Abstract
The rapid growth and poor vascularization of solid tumors expose cancer cells to hypoxia, which promotes the metastatic phenotype by reducing intercellular adhesion and increasing cell motility and invasiveness. In this study, we found that hypoxia increased free NADH levels in cancer cells, promoting CtBP recruitment to the E-cadherin promoter. This effect was blocked by pyruvate, which prevents the NADH increase. Furthermore, hypoxia repressed E-cadherin gene expression and increased tumor cell migration, effects that were blocked by CtBP knockdown. We propose that CtBP senses levels of free NADH to control expression of cell adhesion genes, thereby promoting tumor cell migration under hypoxic stress.
Keywords: NADH, E-cadherin, metastasis, HIF1α, adhesion
The ability of tumors to metastasize is a hallmark of malignancy. A critical event during metastasis is the reduction of adhesion, which facilitates tumor cell invasion into surrounding tissues and vascular channels, ultimately leading to the development of new sites of cancer progression. E-cadherin-mediated cell–cell adhesion is essential for maintaining the homeostasis and architecture of epithelial tissues. Down-regulation of E-cadherin expression occurs concomitantly with dedifferentiation and invasion of epithelial cells during tumorigenesis (1, 2). Consequently, E-cadherin and its associated complex are thought to be key mediators of tumor cell invasion (3).
Highly aggressive, rapidly growing tumors are exposed to hypoxia, which occurs as a consequence of high metabolic activity and inadequate blood supply (4). It has been proposed that hypoxia is the initiating event that sets tumors on the road to metastasis (5). We have shown that the transcriptional corepressor CtBP has the unique ability to sense levels of free nuclear NADH and transmit this information to complexes that regulate gene expression (6). Transcription of E-cadherin and several other cell adhesion proteins is known to be repressed by CtBP (7) via ZEB and other factors believed to interact with the E-cadherin promoter (8, 9). CtBP binding to these transcriptional repressors is induced by elevations in free NADH (6). We propose that the redox-sensing property of CtBP provides a regulatory switch for E-cadherin expression under hypoxic conditions.
In this study, we show that the transcriptional corepressor CtBP has a central role in cancer cell migration in response to hypoxia. Hypoxia increases free NADH levels, which promotes CtBP recruitment to the E-cadherin promoter, repressing E-cadherin gene expression and increasing tumor cell migration. Thus, the pathway we have described links tumor hypoxia to cell migration through NADH regulation of CtBP function.
Results
Loss of E-cadherin expression is regarded as a central event in tumor metastasis, because reduction of cell adhesion between tumor cells facilitates their ability to invade surrounding tissues (3). Malignant tumor cells exhibit uncontrolled growth, eventually outstripping their blood supply, and are exposed to sublethal ischemia, which may further reduce cell adhesion and stimulate invasiveness (5). Our previous study demonstrated that the transcriptional corepressor CtBP has a unique ability to sense the cellular redox change, i.e., CtBP binding to certain transcriptional repressor proteins is regulated by the metabolic state of the cell through the electron carrier redox pair NAD+/NADH. This finding led us propose that CtBP-mediated repression is increased by elevations in nuclear NADH during hypoxia.
Transcription of E-cadherin and several other cell-adhesion proteins is known to be repressed by CtBP (7). Loss of E-cadherin expression directly contributes to tumor metastasis, leading us to ask whether CtBP regulates cell migration during hypoxia. We adopted an in vitro wounding assay to assess the migratory activity of H1299 lung cancer cells under hypoxic conditions. During a 20-h incubation period, cells gradually migrated onto the matrix left from the scraped cell layer (Fig. 1). Hypoxia (1% O2) markedly increased this migration. To test the contribution of CtBP in this process, we used a short interfering (si)RNA capable of reducing levels of CtBP1 and CtBP2 (10) (Fig. 1B). The siRNA knockdown of CtBP completely blocked cell migration.
Fig. 1.
CtBP mediates the hypoxia-induced migration of H1299 lung cancer cells. (A) H1299 cells grown for 20 h in 20% O2 (Control) or 1% O2 (Hypoxia) were assayed for their migration onto the matrix deposited by cells scraped away at the beginning of the assay. The starting lines for the migration and CtBP siRNA treatment (siCtBP) are indicated. (B) Western blot showing CtBP knockdown in H1299 cells by siRNA treatment.
