Abstract
There is increasing indication that interspecific phenotypic differences result from variations in gene-regulatory interactions. Here we provide evidence that mice differ from zebrafish in the way they use homologous key components to regulate pigment cell differentiation. In both zebrafish and mice, one transcription factor, SOX10, controls the expression of another, MITF (microphthalmia-associated transcription factor), which in turn regulates a set of genes critical for pigment cell development and pigmentation. Mutations in either Sox10 or Mitf impair pigment cell development. In Sox10-mutant zebrafish, experimentally induced expression of Mitf fully rescues pigmentation. Using lineage-directed gene transfer, we show that, in the mouse, Mitf can rescue Sox10-mutant precursor cells only partially. In fact, retrovirally mediated, Sox10-independent Mitf expression in mouse melanoblasts leads to cell survival and expression of a number of pigment biosynthetic genes but does not lead to expression of tyrosinase, the rate-limiting pigment gene which critically depends on both Sox10 and Mitf. Hence, compared with fish, mice have evolved a regulation of tyrosinase expression that includes feed-forward loops between Sox10 and tyrosinase regulatory regions. The results may help to explain how some embryos, such as zebrafish, can achieve rapid pigmentation after fertilization, whereas others, such as mice, become pigmented only several days after birth.
Keywords: neural crest, pigmentation, transcription, cell fate, neurocristopathy
Pigmentation patterns of the skin and its appendages such as feathers and hairs have always served as convenient markers for the distinction of one species from another. Still, relatively little is known about the mechanisms that control this species-specific pigmentation from its earliest manifestation during development to the mature pattern of the adult. Given that transcription factors function as critical regulators of cell lineage determination and development, it is not surprising that a number of distinct transcription factors have also been found to regulate the development of pigment cells (1–4). SOX10 and MITF (microphthalmia-associated transcription factor), for instance, are both DNA-binding proteins involved in the development of vertebrate melanocytes, which are pigment-bearing cells that are generated in the neural crest and migrate to various destinations including skin, eye, and inner organs (5). In fact, if either SOX10 or MITF is missing, the pigment lineage is aborted (6–10). Conceivably, subtle interspecific differences in the functions of these two factors might influence the spatial-temporal development of melanocytes and hence determine adult pigmentation.
SOX10 is a member of the SRY-box containing high mobility group DNA-binding proteins (11). Its role in melanocyte development is evident from the effects of spontaneous and induced mutations. In zebrafish, for instance, several alleles of colorless are due to mutations in Sox10 (10). In mice, a spontaneous, truncating mutation in Sox10 is found in Dominant megacolon (7, 8). Heterozygotes with this mutation can have white belly spots and always show a loss of neural crest-derived enteric ganglia, which severely reduces the motility of the large intestine (7). A similar pigmentation/colon phenotype is seen in mice heterozygous for a targeted mutation in Sox10 (12). In humans, SOX10 mutations are associated with Waardenburg–Hirschsprung disease [Online Mendelian Inheritance in Man (OMIM) accession no. 277580] (13) and a complex neurocristopathy that combines peripheral demyelinating neuropathy, central leukodystrophy, Waardenburg syndrome, and Hirschprung disease (PCWH, OMIM accession no. 609136) (14). MITF, on the other hand, is a basic helix–loop–helix leucine zipper transcription factor whose mutations lead to pigmentary abnormalities and, depending on the species, deafness and eye abnormalities but none of the intestinal or demyelinating and dysmyelinating symptoms seen with Sox10 mutations (6, 15). Mitf is mutated in nacre zebrafish (9), in microphthalmia mice (6, 15), and in humans with Waardenburg syndrome type 2A (OMIM accession no. 193510) (16) and Tietz syndrome (OMIM accession no. 103500) (17).
During pigment cell development, both SOX10 and MITF act cell-autonomously because they are normally expressed in pigment cell precursors (6, 7, 10, 18–21), can rescue the respective mutant pigment cells after gene transfer (this report), and are functionally linked in a common pathway. In fact, in vitro, SOX10 regulates, in cooperation with other factors, the expression of Mitf through binding sites in the melanocyte-specific M promoter of Mitf (22–25). Interestingly, in zebrafish, Sox10 is rapidly down-regulated in pigment cell precursors when they start migrating from the neural crest (10, 26). In contrast, in mice, Sox10 expression lasts much longer in developing melanocytes (called melanoblasts) and persists during the cells’ migration and homing into skin (27, 28).
