Abstract
Compartment-specific Ras signaling is an emerging paradigm that may explain the multiplex outputs from a single GTPase. The fission yeast, Schizosaccharomyces pombe, affords a simple system in which to study Ras signaling because it has a single Ras protein, Ras1, that regulates two distinct pathways: one that controls mating through a Byr2-mitogen-activated protein kinase cascade and one that signals through Scd1-Cdc42 to maintain elongated cell morphology. We generated Ras1 mutants that are restricted to either the endomembrane or the plasma membrane. Protein binding studies showed that each could interact with the effectors of both pathways. However, when examined in ras1 null cells, endomembrane-restricted Ras1 supported morphology but not mating, and, conversely, plasma membrane-restricted Ras1 supported mating but did not signal to Scd1-Cdc42. These observations provide a striking demonstration of compartment-specific Ras signaling and indicate that spatial specificity in the Ras pathway is evolutionarily conserved.
Keywords: cancer, Cdc42, Int6, mitogen-activated protein kinase, eIF3
Ras proteins control a wide variety of cellular processes including growth and differentiation. Mutations in RAS genes are associated with 30% of human cancers (1, 2). Mammalian genomes contain three closely related RAS genes that encode four proteins, H-Ras, N-Ras, K-Ras4A, and K-Ras4B. Recent studies demonstrate that the various Ras isoforms are not functionally redundant (3). Although highly homologous in their N-terminal domains, Ras isoforms differ substantially in their C-terminal sequences (4). These C-terminal hypervariable regions include a CAAX motif that is subject to a series of posttranslational modifications and an adjacent region that, in all Ras isoforms except K-Ras4B, contains cysteine residues that are sites of palmitoylation. C-terminal processing directs trafficking of nascent Ras proteins from their site of synthesis in the cytosol to the cytosolic face of the endoplasmic reticulum (ER) and Golgi apparatus and eventually to the plasma membrane (PM). Until recently, Ras proteins were considered to signal exclusively from the PM. We have shown that N-Ras and H-Ras can be activated on and signal from the endomembrane (5–7). This discovery has led to the hypothesis that Ras regulates distinct pathways from different subcellular compartments. The complexity of Ras isoforms and closely related GTPases in metazoan cells has made testing this hypothesis difficult.
Unlike metazoan cells, or even Saccharomyces cerevisiae, the genome of the fission yeast, Schizosaccharomyces pombe, encodes only one Ras protein, Ras1 (8). Nevertheless, Ras1 regulates two effectors that control distinct signaling pathways. Ras1 regulates Byr2, a mitogen-activated protein/ERK kinase kinase (MEKK) homolog that responds to pheromone signaling and controls mating through a mitogen-activated protein kinase (MAPK) pathway (9). Ras1 also regulates Scd1 [also known as Ral1 (10)], an exchange factor for the S. pombe Cdc42 ortholog (11) that regulates morphogenesis to maintain an elongated cell. The Ras1-Scd1 pathway also influences mitosis, in part by interacting with Yin6, a homolog of the mammalian Int6, to control proteasome translocation and assembly (12, 13). Cells with a defective Ras1-Byr2 pathway are sterile but have an elongated cell morphology. Conversely, cells defective in the Ras1-Scd1 pathway are abnormally round, but their mating pheromone signaling remains intact. S. pombe therefore represents a genetically well characterized model organism that offers an ideal simple system in which to study differential signaling of Ras.
Two Cdc25-like exchange factors for Ras1 have been identified as Ste6 and Efc25 (14–16). Interestingly, they are not functionally interchangeable. Whereas Ste6 specifically regulates the Byr2 pathway, Efc25 regulates only the Scd1 pathway. These observations are somewhat surprising because they are antithetical to GTPase signaling models that predict that a GTP-bound GTPase-like Ras should be free to interact with any of its effectors regardless of which of several exchange factors catalyzed the GTP/GDP exchange. Among the possible explanations for this paradox is the possibility that the regulation of the mating versus the morphology pathways occurs on distinct subcellular compartments. To test this idea, we studied the ability of Ras1 to control Byr2 and Scd1 from the PM versus the endomembrane.
Results and Discussion
Ras1 Palmitoylation Mutant Activates the Scd1 Morphology Pathway but Not the Byr2 Mating Pathway.