Next, we asked whether CtBP mediates the influence of hypoxia on E-cadherin expression. Hypoxia (5% O2 for 72 h) had been shown to down-regulate E-cadherin transcription in ovarian cancer cells (11). We measured E-cadherin mRNA in HT29 colon (Fig. 2A) and H1299 lung (Fig. 2B) cancer cells and found that, in these instances, E-cadherin transcripts were also decreased by hypoxia (1% O2 for 16 h). Many hypoxic responses are mediated by HIF-1α, a transcriptional activator that is stabilized under hypoxic conditions (12). Studies of renal clear-cell carcinoma (13) and ovarian tumors (11) show a reciprocal pattern of HIF-1α and E-cadherin expression. Presumably, this pattern could result from an indirect effect of HIF-1α (i.e., stabilization of HIF-1α could induce the synthesis of an E-cadherin repressor, such as snail). Alternatively, HIF-1α stabilization could merely represent exposure of cells to hypoxia, in which case, the E-cadherin repression could represent the effect of an HIF-1α-independent pathway. To distinguish between these possibilities, we treated H1299 cells with cycloheximide to block protein synthesis. Hypoxia reduced the expression of E-cadherin mRNA even in the presence of cycloheximide (Fig. 2C), suggesting that this effect is independent of HIF-1α and is not due to induction of E-cadherin repressors. Furthermore, siRNA knockdown of CtBP blocked the hypoxia-induced decrease of E-cadherin mRNA in both cell lines (Fig. 2 A and B), suggesting that CtBP mediates the hypoxia-induced repression of the E-cadherin gene. To ensure that the effect of hypoxia occurred at the transcriptional level, an E-cadherin luciferase reporter was introduced to the H1299 cells. In this context, hypoxia potentiated ZEB-mediated repression of the E-cadherin reporter (data not shown).
Fig. 2.
siRNA-mediated knockdown of CtBP prevents the decrease of E-cadherin mRNA induced by hypoxia. HT29 colon cancer (A) and H1299 lung cancer (B) cells transfected with siRNA against human CtBP were exposed to hypoxia for 16 h. E-cadherin mRNA levels were measured by real-time RT-PCR. Data were normalized to lamin. (C) Hypoxia repression of E-cadherin transcription is independent of protein synthesis. H1299 lung cancer cells were treated with 50 μg/ml cycloheximide and exposed to hypoxia for 16 h. E-cadherin mRNA levels were measured by RT-PCR.
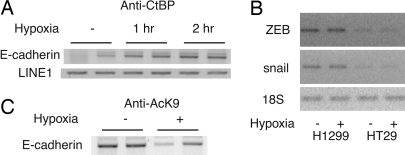
CtBP is a transcriptional corepressor that carries out its function by associating with DNA-binding repressors at particular target sites. To explore the role of CtBP in hypoxia-regulated gene expression, we tested CtBP recruitment to the endogenous E-cadherin promoter in vivo by using chromatin immunoprecipitation (ChIP) assays (14). The E-cadherin promoter contains two E-boxes that have been shown to interact with the transcriptional repressor ZEB (15). Cells exposed to hypoxia (1% O2 for 1 h) showed enhanced CtBP recruitment to the proximal region of the E-cadherin promoter (Fig. 3A). Semiquantatitive RT-PCR experiments indicate that neither ZEB nor snail was induced by acute (data not shown) or 16-h hypoxia treatment in the cell types tested (Fig. 3B), whereas the interaction between ZEB and CtBP was increased by hypoxia treatment in a mammalian two-hybrid assay (data not shown). These results suggest that hypoxia could potentially induce tumor cell migration by enhancing CtBP recruitment to the promoter of the E-cadherin gene. Like other corepressors, CtBP associates with histone deacetylases and other chromatin-modifying enzymes (16). Accordingly, hypoxia also decreased acetylation of His-3 at Lys-9 within this region (Fig. 3C).
Fig. 3.