The finding of spatial-temporal differences in the expression patterns of Sox10 between zebrafish and mice may suggest that there are species-specific differences in the regulatory link between Sox10 and Mitf. Recently, it has been shown that experimentally induced expression of Mitf can fully rescue melanocyte development and pigmentation in Sox10 mutant zebrafish (26). This finding indicates that the major if not only function of Sox10 in zebrafish melanocyte development is the induction of Mitf. Here, we find that melanocyte development in mice does not follow a similarly simple pathway. Using a recently designed method of lineage-directed gene transfer in cultured neural crest cells (29), we show that in contrast to zebrafish, MITF can rescue survival and partial differentiation but not full pigmentation of Sox10-deficient mouse melanoblasts. Our findings indicate that the regulatory circuits that link Sox10, Mitf, and specific downstream pigmentation genes differ between zebrafish and mice and may hence explain why the developmental onset of pigmentation is distinct in these two species.
Results
Sox10 Is Required for the Development of Cultured Mouse Melanoblasts.
To test the role of Sox10 during early melanoblast development, we used a line of mice with a targeted mutation in Sox10, Sox10tm1weg (here called Sox10lacZ), in which a bacterial lacZ gene is inserted into the Sox10 gene (12). This insertion creates a functional null allele of Sox10 and at the same time serves as a convenient marker for tracking Sox10-deficient cells. In a first set of experiments, we confirmed that the bacterial lacZ marker in the targeted Sox10lacZ mice is suitable to track melanoblasts during development in vivo. As shown in Fig. 5, which is published as supporting information on the PNAS web site, in heterozygous Sox10lacZ/+ embryos at embryonic day (E)12.5, whole-mount β-gal labeling showed individual labeled cells underneath the surface ectoderm around the eye and in the area of the trunk where melanoblasts are normally found. In Sox10lacZ/lacZ homozygotes, however, such cells were almost completely absent, consistent with the notion that Sox10 is required for melanoblast survival. Because the initial emergence of β-gal-positive neural crest cells at E8.5–E9.5 is not impaired in Sox10lacZ/lacZ homozygotes and because their numbers become reduced only at E10.5 (12), it appears that Sox10 is first required for melanoblast development between E9.5 and E10.5, that is, long before pigmentation.
Unrelated to the failure in melanocyte development, however, Sox10 deficiency leads to embryonic lethality at around E13.5 (not shown). This fact precluded a detailed in vivo analysis of gene-regulatory interactions between Sox10, Mitf, and downstream target genes during subsequent embryological stages. Hence, we decided to test such interactions in primary neural tube explant cultures (21, 29). Such cultures are established from E9.5 embryos and kept under conditions that faithfully recapitulate the in vivo development of melanocytes with respect to several parameters, including the dependence on critical transcription and signaling factors (21), the rates of cell division (18), and the timing of gene expression and pigmentation (21, 30). As expected, at day 2 of culture, neural crest cells from Sox10lacZ/+ embryos readily expressed β-gal and the Sox10 target, MITF (22–25) (Fig. 1A). In contrast, when cultures were established from Sox10lacZ/lacZ embryos, β-gal-positive cells, although numerous, all lacked expression of MITF (Fig. 1B). To assess, conversely, whether Mitf was required for initiation or maintenance of Sox10 expression, we also tested cultures from embryos heterozygous or homozygous for a functional null allele of Mitf, Mitf mi-ew, whose protein product lacks part of the basic domain (15) and accumulates poorly in mutant cells (18, 21). In fact, SOX10 protein was readily seen in cultures from Mitf mi-ew/+ control embryos (Fig. 1C) as well as in cultures from Mitf mi-ew/mi-ew embryos (Fig. 1D). Thus, the genetic hierarchy between Sox10 and Mitf, suggested from in vivo observations and molecular analyses (12, 20, 23, 24, 26), was maintained in primary cultures, and cells positive for β-gal (representing Sox10 expression) or endogenous SOX10 survived at least initially despite the absence of either functional SOX10 or MITF.
Fig. 1.
Expression of MITF in primary neural crest cell cultures depends on Sox10, but SOX10 expression does not depend on Mitf. Cultures were established from E9.5 embryos of the indicated genotypes and stained for SOX10 and MITF expression 2 days later. β-gal/MITF double-positive cells in Sox10lacZ/+ cultures are shown (white arrows in A), and SOX10/MITF double-positive cells in Mitfmi-ew/+ cultures are shown (white arrows in C). In contrast, Sox10lacZ/lacZ cultures show only β-gal- but not MITF-labeled cells (white arrows in B), whereas Mitfmi-ew/mi-ew cultures show SOX10- but not MITF-labeled cells (D).
Sox10 Acts Through Mitf to Induce Downstream Target Genes.