Cysteine residues immediately upstream of CAAX sequences are palmitoylated in S. cerevisiae and higher metazoans. S. pombe Ras1, like S. cerevisiae Ras2p and mammalian N-Ras, contains a single such cysteine at position 215 (C215), and we have obtained evidence that it is palmitoylated (see Fig. 5, which is published as supporting information on the PNAS web site). To determine the functional significance of palmitoylation at position 215, we constructed expression vectors that use the ras1 promoter to express Ras1 or various Ras1 mutants, including one with a cysteine to serine substitution at position 215 to block palmitoylation. These vectors were integrated chromosomally in ras1 null (ras1Δ) cells via homologous recombination. Immunoblots were performed to screen for strains in which the exogenous Ras1 protein was expressed at a level similar to that of endogenous Ras1 (see Fig. 6, which is published as supporting information on the PNAS web site). Analysis of these strains revealed that whereas, as expected, wild-type Ras1 rescued both the mating and morphological defects, the palmitoylation-deficient mutant (Ras1C215S) rescued only the morphological defect (Fig. 1A). In contrast, the farnesylation-deficient mutant (Ras1C216S) and the double Ras1C215/216S mutant rescued neither defect, demonstrating that, unlike palmitoylation, farnesylation is absolutely required for all Ras1 functions. A palmitoylation-deficient, constitutively active Ras1 protein (8) (Ras1G17V,C215S) restored normal cell morphology without affecting the mating pheromone response (Fig. 1B), demonstrating that the selective rescue of the morphology pathway is not the result of inefficient GTP/GDP exchange. We have shown previously that yin6Δ in combination with ras1Δ creates a synthetic growth defect that results from the specific inactivation of the Scd1-Cdc42 pathway (13). Fig. 1C shows that Ras1C215S, but not Ras1C215/216S or Ras1C216S, rescued the cold-dependent growth defect of yin6Δ ras1Δ cells, confirming the capacity of palmitoylation-deficient Ras to signal down the Scd1-Cdc42 pathway.
Fig. 1.
Ras1C215S activates the Scd1, but not the Byr2, pathway. (A) ras1Δ cells expressing GFP-tagged versions of the indicated proteins from the endogenous ras1 promoter were imaged at either log phase (−) or after 3 days of starvation to induce sexual differentiation (+) and scored for sporulation (asterisks). (Scale bars: 5 μm.) (B) ras1Δ cells expressing GFP-tagged versions of the indicated proteins from the endogenous ras1 promoter were imaged at log phase. GFP-Ras1G17V, but not GFP-Ras1G17V,C215S, induced a hypersexual phenotype (8), marked by the presence of overextended conjugation tubes (arrowhead). (C) Cells lacking both ras1 and yin6 (ras1Δ yin6Δ) were transformed with vectors expressing the indicated GFP-tagged Ras1 proteins from the endogenous ras1 promoter. Transformed cells were serially diluted, spotted on plates, and incubated at the temperatures shown to determine whether various Ras1 proteins can rescue the cold-dependent growth defects of ras1Δ yin6Δ cells. Identical results were obtained in A–C whether Ras1 proteins were either untagged or tagged by a single copy of the hemagglutinin (HA) epitope and whether they were overexpressed by a high-copy vector.
Ras1C215S Can Efficiently Bind both Scd1 and Byr2.
To determine whether the substitution of serine for cysteine at position 215 of Ras1 affects the ability of the GTPase to bind to effectors, we tested Ras1-C215S for interaction with Byr2 and Scd1 by using a yeast two-hybrid assay. Ras1-C215S, like wild-type Ras1, bound both effectors with equivalent efficiency (Fig. 2A). In this assay, only the pool of the wild-type Ras1 fusion protein that enters the yeast nucleus can give a positive result. Although we do not have evidence that this pool is modified by palmitate, the critical interpretation of the result stands as follows: Palmitoylation is not required for the interaction of Ras1 with either Byr2 or Scd1. This conclusion is further supported by in vitro binding experiments in which GST-Byr2 pulled down, in a GTP-dependent fashion, equivalent amounts of wild-type Ras1, Ras1-C215S (Fig. 2B), and Ras1-C216S (Fig. 7, which is published as supporting information on the PNAS web site). Collectively, these two measures of protein-protein interaction suggest that the inability of Ras1-C215S to rescue mating was not because of an intrinsic defect in Byr2 binding.
Fig. 2.
Various Ras1 mutants bind Byr2 and Scd1 with equal efficiency. (A) Protein-protein interactions were measured by the yeast two-hybrid system, and the results of activation of the HIS3 reporter gene are shown. Various Ras1 proteins were fused with the GAD. Byr2 was fused with the Gal4 DNA binding domain (GBD), whereas Scd1ΔN (containing the minimal Ras binding domain of Scd1) was fused with the LexA DNA binding domain (LBD). (B) His-Ras1, His-Ras1C215S (both with a T7 epitope tag) and GST-Byr2 were expressed and purified from E. coli. GST-Byr2 bound to glutathione-Sepharose beads was mixed with the GTPγS- or GDP-loaded Ras1 proteins. Affinity purified proteins associated with Byr2 were then analyzed by immunoblot using an antibody directed to T7. (The binding of Byr2 to Ras1C216 was examined under identical conditions and is shown in Fig. 7.)