Hypoxia increases CtBP recruitment to the E-cadherin promoter and deacetylates histone H3. (A) Exposure of HT29 cells to hypoxia increases CtBP recruitment to the E-cadherin promoter. Cells were exposed to hypoxia and assayed by ChIP. (B) ZEB and snail levels in tumor cells are not affected by hypoxia. ZEB and snail mRNA levels were measured by RT-PCR. 18S serves as control. HT29 colon and H1299 cancer cells were treated with hypoxia for 16 h (shown here) or 90 min (data not shown). (C) Lysine 9, histone H3 acetylation (AcK9) on the E-cadherin promoter was decreased by hypoxia. HT29 cells were incubated in a hypoxia chamber for 1 h and were assayed by ChIP using an anti-AcK9 antibody.
To elucidate the regulatory pathway controlling E-cadherin expression, we measured the free NADH changes that occurred as a result of hypoxia treatment. Hypoxia decreased the free NAD+/NADH ratio, as determined by measurement of the pyruvate and lactate concentrations (17) (Table 1). Given the basal NAD+/NADH ratio of 727, the conversion of NAD+ to NADH should not alter the free NAD+ level significantly. Thus, we estimate that the free NADH concentration increases by ≈5-fold in response to hypoxia.
Table 1.
Hypoxia changes the free cellular NAD+/NADH ratio
| Treatment | Control | Hypoxia |
|---|---|---|
| Free NAD+/NADH ratio | 727 | 153 |
| Estimated NADH signal | ||
| No treatment | 18 | 46 |
| Pyruvate | 6 | 20 |
H1299 cells were exposed to hypoxia, and the free cytoplasmic NAD+/NADH ratios were calculated as described in the text. Nuclear NADH is regulated by hypoxia and pyruvate in H1299 cells. The nuclear NAD(P)H fluorescence (the sum of the NADH and NADPH signals) was measured by two-photon microscopy. The nuclear NADH level was calculated by assuming the NADPH/NADH ratio is 4, as determined by Shigemori et al. (19). Thirty millimolar pyruvate decreased the nuclear NADH signal.
Two-photon microscopy was also used to investigate the nuclear NADH changes. This technique uses long-wavelength excitation to avoid damage to cellular structures and minimize the contribution from other fluorescent molecules (18). Thus, 2-photon microscopy provides a noninvasive and quantitative measure of the nuclear NADH and NADPH levels [designated NAD(P)H, the sum of the NADH and NADPH signals]. Assuming an NADPH/NADH ratio of 4, as determined in ref. 19, we estimate that the nuclear NADH level increases by 3-fold after hypoxia treatment.
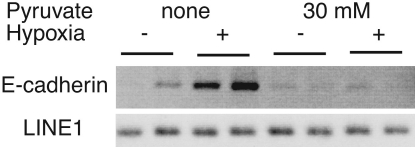
We tested the contribution of NADH to the hypoxia-triggered CtBP recruitment by treating the cells with pyruvate, which prevents the NADH increase. Pyruvate increases the free NAD+/NADH ratio in myoblasts, consistent with the cellular conversion of NADH to NAD+ through lactate dehydrogenase (20). We observed an overall decrease in NAD(P)H upon pyruvate application (Table 1), such that the NAD(P)H levels during hypoxia were equivalent to baseline in the absence of pyruvate. Accordingly, pyruvate treatment blocked the enhanced recruitment of CtBP to E-cadherin promoter (Fig. 4). These data show that hypoxia causes a rise in cellular NAD(P)H that is prevented when the cells are treated with pyruvate and support the view that CtBP recruitment to E-cadherin during hypoxia is regulated by NADH changes.
Fig. 4.
Pyruvate prevents the enhancement of CtBP recruitment to E-cadherin by hypoxia. H1299 human lung cancer cells were pretreated with 30 mM pyruvate to prevent the NADH increase and were assayed by ChIP using an anti-CtBP antibody. Primers surrounding the proximal E-boxes in the E-cadherin promoter (E-cadherin) were used to PCR-amplify the ChIP sample. Human LINE1 serves as a nonspecific control.
We next asked whether CtBP recruitment is regulated by other NADH regulators, such as CoCl2 or NaN3. CoCl2 and NaN3 treatment both increase free NADH. H1299 cells were treated with 200 μM CoCl2 or 10 mM NaN3 for 1 h, and the free cellular NAD+/NADH ratios were determined. The free intracellular NAD+/NADH ratio decreased from 727 (control) to 311 (CoCl2) and 257 (NaN3) (6). Both treatments increased CtBP recruitment to the proximal region of the E-cadherin promoter (Fig. 5), supporting the involvement of NADH in this process.