On the basis of the above experiments and recent results in zebrafish (26), we reasoned that deliberate expression of SOX10 in melanocyte precursors should rescue Mitf expression and pigmentation in Sox10lacZ/lacZ cells and that expression of MITF alone should likewise fully rescue pigment cell development. To achieve lineage-directed gene transfer, we used a method described in ref. 29 that is based on the RCAS (replication-competent avian sarcoma-leukosis virus long terminal repeat with splice acceptor) system (31) and is composed of two principal components. First, the receptor for the virus RCAS, TVA (receptor for subgroup A Rous sarcoma virus), is expressed from a transgene under the control of regulatory regions of Pax3, a paired domain transcription factor gene expressed in neural crest precursor cells at around E8.5–E9 (32), independent of Sox10 or Mitf mutations. Alternatively, cultures are infected with a TVA-adenoviral vector. Second, once TVA is expressed, the cultures are infected with recombinant RCAS virus expressing either GFP or wild-type or mutant transcription factors, and the effects on pigment gene expression and pigmentation are assessed. We have previously demonstrated (29) that cultures derived from Sox10lacZ/lacZ embryos carrying a Pax3-tv-a transgene, which are devoid of pigmented cells, produce mature melanocytes upon infection with RCAS-HA-SOX10. This recombinant virus leads to production of functional SOX10 protein tagged at the amino terminus with a hemagglutinin (HA) tag. To further validate the method, we also infected cultures from Pax3-tv-a transgenic, Sox10+/+ (wild type) embryos with RCAS-GFP and found that they readily generated SOX10/GFP as well as MITF/GFP double-positive cells (Fig. 6, which is published as supporting information on the PNAS web site). These results demonstrate that the method allows for lineage-directed gene transfer into SOX10/MITF-positive melanoblasts.
With this methodology in hand, we then proceeded to analyze the rescue of Sox10lacZ/lacZ cultures with HA-SOX10 protein. To this end, we used cultures from Sox10lacZ/lacZ embryos carrying a Pax3-tv-a transgene and infected them with one of three RCAS viruses: RCAS-HA-SOX10, expressing full-length SOX10; RCAS-HA-SOX10del, expressing a SOX10 protein lacking the transcriptional activation domain; or RCAS-GFP. As shown in Fig. 2, RCAS-HA-SOX10 infection led to MITF expression, here measured after a 10-day incubation period (Fig. 2 A and C), whereas RCAS-HA-SOX10del infection (Fig. 2 B and D) or RCAS-GFP infection (not shown) did not lead to MITF expression. Likewise, no MITF was seen in RCAS-HA-SOX10-infected Mitf mi-ew/mi-ew cultures, which were made to express TVA from an adenoviral vector (Fig. 2F). In fact, in such Mitf mi-ew/mi-ew cultures, there was also no signal for dopachrome tautomerase (DCT), PMEL17, tyrosinase (TYR)-related protein-1 (TYRP1), and TYR (not shown), all of which are melanocyte-specific proteins encoded by their corresponding Mitf target genes (Fig. 2 H, J, and L). Nevertheless, similarly infected wild-type (Mitf+/+) cultures showed SOX10/MITF double-positive cells (Fig. 2E) as well as cells expressing each of the melanocyte proteins along with MITF (Fig. 2 G, I, and K). The results confirmed in primary mouse melanoblasts that SOX10 acts through the induction of MITF to induce downstream pigmentation genes. The results did not answer, however, whether MITF is the sole target of SOX10 to effect pigment cell development and melanogenesis.
Fig. 2.
SOX10 is required to induce MITF, and MITF is required to induce pigmentation genes. (A–D) MITF expression is rescued in Sox10lacZ/lacZ cultures by RCAS-HA-SOX10 (white arrrows in A and C indicate HA-SOX10/MITF double-positive cells) but not by RCAS-HA-SOX10del (D). Neural crest cultures transgenic for Pax3-tv-a were infected with the indicated RCAS viruses at day 1 in culture and labeled 10 days later. (E–L) In Mitf mutant cultures, RCAS-HA-SOX10 cannot induce pigmentation genes. (E, G, I, and K) Wild-type control cultures infected with RCAS-HA-SOX10. White arrows indicate double-positive cells. (F, H, J, and L) Mitfmi-ew/mi-ew cultures were first infected with an adenoviral vector expressing TVA and then with RCAS-HA-SOX10. Although numerous HA-SOX10-expressing cells are present, none of them express MITF, DCT, PMEL17, or TYRP1.
MITF Does Not Rescue Pigmentation in the Absence of Sox10.