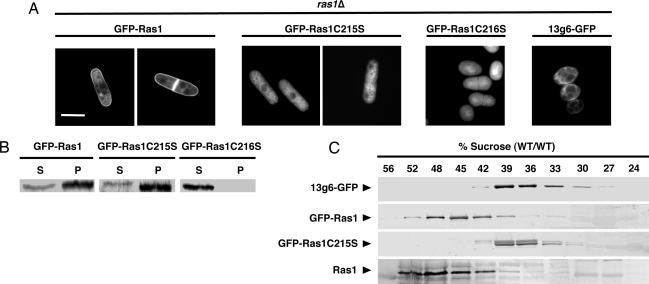
RasC215S Localizes to the Endomembrane but Not the PM.
We next determined the subcellular distribution of various Ras1 proteins by expressing from the endogenous ras1 promoter GFP-tagged forms of these proteins in ras1Δ cells (Fig. 3A). As expected, GFP-Ras1 appeared on the PM, including that forming along the fission plane. Importantly, a weaker fluorescence signal was also observed on internal membranes. In contrast, GFP-Ras1C215S was not detected on the PM, and its localization on intracellular membranes was enhanced relative to that of wild-type Ras1. The latter pattern included the nuclear envelope and associated reticular structures and was similar to that of a GFP-tagged ER-resident protein, 13g6 (17). GFP-Ras1C216S did not associate with any membrane structure but rather appeared diffuse throughout the nucleoplasm and cytosol in a homogeneous distribution that revealed negatively imaged organelles.
Fig. 3.
Ras1C215S localizes to the endomembrane. (A) Vectors expressing GFP-tagged forms of the Ras1 proteins from the endogenous ras1 promoter or the ER marker 13g6 (20) from the nmt1 promoter, were integrated into the chromosome of ras1Δ cells and imaged during log-phase growth. (Scale bar: 5 μm.) (B) High-speed supernatant (S) and membrane pellet (P) fractions of lysates from ras1Δ cells expressing the indicated proteins from the endogenous ras1 promoter were analyzed by immunoblotting with the GFP antibody. (C) Lysates from ras1Δ cells expressing either 13g6-GFP, GFP-Ras1, or GFP-Ras1C215S, or from wild-type cells expressing GFP alone, were applied to a 15–56% (wt/wt) sucrose gradient. After centrifugation, fractions were collected and analyzed by immunoblotting with either the anti-pan-Ras RAS 10 antibody or the GFP antibody.
To confirm this localization by an independent method, we performed subcellular fractionation. Whereas GFP-Ras1C216S was found entirely in the high-speed supernatant, both GFP-Ras1 and GFP-Ras1C215S were predominantly found in the membrane pellet (Fig. 3B), demonstrating that, although not localized to the PM, palmitoylation-deficient Ras1 is efficiently associated with other cellular membranes. Analysis of the membrane fractions by sucrose density gradient revealed that the peaks of endogenous Ras1 and 13g6 could be readily resolved with the Ras1 enriched fractions migrating further into the sucrose gradient, consistent with the behavior of PM-derived vesicles. Nevertheless, there was some overlap in the middle of the gradient, indicating that a portion of the total pool of endogenous Ras1 associated with lighter membranes, consistent with the fluorescent result (Fig. 3A). Endogenous Ras1 migrated in a pattern similar to that of GFP-Ras1. In contrast, the peak of GFP-Ras1C215S was shifted dramatically into the lighter membranes, and the pattern was nearly identical to that of membranes enriched for 13g6 (Fig. 3C). These results indicate that, whereas the bulk of Ras1 is in the PM, some remains associated with the endomembrane and that palmitoylation-deficient Ras1C215S is restricted to endomembrane. Thus, as in metazoan cells, Ras1 that is farnesylated but not palmitoylated is targeted to and accumulates on the ER.
Ras1 at the PM Signals to the Byr2 Mating Pathway but Not the Scd1 Morphology Pathway.
Having thus demonstrated that ER-associated Ras1 signals down the Scd1, but not the Byr2, pathway, we sought to determine the functional capabilities of PM-restricted Ras1. To this end, we sought a C-terminal PM targeting motif that, unlike the CAAX motif, would not direct trafficking through the endomembrane. We reasoned that we should avoid the transmembrane tether of an intrinsic S. pombe PM protein because, as a secretory protein, it would transit the endomembrane en route to the PM and might therefore give an ambiguous result. Accordingly, we sought a protein that might be targeted directly from free polysomes in the cytosol to the PM. We examined the mammalian non-CAAX GTPase Rit that is targeted to the PM by a hydrophobic C terminus, which requires no posttranslational modification (18). Unlike GFP-H-Ras that, at steady state, localized to both the PM and Golgi apparatus, GFP-Rit localized exclusively on the PM (Fig. 4A). Indeed, GFP-Rit gave us the cleanest PM targeting that we have observed to date. Moreover, by extending GFP with the C terminus of Rit (RitC), we showed that this region was necessary and sufficient for stringent PM targeting without any detectable endomembrane localization (Fig. 4A). We therefore made a cyan fluorescent protein (CFP)-tagged Ras1-RitC chimeric protein by replacing the C-terminal hypervariable domain and CAAX box of Ras1 with RitC (CFP-Ras1-RitC).