Fig. 5.
CtBP recruitment to the E-cadherin promoter is increased by agents that increase free NADH. CoCl2 or NaN3 stimulates CtBP recruitment to the E-cadherin promoter in H1299 cells. ChIP assays were performed as described.
To further investigate the role of the CtBP-ZEB-E-cadherin pathway in hypoxia-induced tumor cell migration, we tested whether overexpression of an E-cadherin promoter fragment that had been shown to interact with ZEB (8) relieved the hypoxia-induced CtBP recruitment to the endogenous E-cadherin gene. Introduction of a plasmid containing the −80 to +10 region of human E-cadherin promoter (21) blocked the decrease of E-cadherin mRNA in the hypoxia-treated tumor cells (Fig. 6A). In concert, expression of the exogenous E-cadherin promoter fragment attenuated the hypoxia-induced migration (Fig. 6B). A promoter fragment containing mutations in the two E-boxes that precluded ZEB binding (8) had no effect on either the hypoxia-driven decrease in E-cadherin transcription or cell migration.
Fig. 6.
The hypoxia-mediated reduction of E-cadherin expression and tumor cell migration involves the E-box-containing region of the promoter. H1299 cells were transfected with a plasmid containing the −80 to +10 region of the human E-cadherin promoter containing the two wild-type E-boxes (WT) or a version containing mutated E-boxes (mt). E-cadherin mRNA was measured 16 h after exposure to hypoxia by real-time RT-PCR (A) and cell migration from the edge of the dark field was scored 20 h after exposure to hypoxia (B).
Discussion
CtBP plays a key role in cellular regulation by binding to a variety of transcriptional repressors critical for development and tumorigenesis (22). Microarray analysis of cells from CtBP knockout mice indicates that several cell adhesion genes are under CtBP control (7). Of these, the best characterized is E-cadherin. We have uncovered an aspect of hypoxia regulation that links the repression of E-cadherin expression to the redox-sensing properties of CtBP. We show that the transcriptional corepressor CtBP, expressed in human cancer cells, has a central role in cell motility. Hypoxia increases free NADH levels, which promotes CtBP recruitment to the E-cadherin promoter, repressing E-cadherin gene expression and increasing tumor cell migration. Thus, the pathway we have described links tumor hypoxia to cell migration through NADH regulation of CtBP function.
With the progressive and rapid growth of malignant tumors, cancer cells in the ischemic condition are exposed to hypoxia and are, therefore, transformed to the metastatic phenotype through a reduction in intercellular adhesion as well as an increase in cell motility and invasiveness. The expression and localization of HIF-1 is a good indicator of tissue hypoxia (23). It has recently been reported that increased levels of HIF-1 are found in human cancers and are significantly correlated with short patient survival (24–27). We propose that the hypoxic microenvironment influences the functional activity of the transcriptional corepressor CtBP. The finding that the nuclear HIF-1 expression is correlated topologically with loss of E-cadherin expression in human cancers links hypoxia to the down-regulation of E-cadherin. Carcinomas, representing the majority of human cancers, develop from epithelial cells. Epithelial cells possess prominent cell–cell and cell–matrix adhesions and are programmed to undergo apoptosis upon their release from the extracellular matrix, preventing the colonization of mislocalized cells. As they progress to malignancy, carcinoma cells lose the expression of certain epithelial-specific genes. We hypothesize that hypoxia may affect cell attachment and invasiveness, at least partially, through CtBP.
The ability of CtBP to detect changes in NADH and transmit this information to specific promoter elements appears to underlie this regulation. Of note, our studies suggest that the free NADH levels are more important for CtBP regulation than NAD+. Thus, CtBP differs from the Sir2 enzymes, which are regulated predominantly by NAD+ (28, 29). This enhanced sensitivity to NADH supports our earlier analysis of CtBP binding using fluorescence resonance energy transfer (30). X-ray crystallographic analyses also support the idea that NADH and NAD+ induce different CtBP structures (31–33), which could conceivably influence the interaction with ZEB and other transcriptional repressors.