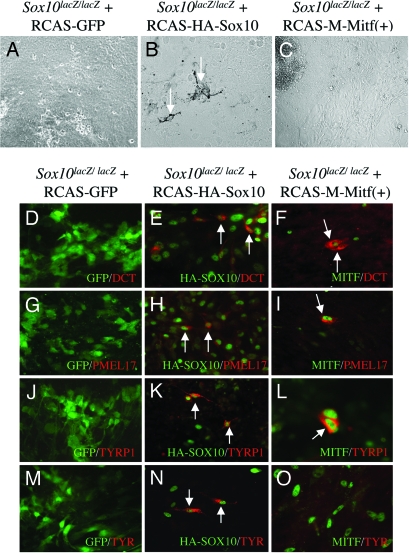
To address the question of whether SOX10 acts exclusively through MITF, we then tested whether MITF alone was capable of rescuing Sox10lacZ/lacZ cultures. To this end, we generated RCAS-M-MITF(+) from which the “melanocyte-specific”, 419-residue isoform of M-MITF(+) is expressed. This isoform contains a stretch of alternatively spliced six residues [referred to by the (+) sign] upstream of the basic domain that have previously been shown to be important both in vivo (15) and in vitro (33). In a first experiment, we kept the cultures for 14 days to allow for the appearance of pigmented cells. As shown in Fig. 3A, Sox10lacZ/lacZ cultures control-infected with RCAS-GFP did not produce pigmented cells, whereas those infected with RCAS-HA-SOX10 did [8 of 8 cultures (100%) contained mature melanocytes] (Fig. 3B). In contrast, when infected with RCAS-M-MITF(+), no pigmented cells could be detected [0 of 26 cultures (0%) contained melanocytes] (Fig. 3C). This result suggested that experimental expression of MITF did not overcome the lack of functional SOX10.
Fig. 3.
SOX10 but not MITF can rescue the generation of pigmented, mature melanocytes in Sox10lacZ/lacZ cultures. (A–C) Rescue of pigmentation. Neither RCAS-GFP (A) nor RCAS-M-MITF(+) (C) infection leads to pigmented cells but RCAS-HA-SOX10 infection does (B). (D–O) Rescue of pigmentation gene expression. In Sox10lacZ/lacZ cultures, RCAS-HA-SOX10 infection leads to the induction of several pigmentation genes including TYR, whereas RCAS-M-MITF(+) infection only induces DCT, PMEL17, and TYRP1 but not TYR. (D, G, J, and M) RCAS-GFP control-infected cultures show no induction of the indicated pigmentation genes. (E, H, K, and N) RCAS-HA-SOX10 infection rescues expression of the indicated pigmentation genes. White arrows mark positive cells. (F, I, L, and O) RCAS-M-MITF(+) induces DCT, PMEL17, and TYRP1 but not TYR. White arrows mark positive cells.
MITF Induces Several Pigmentation Genes but Not TYR in the Absence of Sox10.
To investigate the stage at which RCAS-M-MITF(+)-infected Sox10lacZ/lacZ cultures were blocked in their generation of pigmented cells, we then tested them and appropriate controls for expression of the above-mentioned melanocyte-specific proteins DCT, PMEL17, and TYRP1 as well as TYR, the rate-limiting enzyme of melanin synthesis. None of these proteins was expressed in RCAS-GFP-infected Sox10lacZ/lacZ cultures (Fig. 3 D, G, J, and M), but all of them were present in RCAS-HA-SOX10-infected cultures [2 of 2 cultures (100%) each for DCT, PMEL17, TYRP1, and TYR] (Fig. 3 E, H, K, and N). In contrast, RCAS-M-MITF(+)-infected Sox10lacZ/lacZ cultures were rescued only partially: 3 of 3 cultures (100%) showed DCT-positive cells (as represented in Fig. 3F), 4 of 4 cultures (100%) were positive for PMEL17 (as represented in Fig. 3I), and 3 of 4 cultures (75%) were positive for TYRP1 (a positive culture is represented in Fig. 3L). TYR, in contrast, remained undetectable [0 of 15 cultures (0%) were positive] (represented in Fig. 3O). To exclude the possibility, however remote, that this latter result was due to some deleterious effect of RCAS-M-MITF(+) on TYR expression, we also tested whether RCAS-M-MITF(+) would interfere with the expression of melanogenic genes and pigmentation in Mitf mi-ew/mi-ew cultures. We found, however, that TYR was now expressed [6 of 6 cultures (100%) positive], as were DCT, PMEL17 and TYRP1, and the cultures generated pigmented cells (Fig. 7, which is published as supporting information on the PNAS web site). These findings showed that RCAS-derived, recombinant MITF was not only not inhibitory but also, in fact, fully functional. In conclusion, then, murine Sox10lacZ/lacZ melanocyte precursors can be rescued by SOX10 all of the way to mature melanocytes, but in contrast to observations in zebrafish (26), experimental expression of MITF, a major transcriptional target of SOX10, leaves them stuck in their development at a stage just before expression of the critical pigment gene tyrosinase.