Fig. 4.
Ras1 localized to the PM activates the Byr2, but not the Scd1, pathway. (A) The last C-terminal 62 aa of the Rit protein (RitC) was fused to GFP, transiently expressed in Madin Darby canine kidney (MDCK) cells, and imaged by confocal microscopy. GFP, GFP-H-Ras, and GFP-Rit proteins were also examined as controls. Although GFP-H-Ras appeared on both the PM and endomembrane (e.g., Golgi apparatus), GFP-Rit appeared exclusively on the PM. (Scale bar: 10 μm.) (B) ras1Δ cells coexpressing YFP-tagged 13g6 off the nmt1 promoter and either CFP-tagged Ras1-RitC or CFP-tagged Ras1C215S off the endogenous ras1 promoter were imaged by confocal microscopy. The CFP signal was pseudocolored green, whereas the YFP signal was pseudocolored red. Yellow in the merged images marks regions where the tested proteins overlap in their subcellular localization. (C) ras1Δ cells expressing either GFP-tagged 13g6 from the nmt1 promoter or GFP-Ras1-RitC from the endogenous ras1 promoter were analyzed by sucrose gradients as in Fig. 3, with the exception that a more narrowly focused gradient was used. (D) ras1Δ cells expressing various GFP-tagged Ras1 proteins were plated and imaged at either log phase (−) or under conditions of sexual differentiation (+) as in Fig. 1. Asterisks indicate sporulating cells. (Scale bar: 5 μm.) Similar results were obtained when these proteins were tagged by a single hemagglutinin epitope and when these proteins were expressed from a high-copy vector (data not shown).
We coexpressed CFP-Ras1-RitC or CFP-tagged Ras1C215S in ras1Δ cells with a yellow fluorescent protein (YFP)-tagged 13g6 to mark the ER (Fig. 4B). All of the membranes marked by 13g6-YFP showed colocalization with CFP-Ras1C215S, indicating that palmitoylation-deficient Ras1 has significant affinity for the ER. CFP-Ras1C215S marked intracellular membranes, as well as cytoplasm, not labeled by 13g6-YFP, indicating that, although there is significant overlap, the subcellular compartments marked by the two proteins are not identical. In contrast, CFP-Ras1-RitC was observed at the edge of the cell and showed no overlap with 13g6-YFP. It should be noted that, as is the case with S. cerevisiae, much of the ER of S. pombe is found immediately subjacent to the PM such that electron microscopy is required for clear resolution of the two membrane compartments (19, 20) (see Fig. 8, which is published as supporting information on the PNAS web site), and decoration of the ER in these cells with fluorescent probes sometimes gives the appearance of PM at the resolving power of the light microscope. One distinction we have observed in the two similar patterns is that fluorescent probes that decorate the PM give a smooth linear pattern, whereas those that decorate ER give a lumpy pattern at the cell edge.
The distribution of CFP-Ras1-RitC in the PM was confirmed by subcellular fractionation on sucrose gradients that revealed CFP-Ras1-RitC to be enriched in heavier fractions (Fig. 4C). Like palmitoylation-deficient Ras1, Ras1-RitC bound both Scd1 and Byr2 with efficiencies equal to that of wild-type Ras1 (Fig. 2A). Importantly, functional analysis in ras1Δ cells revealed that, whereas GFP-Ras1C215S rescued the Scd1 morphology but not the Byr2 mating pathway, the converse was true of GFP-Ras1-RitC (Fig. 4D). Moreover, the inability of GFP-Ras1-RitC to rescue the growth defect of yin6Δ ras1Δ cells confirmed its deficiency in Scd1 signaling (data not shown). The inability of PM-restricted Ras1-RitC to signal to Scd1 suggests that it is the minor pool of endogenous Ras1 found on internal membranes (Fig. 3A), which signals down the morphology pathway.
Our results demonstrate that Ras1 restricted to the ER signals to the Scd1-Cdc42 pathway but is incapable of signaling down the Byr2-mitogen-activated protein kinase (MAPK) pathway. The observation that constitutively active, endomembrane-restricted Ras1G17V,C215S also selectively activated the morphology pathway and demonstrates that compartmentalization occurs at the level of Ras1 rather than at the level of the GEF specific for this pathway. Conversely, Ras1 restricted to the PM signals via the Byr2-MAPK pathway but does not activate Scd1 and Cdc42. Because pheromone receptors relay mating signals across the PM, which activate Byr2, it seems appropriate that PM-associated Ras1 regulates this pathway. In support of this idea, Byr2 has been shown to associate with the PM upon sexual differentiation (21). We and others (12, 22) have shown that the Ras1-Scd1 pathway is involved in protein trafficking. Thus it is logical to expect that this pathway operates at the endomembrane. Consistent with this notion are the observations that mammalian Cdc42, when dissociated from RhoGDI, is localized predominantly on the endomembrane (23) and that the subcellular localization of S. pombe Cdc42 is indistinguishable from that of Ras1-C215S (24). Thus, downstream elements of the various Ras signaling pathways localize to the compartments upon which they are regulated by Ras and may therefore determine the spatial aspect of signaling.