The pathway we have discovered is independent of the well characterized example of hypoxia regulation by HIF-1α. Our studies suggest a mechanism in which the formation of a repressor–CtBP complex is regulated by changes in the nuclear levels of free NADH. Over the time course that we have examined, this regulation appears to depend on preexisting transcriptional repressors, such as ZEB. This hypothesis is consistent with the recent report by Eger et al. (34) indicating that ZEB is the predominant regulator of E-cadherin in human breast cancer cells. It is possible, however, that other E-cadherin repressors could also be induced by hypoxia over the long-term (11) and could additionally contribute to tumor migration.
Down-regulation of E-cadherin was reported in a substantial percentage of carcinomas or borderline tumors when compared with benign epithelium (35). Reduced expression of E-cadherin and catenins has been observed in various human malignancies, often being associated with reduced differentiation and the presence of lymph node or blood–bone metastasis (36, 37). Our study provides a molecular mechanism for the observation that tumors induced by E1A/ras are more metastatic if CtBP interaction with E1A is abrogated (38). In head and neck cancers, cervical carcinomas, and soft tissue sarcomas, the presence of hypoxia may be associated with increased invasiveness and a propensity for metastasis (39, 40). Hypoxia down-regulates E-cadherin mRNA and protein levels in several tumor types (11, 41), and we suggest that this regulation depends on the redox-sensing properties of CtBP. Hypoxia has been regarded as a central factor underlying tumor metastasis, and hypoxic tumor cells are more invasive (39, 40). It is conceivable, therefore, that interfering with CtBP might perturb the spread of highly aggressive metastasizing tumors.
Materials and Methods
Chemicals.
Cycloheximide, CoCl2, NaN3, pyruvate, lactate, lactate dehydrogenase, and antibody to α-tubulin were purchased from Sigma. Anti-CtBP and histone H3 acetylation antibodies were from Upstate Biotechnology, Lake Placid, NY.
Plasmids.
ZEB expression vector was kindly provided by Douglas Dean (Washington University School of Medicine, St. Louis). The luciferase reporter construct containing the −80 to +10 region of the human E-cadherin promoter was a gift from S. Frisch (West Virginia University, Morgantown, WV) (8). siRNA oligonucleotides for CtBP (5′-GGG AGG ACC UGG AGA AGU UdTdG/dGdTCCC UCC UGG ACC UCU UCAA-5′) were from Dharmacon Research, Lafayette, CO. Scramble II Duplex was used as control.
Luciferase Assays.
Cells (5 × 104 per well, in 24-well plates) were transfected by Lipofectamine 2000 (Invitrogen) per the manufacturer’s instructions. After 24 h, plates were placed at 1% O2 for 16 h or left in normoxia. Cells were lysed in 100 μl of lysis buffer, and luciferase activity was determined by mixing 10 μl of cell extracts and 100 μl of luciferase assay reagent. Light production was measured for 10 s in a luminometer (6).
Cell Culture.
The human non-small-cell lung cancer cell line H1299 and colon carcinoma cell line HT29 were purchased from the American Type Culture Collection and cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% FCS (HyClone) and antibiotics (Invitrogen). Incubation was carried out at 37°C under 5% CO2 in air. The hypoxia chamber (1% O2, 5% CO2, and 94% N2) is described in ref. 6. HT29 or H1299 cells were transfected with 100 nM oligonucleotides, and, 3 days later, the expression of human CtBP was assayed by Western blotting using an anti-CtBP antibody.
Cell Migration Assays.
The wound closure assay was performed as described by Andre et al. (42). A wound was induced on the confluent monolayer cells by scraping a gap with a micropipette tip, and the speed of wound closure was monitored by light microscopy. Cell migration was measured by the traveling distance from the original wound edge after 20 h of incubation at 37°C under 20% O2 as control or under 1% O2 as hypoxia. Photographs were taken by using phase-contrast microscopy.
Western Blotting.