Discussion
The above results clearly show that distinct species differ in the way they use homologous regulators and their regulated targets for pigment cell development. In zebrafish, Sox10, Mitf, and downstream pigment genes are linked in a linear, seemingly simple regulatory chain in which Sox10 controls the expression of Mitf, which in turn is sufficient to regulate pigmentation gene expression and pigmentation. In mice, the situation is apparently more complex in that the generation of melanocytes requires both Sox10 and Mitf, and neither gene alone can overcome the lack of the other to generate Tyr-expressing, mature melanocytes (schematically illustrated in Fig. 4). Interestingly, similar species-specific differences are seen in other parts of the pigmentation pathway. For instance, in zebrafish, mutations in the tyrosine receptor kinase KIT or the G-coupled, endothelin receptor type B (EDNRB) are still compatible with the generation of at least some subclasses of melanocytes (2), implying that the necessary pigmentation genes including Tyr are expressed. In contrast, in the mouse, Kit−/− melanoblasts lack Tyr expression (although they express other pigmentation genes, at least in culture), can only be rescued to express Tyr, and become pigmented, by deliberate stimulation of the mitogen-activated protein kinase pathway by using unrelated ligand/receptor systems such as HGF (hepatocyte growth factor)/MET (met protooncogene) (21), constitutively active RET (ret protooncogene) (34), or cAMP-elevating drugs (21). The situation is even more complex with Ednrb. As with Kit, the absence of Ednrb in cultured mouse melanoblasts leads to a lack of Tyr expression, but the rescue of pigmentation requires two separate signaling steps, a first one to rescue Tyr expression, itself insufficient to support pigmentation, and a second one thought to induce the enzyme’s activation (35). Hence, the species-specific differences in the role of Sox10 and Mitf described above extend to critical signaling steps. These differences have interesting molecular and developmental implications.
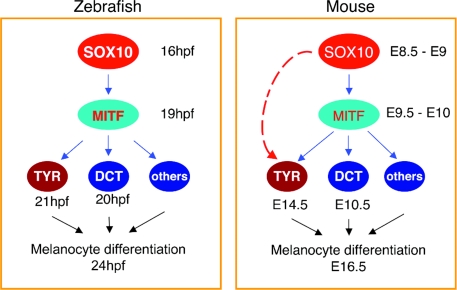
Fig. 4.
The distinction in the transcriptional regulatory hierarchy of Sox10 and Mitf for melanocyte development and differentiation in zebrafish and mice. Examples of the transcriptional regulatory network models are based on ref. 42. (Left) A simple regulatory chain model in zebrafish melanocyte development. Here, Sox10 directly activates Mitf, and Mitf, independent of further action of Sox10, rapidly stimulates downstream target genes and hence pigmentation. (Right) A feed-forward loop network model operating during mouse melanocyte development. Here, Sox10 directly regulates Mitf and cooperates with Mitf and Sox10 or Sox10-dependent regulators to activate downstream target genes, including Tyr. Thus, this model allows for temporal control of melanogenic gene expression.
Previous in vitro analysis of the mouse Dct promoter has provided evidence for cooperation of SOX10 and MITF over discrete juxtaposed binding sites (ref. 36 and references therein). Moreover, Sox10 shows haploinsufficiency for Dct expression (20) and pigment cell development in mice because approximately half of Sox10lacZ/+ mice display a white belly spot (7, 12). Nevertheless, as demonstrated in our culture assays, Sox10 is dispensable for Dct expression provided MITF levels are maintained. Cooperation between SOX10 and MITF has not been reported for the Tyr promoter, however, and yet our results show that Tyr expression codepends on these two factors. In support of this notion, previous transgenic observations have already suggested that the regulation of Tyr in vivo may be more complex than that of Dct. Although a 3.2-kb Dct promoter fragment in transgenic mice leads to an expression pattern reasonably reflecting that of the endogenous gene (37), faithful Tyr expression has only been achieved by using large chromosomal but not smaller fragments (38).
It is remarkable that many of the differences in pigment cell development between zebrafish and mice center on Tyr expression and/or its activation. This is likely so because TYR activity, used in the conversion of the amino acid tyrosine to l-3,4-dihydroxyphenylalanine (l-DOPA), is not only the rate-limiting first step in melanin synthesis, but it also leads to products such as dopachrome, which are potentially cytotoxic unless synthesized within specific organelles (melanosomes) and further metabolized by other melanogenic enzymes (reviewed in ref. 39). Thus, it is critical that Tyr is not activated before the remainder of the melanin synthetic machinery is in place. In zebrafish, both Dct and Tyr are expressed just after Mitf expression (9, 40), and melanin synthesis begins as early as 24 h after fertilization (2, 40), that is, before the cells’ completion of migration from the neural crest to their final destinations. In mice, Sox10, Mitf, Dct, Pmel17, Tyrp1, and Tyr are expressed in a characteristic temporal sequence (21). Dct, for instance, is coexpressed with Sox10 and Mitf, but Tyr is not found for another 4–5 days despite continued Sox10 and Mitf expression (21, 30). Consequently, the cells remain unpigmented until migration is completed at E16.5. The molecular mechanisms of this temporal delay in Tyr expression are not clear but likely involve the above-mentioned signaling pathways and the observed dual dependence on Sox10 and Mitf. For instance, it is conceivable that in mice, Tyr expression is delayed because expression of one or several Sox10/Mitf-dependent, intermediate regulatory genes is first required; in zebrafish, the putative homologs of these genes are either not Sox10/Mitf-dependent or are not critical for Tyr expression. In any event, the consequence of the differences in pigment gene expression between zebrafish and mice is a pronounced difference in the timing of pigmentation onset. In zebrafish, pigmentation occurs early on, providing a clear evolutionary advantage for camouflage and light protection in the free living larvae. In mice, however, early pigmentation is, if not damaging, at least not necessary because mammalian embryos develop safely inside their mothers. It is well possible that developmental regulatory networks in fish and mice are not intrinsically “simple” or “complex” but simply reflect distinct evolutionary demands on the respective cellular systems. In this sense, subtle changes in the regulatory networks may account for many of the pigmentary variations that we can enjoy in nature.