In conclusion, the genetically tractable and relatively simple system offered by S. pombe has allowed a clear demonstration of compartmentalized Ras signaling. These results support the observation in S. cerevisiae that one Ras effector, Eri1, which is a component of GPI-GlcNAc transferase, is restricted to the ER (25, 26). Our observations also support those observations reported in mammalian cells (27) and reveal that spatially restricted Ras signaling has been conserved through evolution.
Materials and Methods
S. pombe Strains and Microbial Manipulations.
The wild-type strain used in this study is SP870 (11). ras1Δ (strains SPRU and SPRN) and ras1Δ yin6Δ (RAS1UYIN6K) strains were all derived from SP870 as described (9, 11, 13). Cells were grown in either yeast extract medium (YEAU) or synthetic minimal medium (MM) (28). To express genes controlled by the thiamine-repressable nmt1 promoter, thiamine-free MM medium was used. All expression studies were done with vectors integrated into the ars1 locus of the S. pombe chromosome, which was done by transforming cells with MluI-linearized vectors. All experiments were carried out with cells pregrown to early logarithmic phase (2–5 × 106 cells per ml). To spot cells on plates, equal numbers of cells were serially diluted 1:5.
Mammalian Cell Culture and Transfection.
Madin Darby canine kidney (MDCK) cells (American Type Culture Collection) were grown in DMEM containing 10% FBS (CellGro, Herndon, VA) at 5% CO2 and 37°C. All transfections (0.5 μg DNA per 35-mm dish) were performed 1 day after plating at 50% confluence by using SuperFectTM (Qiagen). Transiently transfected cells were analyzed 1 day after transfection. To facilitate microscopy (see Microscopy), cells were imaged in the same 35-mm culture dish that incorporated a #1.5 glass coverslip with a sealed 15-mm cutout on the bottom (MatTek, Ashland, MA).
Plasmid Constructions.
pSLF173, pGADGH, pVJL11, pLBDSCD1ΔN, pREP1, pARTCMRAS1, pARTCMRAS1G17V, pARTCMYIN6, and pGBD are as described (8, 9, 12, 13, 16, 29, 30). pREP4113G6GFP was a kind gift from the Cande laboratory (17). pKH3RIT was generously provided by the Andres laboratory (University of Kentucky). pRPGFP and pRPGFPRAS1 carry the coding sequences for GFP and GFP-Ras1. The promoter in these plasmids was derived from 338 bp of the ras1 5′ flanking sequence, which contains the ras1 promoter (P. Papadaki and E.C.C., unpublished work). pTRCHISB was from Invitrogen. The Ras1C215S, Ras1C216S, and Ras1C215/216S mutants were generated via PCR by using either pARTCMRAS1 or pARTCMRAS1G17V as the template. The forward primer used was 5′-CGGGATCCGATGAGGTCTACCTACTTAAG-3′; the reverse primers were 5′-CGGGATCCCTAACATATAACACAAGATTTAGTTGA-3′ (for C215S), 5′-CGGGATCCCTAACATATAACAGAACATTTAGT-3′ (for C216S), and 5′-CGGGATCCCTAACATATAACAGAAGATTTAGTTGA-3′ (for C215/216S). The PCR products generated with the pARTCMRAS1 template were digested with BamHI and cloned into pREP1, pRPGFP, pGADGH, and pTRCHISB. The PCR products generated with the pARTCMRAS1G17V template were digested with BamHI and cloned into pRPGFP and pGADGH. ras1 was amplified by PCR using pARTCMRAS1 as a template, digested with BamHI, and ligated into pREP1, pGADGH, and pTRCHISB to create pREP1RAS1, pGADGHRAS1, and pTRCHISRas1, respectively. ras1G17V was released from pARTCMRAS1G17V by digesting with BamHI and SacI, and the fragment was ligated into pRPGFP to create pRPGFPRAS1G17V.