Cells were lysed in 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, and 0.5% sodium deoxycholate including proteinase inhibitors complete (Roche Diagnostics). The lysates were centrifuged at 13,000 × g for 20 min at 4°C, and the supernatants were separated by SDS/PAGE (8% acrylamide) and transferred onto PVDF membranes (Millipore). The membranes were blocked in TBST (0.2 mol/liter NaCl, 10 mmol/liter Tris, pH 7.4, and 0.2% Tween-20) containing 5% nonfat dry milk and 0.02% NaN3 for 1 h and then incubated with an antibody against CtBP or α-tubulin in TBST containing 1% nonfat dry milk. The membranes were then incubated with goat anti-rabbit (for CtBP) or goat anti-mouse (for α-tubulin) immunoglobin (Bio-Rad) in TBST containing 1% nonfat dry milk. Bound antibodies were detected with an enhanced chemiluminescence system (Amersham Pharmacia Biosciences).
RT-PCR.
RNA was extracted by using an RNeasy kit (Qiagen, Valencia, CA). DNase I (Ambion)-treated RNA was used for reverse transcription. RNA was incubated with oligodT primers (Invitrogen) and 1 mM dNTPs at 65°C for 5 min, cooled on ice, and incubated with 10 mM DTT, 5× buffer, and RNasin (Promega) RNase inhibitor at 42°C for 2 min. Reverse transcription was performed by using Super transcript II (Invitrogen) at 42°C for 50 min. Reverse transcriptase was inactivated at 70°C for 15 min, and the product was treated with RNase A at 37°C for 30 min before PCR amplification. Real-time PCR was used to quantify the reverse-transcribed products.
ChIP Assays.
HT29 and H1299 cells were treated with CoCl2, NaN3, or hypoxia for 1 h. ChIP assays (14) were performed by using an anti-CtBP antibody or an anti-AcK9 histone H3 antibody (Upstate Biotechnology). Primers surrounding the proximal E-box on E-cadherin promoter (16) were used to PCR-amplify the ChIP sample. Human LINE1 serves as a nonspecific control.
Determination of NAD+/NADH Ratio.
Cells were deproteinized, and the concentrations of lactate and pyruvate were determined as described in ref. 6. In brief, 1 × 106 cells were homogenized in 100 μl of acid-extraction buffer (1 M HClO3) and neutralized with 50 μl of 2 M KHCO3. The concentration of the metabolic intermediates were measured fluorimetrically after an enzymatic cycling reaction using 5 μl of sample. Values for both pyruvate and lactate were detected within the linear range. The free cytoplasmic NAD+/NADH ratio was calculated as described by Williamson et al. (17). Because there should be no barrier to diffusion of NAD+/NADH across the nuclear membrane, we presume that the ratios of free NAD+/NADH in the cytoplasm and nucleus are the same. We have, therefore, determined the ratios of free nucleotides in the former compartment to approximate nuclear values. These determinations were based on methodology described by Krebs and colleagues (17), in which the concentrations of the oxidized and reduced substrates of an NAD+-linked dehydrogenase are used to calculate the NAD+/NADH ratio according to the equation [pyruvate][NADH][H+]/[lactate] [NAD+] = K, where K is the equilibrium constant for the cytoplasmic lactate dehydrogenase.
Determination of the Free Nuclear NADH Concentration.
Two-photon microscopy was performed to measure the nuclear autofluorescent NAD(P)H intensity as described in ref. 43. Cells were grown for 2 days on glass 35-mm dishes (MatTek, Ashland, MA) and maintained at 37°C and 5% CO2 in a humidified chamber on the microscope stage (Zeiss). Hypoxia was induced by continuous flow of gas (5% CO2/95% N2) through the heating unit input valve. NAD(P)H intensity was imaged by using a ×40 1.3 N.A. oil immersion lens (Zeiss), a 710-nm mode-locked Ti:Saph laser (≈3.5 mW at the sample) (Coherent, Santa Clara, CA), and fluorescence collection through a nondescanned detector with a custom 380- to 550-nm filter (Chroma, Rockingham, VT) (44). Images were analyzed with the program metamorph 5.0 (Molecular Devices).
Acknowledgments
We thank D. Dean and S. Frisch for providing the ZEB expression and E-cadherin promoter vectors and R. Veech, P. Stork, Y. Liu, and G. Mandel for helpful comments. This work was supported by National Institutes of Health Grants P01DK044239 (to R.H.G.) and K01CA096561 (to Q.Z.).