Materials and Methods
Mice and Genotyping.
Sox10tm1weg mice (12), here called Sox10lacZ mice (background C57BL/6), and mice carrying the allele Mitf mi-ew (background NAW) (18) or Tg(Pax3-tv-a) (29), expressing the avian TVA leucosis virus receptor under the control of a Pax3 regulatory region, were used. To generate Tg(Pax3-tv-a)/+;Sox10lacZ/+ mice, Tg(Pax3-tv-a)/+ mice were bred with Sox10lacZ/+ mice. Tg(Pax3-tv-a)/+;Sox10lacZ/lacZ embryos were generated from crosses between Tg(Pax3-tv-a)/Tg(Pax3-tv-a);Sox10lacZ/+ mice and Sox10lacZ/+ mice and genotyped as described (29).
Neural Tube Explant Culture and RCAS Virus Infection.
“Gateway” site-specific recombination RCAS vectors and RCAS-HA-SOX10 have been described (29, 41). RCAS-HA-SOX10del [truncated at residue 190 and hence lacking a transcription activation domain (20)] and RCAS-M-Mitf(+) vectors were generated by using the same vector backbone and corresponding cDNAs. Recombinant viruses were produced as described (41), and supernatants containing 106 infectious particles per ml were used. Trunk neural tube explants were prepared and cultured as described (21) except that media were further supplemented with 25% Wnt3a-conditioned medium (gift of R. Nusse, Stanford University, Stanford, CA), 15 nM endothelin-3, 200 pM basic FGF (R & D Systems), and 20 nM 12-O-tetradecanoylphorbol-13-acetate to ensure optimal survival of mutant melanoblasts. After 1 day in culture, cells were infected by two to three rounds of viral infection (each round for 3–4 h). Mitf mi-ew cultures were infected with Adeno-tv-a virus (a gift of M. Herlyn, The Wistar Institute, Philadelphia) and were allowed to grow for 1 day before exposure to the respective RCAS viruses.
X-Gal Staining, Antibodies, and Immunostaining.
β-gal staining for embryos of Sox10lacZ/+ and Sox10lacZ/lacZ and antibody labeling of neural crest cultures were performed as described (21). Anti-HA antibody was from Covance (Berkeley, CA).
Supplementary Material
Acknowledgments
We thank Dr. Michael Wegner (Universitat Erlangen-Nurnberg, Erlangen, Germany) for providing Sox10lacZ mice; Dr. Roel Nusse for Wnt3a; Drs. Dong Fang and Meenhard Herlyn (The Wistar Institute) for Adeno-tv-a; Dr. Doug Foster (University of Minnesota, Minneapolis) for DF-1 cells; and Dr. Harold Varmus (Memorial Sloan–Kettering Cancer Center, New York) for the pCI tv-a800 vector. We also thank Dr. Arturo Incao for technical assistance; Drs. Stacie K. Loftus and Ramin M. Hakami; Karen J. Dunn [National Human Genome Research Institute (NHGRI)] for sharing reagents; and Amy Chen for microinjection of the transgenic constructs. Julia Fekecs provided expert support in the figure preparation. All animal research was covered under protocols G94-7 (to W.J.P.) and 1201-05 (to H.A.), and we thank the Animal Health and Care Staff [National Institute of Neurological Disease and Stroke (NINDS) and NHGRI] for expert animal care. This research was supported in part by the Intramural Research Program of NHGRI and NINDS.