pARTCMRAS1G17V was also digested with SacI, blunt-ended, and then digested with BamHI to release the ras1G17V fragment, which was ligated into pGADGH. To create GFP-Rit, a fragment encoding the Rit protein was released from pKH3RIT by BglII and ligated into pEGFPC1 to form pEGFPC1RIT. To generate GFP-RitC, a fragment encoding the last 62 C-terminal amino acids of Rit was amplified by PCR using pEGFPC1RIT as a template. The forward and reverse primers were 5′-GCGGAATTCGTGTCCCTTTTTTGAGACATCTGCTG-3′ and 5′-GAAGAAGAAAGATTCAGTAACTTGAGGATCCCGG-3′. The fragment was digested with EcoRI and BamHI and ligated into pEGFPC1 to form pEGFPC1RITC. To generate the Ras1-RitC chimera, a fragment encoding the last 62 C-terminal amino acids of the human Rit protein was amplified by PCR using pKH3RIT as a template. The forward and reverse primers were 5′-CTAGTCTAGATGTCCCTTTTTTGAGACAT-3′ and 5′-CGAGCTCTCAAGTTACTGAATCTTTCTTC-3′. In addition, a fragment encoding a truncated form of Ras1 lacking its last 48 C-terminal amino acids was amplified by PCR using primers 5′-CGGGATCCGATGAGGTCTACCTACTTAAG-3′ and 5′-CTAGTCTAGAGCGACGGATCGTGCGAACCA-3′. The PCR fragment encoding the truncated form of Ras1 was digested with BamHI and XbaI and then ligated into pBluescript SK− (Stratagene) to form pBSRAS1ΔC. The PCR fragment encoding the Rit C terminus was digested with XbaI and SacI, then ligated into pBSRAS1ΔC to form pBSRAS1RIT. A BamHI-SacI fragment encoding the Ras1-RitC chimera was released from pBSRAS1RIT and ligated into pRPGFP to form pRPGRAS1RIT. To create the CFP-tagged version of the Ras1-RitC chimera, a fragment encoding CFP was amplified by PCR using pECFP (Clontech) as a template. The forward and reverse primers were 5′-AACTGCAGATGGTGAGCAAGGGCGAGGAGCTG-3′ and 5′-CTAGCTAGCTTGTACAGCTCGTCCATGCCGAG-3′. In addition, GFP was released from pRPGFP by digesting with PstI and NheI to create pRP. The CFP PCR product was digested with PstI and NheI and ligated into pRP to create pRPCFP. The BamHI-SacI fragment encoding the Ras1-RitC chimera was then ligated into pRPCFP to create pRPCFPRAS1RIT. The BamHI-digested fragment encoding Ras1C215S was ligated into pRPCFP to create pRPCFPRAS1C215S. To create 13g6 with a C-terminal YFP fusion, a fragment encoding 13g6 was amplified by PCR using pREP4113G6GFP as a template. The forward and reverse primers were 5′-CCGCTCGAGATGAGGTTTGCATTTTTTGG-3′ and 5′-ATAGTTTAGCGGCCGCATCGTAAACGGACCATGATGG-3′. The 13g6 PCR product was then digested with XhoI and NotI and then ligated into pSLF173 to create pSLF13G6. In addition, a fragment encoding YFP was amplified by PCR using pEYFP (Clontech) as a template. The forward and reverse primers were 5′-ATAAGAATGCGGCCGCGTGAGCAAGGGCGAGGAGCTG-3′ and 5′-GAAGATCTGTTTACTTGTACAGCTCGTCCATGCCGAG-3′. The YFP PCR product was then digested with NotI and BglII and then ligated into pSLF13G6 to create pSLF13G6YFP.
Expression of Various Ras1 Proteins at Endogenous Levels.
ras1Δ cells (strain SPRN) were transformed with MluI-linearize pRPGFP vectors that express various Ras1 proteins off the endogenous ras1 promoter. Transformed colonies were first selected to ensure that the linearized vector had been integrated chromosomally, and these clones of cells were further screened by Western blot by using the pan anti-Ras monoclonal antibody RAS10 (1:500; Upstate Biotechnology, Lake Placid, NY) to seek cells in which the transgene was expressed at levels comparable to that of endogenous Ras1.
Yeast Two-Hybrid Assay.
The binding was tested by measuring the activation of both the lacZ and HIS3 reporter genes (11). Ras1 mutant proteins with abnormal C-termini as well as normal Ras1 proteins were fused with the Gal4 activation domain (GAD) as described earlier. Vectors expressing Byr2 and Scd1ΔN were as described (31, 32). pLBDSCD1ΔN autoactivates the HIS3 reporter gene, so 3-AT (3-aminotriazole) was added to reduce background.
GST Pull-Down Assay.
