the space inside.
Acknowledgments
This work was supported by the Skaggs Institute and National Institutes of Health Grant GM 27953. D.A. and T.I. are Skaggs Postdoctoral Fellows.
Abbreviation
- NICS
nucleus-independent chemical shift.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Högberg A. G. S. J. Am. Chem. Soc. 1980;102:6046–6050. [Google Scholar]
- 2.Aoyama Y., Tanaka Y., Toi H., Ogoshi H. J. Am. Chem. Soc. 1988;110:634–635. [Google Scholar]
- 3.Shivanyuk A., Rebek J., Jr. J. Am. Chem. Soc. 2003;125:3432–3433. doi: 10.1021/ja027982n. [DOI] [PubMed] [Google Scholar]
- 4.Palmer L. C., Rebek J., Jr. Org. Lett. 2005;7:787–789. doi: 10.1021/ol047673x. [DOI] [PubMed] [Google Scholar]
- 5.Avram L., Cohen Y. J. Am. Chem. Soc. 2004;126:11556–11563. doi: 10.1021/ja047698r. [DOI] [PubMed] [Google Scholar]
- 6.Cram D. J., Choi H. J., Bryant J. A., Knobler C. B. J. Am. Chem. Soc. 1992;114:7748–7765. [Google Scholar]
- 7.Soncini P., Bonsignore S., Dalcanale E., Ugozzoli F. J. Org. Chem. 1992;57:4608–4612. [Google Scholar]
- 8.Rudkevich D. M., Hilmersson G., Rebek J., Jr. J. Am. Chem. Soc. 1998;120:12216–12225. [Google Scholar]
- 9.Far A. R., Shivanyuk A., Rebek J., Jr. J. Am. Chem. Soc. 2002;124:2854–2855. doi: 10.1021/ja012453p. [DOI] [PubMed] [Google Scholar]
- 10.Heinz T., Rudkevich D. M., Rebek J., Jr. Nature. 1998;394:764–766. [Google Scholar]
- 11.Körner S., Tucci F. C., Rudkevich D. M., Heinz T., Rebek J., Jr. Chem. A Eur. J. 1999;6:187–195. doi: 10.1002/(sici)1521-3765(20000103)6:1<187::aid-chem187>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Chen J., Körner S., Craig S. L., Rudkevich D. M., Rebek J., Jr. Nature. 2002;415:385–386. doi: 10.1038/415385b. [DOI] [PubMed] [Google Scholar]
- 13.Heinz T., Rudkevich D. M., Rebek J., Jr. Angew. Chem. Int. Ed. 1999;38:1136–1139. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1136::AID-ANIE1136>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Chen J., Körner S., Craig S. L., Lin S., Rudkevich D. M., Rebek J., Jr. Proc. Natl. Acad. Sci. USA. 2002;99:2593–2596. doi: 10.1073/pnas.052706499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanaka M., Shivanyuk A., Rebek J., Jr. Proc. Natl. Acad. Sci. USA. 2004;101:2669–2672. doi: 10.1073/pnas.0308584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholls A., Sharp K., Honig B. Proteins Struct. Funct. Genet. 1991;11:281ff. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 17.Schleyer P. v. R., Maerker C., Dransfeld A., Jiao H., Hommes N. J. R. v. E. J. Am. Chem. Soc. 1996;118:6317–6318. doi: 10.1021/ja960582d. [DOI] [PubMed] [Google Scholar]
- 18.Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery J. A., Jr., Vreven T., Kudin K. N., Burant J. C., et al. gaussian 03. Pittsburgh: Gaussian; 2002. [Google Scholar]
- 19.Scarso A., Trembleau L., Rebek J., Jr. Angew. Chem. Intl. Ed. 2003;42:5499–5502. doi: 10.1002/anie.200352235. [DOI] [PubMed] [Google Scholar]
- 20.Scarso A., Trembleau L., Rebek J., Jr. J. Am. Chem. Soc. 2004;126:13512–13518. doi: 10.1021/ja047952f. [DOI] [PubMed] [Google Scholar]
- 21.Trembleau L., Rebek J. Science. 2003;301:1219–1220. doi: 10.1126/science.1086644. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi S., Kuroyama Y., Sonogashira K., Hagihara N. Synthesis. 1980;8:628–630. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.