Abbreviations
- En
embryonic day n
- RCAS
replication-competent avian sarcoma-leukosis virus long terminal repeat with splice acceptor
- TVA
gene product of tv-a, encoding the receptor for subgroup A avian leucosis virus-derived retrovirus
- HA
hemagglutinin
- DCT
dopachrome tautomerase
- TYR
tyrosinase.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Goding C. R. Genes Dev. 2000;14:1712–1728. [PubMed] [Google Scholar]
- 2.Rawls J. F., Mellgren E. M., Johnson S. L. Dev. Biol. 2001;240:301–314. doi: 10.1006/dbio.2001.0418. [DOI] [PubMed] [Google Scholar]
- 3.Steingrimsson E., Copeland N. G., Jenkins N. A. Cell. 2005;121:9–12. doi: 10.1016/j.cell.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Bennett D. C., Lamoreux M. L. Pigm. Cell. Res. 2003;16:333–344. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 5.Le Douarin N. M., Kalcheim C. The Neural Crest. Cambridge, U.K.: Cambridge Univ. Press; 1999. [Google Scholar]
- 6.Hodgkinson C. A., Moore K. J., Nakayama A., Steingrimsson E., Copeland N. G., Jenkins N. A., Arnheiter H. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- 7.Southard-Smith E. M., Kos L., Pavan W. J. Nat. Genet. 1998;18:60–64. doi: 10.1038/ng0198-60. [DOI] [PubMed] [Google Scholar]
- 8.Herbarth B., Pingault V., Bondurand N., Kuhlbrodt K., Hermans-Borgmeyer I., Puliti A., Lemort N., Goossens M., Wegner M. Proc. Natl. Acad. Sci. USA. 1998;95:5161–5165. doi: 10.1073/pnas.95.9.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lister J. A., Robertson C. P., Lepage T., Johnson S. L., Raible D. W. Development (Cambridge, U.K.) 1999;126:3757–3767. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- 10.Dutton K. A., Pauliny A., Lopes S. S., Elworthy S., Carney T. J., Rauch J., Geisler R., Haffter P., Kelsh R. N. Development (Cambridge, U.K.) 2001;128:4113–4125. doi: 10.1242/dev.128.21.4113. [DOI] [PubMed] [Google Scholar]
- 11.Wegner M. Pigm. Cell. Res. 2005;18:74–85. doi: 10.1111/j.1600-0749.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 12.Britsch S., Goerich D. E., Riethmacher D., Peirano R. I., Rossner M., Nave K. A., Birchmeier C., Wegner M. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pingault V., Bondurand N., Kuhlbrodt K., Goerich D. E., Prehu M. O., Puliti A., Herbarth B., Hermans-Borgmeyer I., Legius E., Matthijs G., et al. Nat. Genet. 1998;18:171–173. doi: 10.1038/ng0298-171. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K., Khajavi M., Ohyama T., Hirabayashi S., Wilson J., Reggin J. D., Mancias P., Butler I. J., Wilkinson M. F., Wegner M., Lupski J. R. Nat. Genet. 2004;36:361–369. doi: 10.1038/ng1322. [DOI] [PubMed] [Google Scholar]
- 15.Steingrimsson E., Moore K. J., Lamoreux M. L., Ferre-D’Amare A. R., Burley S. K., Zimring D. C., Skow L. C., Hodgkinson C. A., Arnheiter H., Copeland N. G., et al. Nat. Genet. 1994;8:256–263. doi: 10.1038/ng1194-256. [DOI] [PubMed] [Google Scholar]
- 16.Tassabehji M., Newton V. E., Read A. P. Nat. Genet. 1994;8:251–255. doi: 10.1038/ng1194-251. [DOI] [PubMed] [Google Scholar]
- 17.Amiel J., Watkin P. M., Tassabehji M., Read A. P., Winter R. M. Clin. Dysmorphol. 1998;7:17–20. [PubMed] [Google Scholar]
- 18.Opdecamp K., Nakayama A., Nguyen M. T., Hodgkinson C. A., Pavan W. J., Arnheiter H. Development (Cambridge, U.K.) 1997;124:2377–2386. doi: 10.1242/dev.124.12.2377. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama A., Nguyen M. T., Chen C. C., Opdecamp K., Hodgkinson C. A., Arnheiter H. Mech. Dev. 1998;70:155–166. doi: 10.1016/s0925-4773(97)00188-3. [DOI] [PubMed] [Google Scholar]
- 20.Potterf S. B., Mollaaghababa R., Hou L., Southard-Smith E. M., Hornyak T. J., Arnheiter H., Pavan W. J. Dev. Biol. 2001;237:245–257. doi: 10.1006/dbio.2001.0372. [DOI] [PubMed] [Google Scholar]