His-Ras1, His-Ras1C215S, His-Ras1C216S, and His-Ras1C215/216S were expressed in Escherichia coli BL21 DE3 cells and grown to midlog phase (6 × 108 cells per ml) in 50 -ml cultures of LB media containing both ampicillin and chloramphenicol. After induction with isopropyl-β-d-thiogalactoside (IPTG) (Sigma), lysates were prepared in PBS buffer plus 1% Triton X-100 and phenylmethylsulfonyl fluoride (PMSF; Sigma). After treatment with DNase, crude lysates were centrifuged at 2,700 × g for 10 min. For GTPγS or GDP treatment, 2 μl of 0.5 M EDTA and 1 μl of 10 mM GTPγS or GDP were added to 100 μl of supernatant and incubated at 30°C for 15 min. The reaction was terminated by placing the samples on ice and by adding 6 μl of 1 M MgCl2. Supernatants were then incubated with 100 μl of His-Bind Resin (Novagen) for 1 h at 4°C. Resin was washed with binding and wash buffers and incubated in elution buffer for 1 h at 4°C. GST-Byr2 was expressed using pGEX2TBYR2RBD (33), and lysates were similarly prepared from E. coli. Supernatants were incubated with 20 μl of glutathione Sepharose beads (Amersham Pharmacia Biotech) for 1 h at 4°C. Beads were washed with and resuspended in the lysis buffer [25 mM Tris·HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 1% Nonidet P-40, and 5% glycerol], then added to the GTPγS- or GDP-loaded His-Ras1, His-Ras1C215S, His-Ras1C216S, or His-Ras1C215/216S supernatants and incubated at 4°C for 1 h. Proteins were analyzed by immunoblot using the T7 antibody (1:10,000; Novagen) and Coomassie blue staining.
Subcellular Fractionation.
ras1Δ cells carrying integrated forms of vectors expressing either GFP-Ras1, GFP-Ras1C215S, or GFP-Ras1C216S off the endogenous ras1 promoter were grown in 100 ml of synthetic minimal medium (MM) to early log phase and broken with glass beads in lysis buffer [PBS (pH 6.5), PMSF, and yeast protease inhibitors; Sigma]. Lysates were first cleared by centrifugation (2,700 × g, 10 min at 4°C) and then subjected to ultracentrifugation (106,000 × g, 2 h at 4°C) to obtain total membrane pellet and supernatant fractionations. Supernatant and membrane pellet fractions were analyzed by immunoblotting with a GFP antibody (1:500; Molecular Probes). Wild-type cells carrying an integrated form of a vector expressing GFP off the endogenous ras1 promoter, ras1Δ cells carrying integrated forms of vectors expressing either 13g6-GFP off the nmt1 promoter, or GFP-Ras1 or GFP-Ras1C215S off the endogenous ras1 promoter were grown in 100 ml of MM and lysed as described above. Cleared supernatants were applied to a 4.2-ml 15–56% (wt/wt) sucrose gradient prepared in lysis buffer. After centrifugation for 18 h (200,000 × g, 4°C), 300-μl fractions were collected from the bottom. Each fraction was analyzed by immunoblotting with the GFP antibody. The endogenous Ras1 was detected by the RAS10 monoclonal antibody.
Microscopy.
Living cells were examined with a Zeiss Axiovert 200 epifluorescence microscope equipped with a Q-Imaging Retiga EX camera and openlab imaging software (ver. 3.1.5; Improvision. Lexington, MA) or with a Zeiss 510 inverted laser scanning confocal microscope.
Supplementary Material
Acknowledgments
We thank Z. Cande, J. Armstrong, D. Andres, and P. Papadaki for providing materials critical for our studies; S. Quatela and S. Chahar for technical assistance; and G. Chamness for discussion and careful reading of the manuscript. We also thank M. Morphew and J. R. McIntosh for the super electron microscopy support. E.C.C. is supported by National Institutes of Health Grants CA90464 and CA107187, U.S. Department of Defense Grant BC021935, and Susan Komen Foundation Grant PDF0402733. M.P. is supported by National Institutes of Health Grants GM55279, CA116034, and CA118495 and the Burroughs–Wellcome Fund. The electron microscopy work is supported by National Institutes of Health Grant RR00592 (to J. R. McIntosh of the National Center for Research Resources, Bethesda, MD).