Abbreviations
- ChIP
chromatin immunoprecipitation
- siRNA
small interfering RNA.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Perl A. K., Wilgenbus P., Dahl U., Semb H., Christofori G. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 2.Meiners S., Brinkmann V., Naundorf H., Birchmeier W. Oncogene. 1998;16:9–20. doi: 10.1038/sj.onc.1201486. [DOI] [PubMed] [Google Scholar]
- 3.Beavon I. R. Eur. J. Cancer. 2000;36:1607–1620. doi: 10.1016/s0959-8049(00)00158-1. [DOI] [PubMed] [Google Scholar]
- 4.Hockel M., Vaupel P. J. Natl. Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 5.Beavon I. R. Mol. Pathol. 1999;52:179–188. doi: 10.1136/mp.52.4.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Q., Piston D. W., Goodman R. H. Science. 2002;295:1895–1897. doi: 10.1126/science.1069300. [DOI] [PubMed] [Google Scholar]
- 7.Grooteclaes M., Deveraux Q., Hildebrand J., Zhang Q., Goodman R. H., Frisch S. M. Proc. Natl. Acad. Sci. USA. 2003;100:4568–4573. doi: 10.1073/pnas.0830998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grooteclaes M. L., Frisch S. M. Oncogene. 2000;19:3823–3828. doi: 10.1038/sj.onc.1203721. [DOI] [PubMed] [Google Scholar]
- 9.Comijn J., Berx G., Vermassen P., Verschueren K., van Grunsven L., Bruyneel E., Mareel M., Huylebroeck D., van Roy F. Mol. Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q., Yoshimatsu Y., Hildebrand J., Frisch S. M., Goodman R. H. Cell. 2003;115:177–186. doi: 10.1016/s0092-8674(03)00802-x. [DOI] [PubMed] [Google Scholar]
- 11.Imai T., Horiuchi A., Wang C., Oka K., Ohira S., Nikaido T., Konishi I. Am. J. Pathol. 2003;163:1437–1447. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G. L., Jiang B. H., Rue E. A., Semenza G. L. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnamachary B., Zagzag D., Nagasawa H., Rainey K., Okuyama H., Baek J. H., Semenza G. L. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 14.Alliston T., Ko T. C., Cao Y., Liang Y. Y., Feng X. H., Chang C., Derynck R. J. Biol. Chem. 2005;280:24227–24237. doi: 10.1074/jbc.M414305200. [DOI] [PubMed] [Google Scholar]
- 15.Remacle J. E., Kraft H., Lerchner W., Wuytens G., Collart C., Verschueren K., Smith J. C., Huylebroeck D. EMBO J. 1999;18:5073–5084. doi: 10.1093/emboj/18.18.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y., Sawada J., Sui G., Affar el B., Whetstine J. R., Lan F., Ogawa H., Luke M. P., Nakatani Y. Nature. 2003;422:735–738. doi: 10.1038/nature01550. [DOI] [PubMed] [Google Scholar]
- 17.Williamson D. H., Lund P., Krebs H. A. Biochem. J. 1967;103:514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson G. H., Knobel S. M., Arkhammar P., Thastrup O., Piston D. W. Proc. Natl. Acad. Sci. USA. 2000;97:5203–5207. doi: 10.1073/pnas.090098797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shigemori K., Ishizaki T., Matsukawa S., Sakai A., Nakai T., Miyabo S. Am. J. Physiol. 1996;270:L803–L809. doi: 10.1152/ajplung.1996.270.5.L803. [DOI] [PubMed] [Google Scholar]
- 20.Fulco M., Schiltz R. L., Iezzi S., King M. T., Zhao P., Kashiwaya Y., Hoffman E., Veech R. L., Sartorelli V. Mol. Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 21.Giroldi L. A., Bringuier P. P., de Weijert M., Jansen C., van Bokhoven A., Schalken J. A. Biochem. Biophys. Res. Commun. 1997;241:453–458. doi: 10.1006/bbrc.1997.7831. [DOI] [PubMed] [Google Scholar]
- 22.Chinnadurai G. Mol. Cell. 2002;9:213–224. doi: 10.1016/s1097-2765(02)00443-4. [DOI] [PubMed] [Google Scholar]
- 23.Vukovic V., Haugland H. K., Nicklee T., Morrison A. J., Hedley D. W. Cancer Res. 2001;61:7394–7398. [PubMed] [Google Scholar]