- 21.Hou L., Panthier J. J., Arnheiter H. Development (Cambridge, U.K.) 2000;127:5379–5389. doi: 10.1242/dev.127.24.5379. [DOI] [PubMed] [Google Scholar]
- 22.Bondurand N., Pingault V., Goerich D. E., Lemort N., Sock E., Caignec C. L., Wegner M., Goossens M. Hum. Mol. Genet. 2000;9:1907–1917. doi: 10.1093/hmg/9.13.1907. [DOI] [PubMed] [Google Scholar]
- 23.Lee M., Goodall J., Verastegui C., Ballotti R., Goding C. R. J. Biol. Chem. 2000;275:37978–37983. doi: 10.1074/jbc.M003816200. [DOI] [PubMed] [Google Scholar]
- 24.Potterf S. B., Furumura M., Dunn K. J., Arnheiter H., Pavan W. J. Hum. Genet. 2000;107:1–6. doi: 10.1007/s004390000328. [DOI] [PubMed] [Google Scholar]
- 25.Verastegui C., Bille K., Ortonne J. P., Ballotti R. J. Biol. Chem. 2000;275:30757–30760. doi: 10.1074/jbc.C000445200. [DOI] [PubMed] [Google Scholar]
- 26.Elworthy S., Lister J. A., Carney T. J., Raible D. W., Kelsh R. N. Development (Cambridge, U.K.) 2003;130:2809–2818. doi: 10.1242/dev.00461. [DOI] [PubMed] [Google Scholar]
- 27.Osawa M., Egawa G., Mak S. S., Moriyama M., Freter R., Yonetani S., Beermann F., Nishikawa S. Development (Cambridge, U.K.) 2005;132:5589–5599. doi: 10.1242/dev.02161. [DOI] [PubMed] [Google Scholar]
- 28.Hakami R. M., Hou L., Baxter L. L., Loftus S. K., Southard-Smith E. M., Incao A., Cheng J., Pavan W. J. Mech. Dev. 2006;123:124–134. doi: 10.1016/j.mod.2005.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou L., Loftus S. K., Incao A., Chen A., Pavan W. J. Dev. Dyn. 2004;229:54–62. doi: 10.1002/dvdy.10468. [DOI] [PubMed] [Google Scholar]
- 30.Steel K. P., Davidson D. R., Jackson I. J. Development (Cambridge, U.K.) 1992;115:1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- 31.Fisher G. H., Orsulic S., Holland E., Hively W. P., Li Y., Lewis B. C., Williams B. O., Varmus H. E. Oncogene. 1999;18:5253–5260. doi: 10.1038/sj.onc.1203087. [DOI] [PubMed] [Google Scholar]
- 32.Magnaghi P., Roberts C., Lorain S., Lipinski M., Scambler P. J. Nat. Genet. 1998;20:74–77. doi: 10.1038/1739. [DOI] [PubMed] [Google Scholar]
- 33.Bismuth K., Maric D., Arnheiter H. Pigm. Cell. Res. 2005;18:349–359. doi: 10.1111/j.1600-0749.2005.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwamoto T., Takahashi M., Ohbayashi M., Nakashima I. Exp. Cell Res. 1992;200:410–415. doi: 10.1016/0014-4827(92)90189-f. [DOI] [PubMed] [Google Scholar]
- 35.Hou L., Pavan W. J., Shin M. K., Arnheiter H. Development (Cambridge, U.K.) 2004;131:3239–3247. doi: 10.1242/dev.01193. [DOI] [PubMed] [Google Scholar]
- 36.Murakami H., Arnheiter H. Pigm. Cell. Res. 2005;18:265–277. doi: 10.1111/j.1600-0749.2005.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkie A. L., Jordan S. A., Jackson I. J. Development (Cambridge, U.K.) 2002;129:3349–3357. doi: 10.1242/dev.129.14.3349. [DOI] [PubMed] [Google Scholar]
- 38.Giraldo P., Montoliu L. Pigm. Cell. Res. 2002;15:258–264. doi: 10.1034/j.1600-0749.2002.02030.x. [DOI] [PubMed] [Google Scholar]
- 39.Tadokoro T., Kobayashi N., Beer J. Z., Zmudzka B. Z., Wakamatsu K., Miller S. A., Lamoreux M. L., Ito S., Hearing V. J. In: Mechanisms of Suntanning. Ortonne J. P., Ballotti R., editors. London: Martin Dunitz; 2002. pp. 67–76. [Google Scholar]
- 40.Camp E., Lardelli M. Dev. Genes Evol. 2001;211:150–153. doi: 10.1007/s004270000125. [DOI] [PubMed] [Google Scholar]
- 41.Loftus S. K., Larson D. M., Watkins-Chow D., Church D. M., Pavan W. J. DNA Res. 2001;8:221–226. doi: 10.1093/dnares/8.5.221. [DOI] [PubMed] [Google Scholar]
- 42.Lee T. I., Rinaldi N. J., Robert F., Odom D. T., Bar-Joseph Z., Gerber G. K., Hannett N. M., Harbison C. T., Thompson C. M., Simon I., et al. Science. 2002;298:799–804. doi: 10.1126/science.1075090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.