Abbreviations
- CFP
cyan fluorescent protein
- ER
endoplasmic reticulum
- GAD
Gal4 activation domain
- PM
plasma membrane
- RitC
C terminus of Rit
- YFP
yellow fluorescent protein.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Bos J. L. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 2.Rodenhius S. Semin. Cancer Biol. 1992;3:241–247. [PubMed] [Google Scholar]
- 3.Malumbres M., Pellicer A. Front. Biosci. 1998;3:d887–d912. doi: 10.2741/a331. [DOI] [PubMed] [Google Scholar]
- 4.Brandt-Rauf P. W., Carty R. P., Chen J., Avitable M., Lubowsky J., Pincus M. R. Proc. Natl. Acad. Sci. USA. 1988;85:5869–5873. doi: 10.1073/pnas.85.16.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choy E., Chiu V. K., Silletti J., Feoktistov M., Morimoto T., Michaelson D., Ivanov I. E., Philips M. R. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 6.Chiu V. K., Bivona T., Hach A., Sajous J. B., Silletti J., Wiener H., Johnson R. L., Cox A. D., Philips M. R. Nat. Cell Biol. 2002;4:343–350. doi: 10.1038/ncb783. [DOI] [PubMed] [Google Scholar]
- 7.Bivona T. G., Perez De Castro I., Ahearn I. M., Grana T. M., Chiu V. K., Lockyer P. J., Cullen P. J., Pellicer A., Cox A. D., Philips M. R. Nature. 2003;424:694–698. doi: 10.1038/nature01806. [DOI] [PubMed] [Google Scholar]
- 8.Fukui Y., Kozasa T., Kaziro Y., Takeda T., Yamamoto M. Cell. 1986;44:329–336. doi: 10.1016/0092-8674(86)90767-1. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Xu H.-P., Riggs M., Rodgers L., Wigler M. Mol. Cell. Biol. 1991;11:3554–3563. doi: 10.1128/mcb.11.7.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukui Y., Yamamoto M. Mol. Gen. Genet. 1988;215:26–31. doi: 10.1007/BF00331298. [DOI] [PubMed] [Google Scholar]
- 11.Chang E. C., Barr M., Wang Y., Jung V., Xu H. P., Wigler M. H. Cell. 1994;79:131–141. doi: 10.1016/0092-8674(94)90406-5. [DOI] [PubMed] [Google Scholar]
- 12.Yen H.-C. S., Gordon C., Chang E. C. Cell. 2003;112:207–217. doi: 10.1016/s0092-8674(03)00043-6. [DOI] [PubMed] [Google Scholar]
- 13.Yen H.-C. S., Chang E. C. Proc. Natl. Acad. Sci. USA. 2000;97:14370–14375. doi: 10.1073/pnas.97.26.14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes D. A., Fukui Y., Yamamoto M. Nature. 1990;344:355–357. doi: 10.1038/344355a0. [DOI] [PubMed] [Google Scholar]
- 15.Tratner I., Fourticq-Esqueoute A., Tillit J., Baldacci G. Gene. 1997;193:203–210. doi: 10.1016/s0378-1119(97)00115-7. [DOI] [PubMed] [Google Scholar]
- 16.Papadaki P., Pizon V., Onken B., Chang E. C. Mol. Cell. Biol. 2002;22:4598–4606. doi: 10.1128/MCB.22.13.4598-4606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brazer S.-C. W., Williams H. P., Chappell T. G., Cande W. Z. Yeast. 2000;16:149–166. doi: 10.1002/(SICI)1097-0061(20000130)16:2<149::AID-YEA514>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Lee C.-H. J., Della N. G., Chew C. E., Zack D. J. J. Neurosci. 1996;16:6784–6794. doi: 10.1523/JNEUROSCI.16-21-06784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prinz W. A., Grzyb L, Veenhuis M., Kahana J. A., Silver P. A., Rapoport T. A. J. Cell Biol. 2000;150:461–474. doi: 10.1083/jcb.150.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pidoux A. L., Armstrong J. J. Cell Sci. 1993;105:1115–1120. doi: 10.1242/jcs.105.4.1115. [DOI] [PubMed] [Google Scholar]
- 21.Ozoe F., Kurokawa R., Kobayashi Y., Jeong H. T., Tanaka K., Sen K., Nakagawa T., Matsuda H., Kawamukai M. Mol. Cell. Biol. 2002;22:7105–7119. doi: 10.1128/MCB.22.20.7105-7119.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray J. M., Johnson D. I. J. Biol. Chem. 2001;276:3004–3009. doi: 10.1074/jbc.M007389200. [DOI] [PubMed] [Google Scholar]
- 23.Michaelson D., Silletti J., Murphy G., D'Eustachio P., Rush M., Philips M. R. J. Cell Biol. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray J. M., Johnson D. I. Genetics. 2000;154:155–165. doi: 10.1093/genetics/154.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobering A. K., Romeo M. J., Vay H. A., Levin D. E. Mol. Cell. Biol. 2003;23:4983–4990. doi: 10.1128/MCB.23.14.4983-4990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobering A. K., Watanabe R., Romeo M. J., Yan B. C., Specht C. A., Orlean P., Riezman H., Levin D. E. Cell. 2004;117:637–648. doi: 10.1016/j.cell.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Bivona T. G., Philips M. R. Curr. Opin. Cell Biol. 2003;15:136–142. doi: 10.1016/s0955-0674(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 28.Chen C.-R., Li Y.-C., Chen J., Hou M. C., Papadaki P., Chang E. C. Proc. Natl. Acad. Sci. USA. 1999;96:517–522. doi: 10.1073/pnas.96.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Criekinge W. V., Beyaert R. Biol. Proced. Online. 1999;2:1–38. doi: 10.1251/bpo16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basi G., Schmid E., Maundrell K. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- 31.Li Y.-C., Chang E. C. Genetics. 2003;165:477–488. doi: 10.1093/genetics/165.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Aelst L., Barr M., Marcus S., Polverino A., Wigler M. Proc. Natl. Acad. Sci. USA. 1993;90:6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheffzek K., Grunewald P., Wohlgemuth S., Kabsch W., Tu H., Wigler M., Wittinghofer A., Herrmann C. Structure (London) 2001;9:1043–1050. doi: 10.1016/s0969-2126(01)00674-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.