- 24.Zhong H., De Marzo A. M., Laughner E., Lim M., Hilton D. A., Zagzag D., Buechler P., Isaacs W. B., Semenza G. L., Simons J. W. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 25.Semenza G. L. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 26.Ravi R., Mookerjee B., Bhujwalla Z. M., Sutter C. H., Artemov D., Zeng Q., Dillehay L. E., Madan A., Semenza G. L., Bedi A. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 27.Birner P., Schindl M., Obermair A., Breitenecker G., Oberhuber G. Clin. Cancer Res. 2001;7:1661–1668. [PubMed] [Google Scholar]
- 28.Imai S., Armstrong C. M., Kaeberlein M., Guarente L. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 29.Landry J., Sutton A., Tafrov S. T., Heller R. C., Stebbins J., Pillus L., Sternglanz R. Proc. Natl. Acad. Sci. USA. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fjeld C. C., Birdsong W. T., Goodman R. H. Proc. Natl. Acad. Sci. USA. 2003;100:9202–9207. doi: 10.1073/pnas.1633591100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar V., Carlson J. E., Ohgi K. A., Edwards T. A., Rose D. W., Escalante C. R., Rosenfeld M. G., Aggarwal A. K. Mol. Cell. 2002;10:857–869. doi: 10.1016/s1097-2765(02)00650-0. [DOI] [PubMed] [Google Scholar]
- 32.Nardini M., Spano S., Cericola C., Pesce A., Massaro A., Millo E., Luini A., Corda D., Bolognesi M. EMBO J. 2003;22:3122–3130. doi: 10.1093/emboj/cdg283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q., Fjeld C. C., Nottke A. C., Goodman R. H. in CtBP Family Proteins, ed. Chinnadurai, G. Georgetown, TX: Landes Bioscience; 2005. [Google Scholar]
- 34.Eger A., Aigner K., Sonderegger S., Dampier B., Oehler S., Schreiber M., Berx G., Cano A., Beug H., Foisner R. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 35.Darai E., Scoazec J. Y., Walker-Combrouze F., Mlika-Cabanne N., Feldmann G., Madelenat P., Potet F. Hum. Pathol. 1997;28:922–928. doi: 10.1016/s0046-8177(97)90007-1. [DOI] [PubMed] [Google Scholar]
- 36.Tomlinson J. S., Alpaugh M. L., Barsky S. H. Cancer Res. 2001;61:5231–5241. [PubMed] [Google Scholar]
- 37.Cai J., Ikeguchi M., Tsujitani S., Maeta M., Liu J., Kaibara N. Gastric Cancer. 2001;4:66–74. doi: 10.1007/pl00011726. [DOI] [PubMed] [Google Scholar]
- 38.Boyd J. M., Subramanian T., Schaeper U., La Regina M., Bayley S., Chinnadurai G. EMBO J. 1993;12:469–478. doi: 10.1002/j.1460-2075.1993.tb05679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundfor K., Lyng H., Rofstad E. K. Br. J. Cancer. 1998;78:822–827. doi: 10.1038/bjc.1998.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brizel D. M., Sibley G. S., Prosnitz L. R., Scher R. L., Dewhirst M. W. Int. J. Radiat. Oncol. Biol. Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 41.Kokura S., Yoshida N., Imamoto E., Ueda M., Ishikawa T., Uchiyama K., Kuchide M., Naito Y., Okanoue T., Yoshikawa T. Cancer Lett. 2004;211:79–87. doi: 10.1016/j.canlet.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 42.Andre F., Rigot V., Thimonier J., Montixi C., Parat F., Pommier G., Marvaldi J., Luis J. Int. J. Cancer. 1999;83:497–505. doi: 10.1002/(sici)1097-0215(19991112)83:4<497::aid-ijc11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 43.Rocheleau J. V., Head W. S., Nicholson W. E., Powers A. C., Piston D. W. J. Biol. Chem. 2002;277:30914–30920. doi: 10.1074/jbc.M202314200. [DOI] [PubMed] [Google Scholar]
- 44.Rocheleau J. V., Head W. S., Piston D. W. J. Biol. Chem. 2004;279:31780–31787. doi: 10.1074/jbc.M314005200. [DOI] [PubMed] [Google Scholar]