Abstract
Natural killer (NK) T cells using an invariant Vα14 (Vα14i) T cell receptor rearrangement form a distinct immunoregulatory T cell lineage. Several studies indicated that a NK1.1− Vα14i NKT precursor cell differentiates and expands within the thymus before export to the peripheral tissues occurs. However, little is known about the signals that cause the emigration of Vα14i NKT cells from the thymus to the periphery. Here we show that signaling of lymphotoxin (LT) αβ through the LTβ receptor (LTβR) is indispensable for regulating peripheral but not thymic Vα14i NKT cell numbers. Homing to and homeostatic proliferation of thymic Vα14i NKT cells in peripheral organs, however, was not dependent on LTβR. Instead, our data indicate that a LTβR-expressing thymic stromal cell regulates the thymic emigration of Vα14i NKT cells but not conventional T cell receptor αβ cells.
Keywords: innate immunity, TNF cytokines, development, LTβR
Lymphotoxin (LT) α and β are members of the TNF family that form biologically active homotrimers or heterotrimers. LTα can be secreted as a homotrimer that can bind with equal affinity to either TNF receptor 1 or 2 (1). LTα can also be membrane-bound by association with LTβ to form LTαβ (2, 3). This heterotrimer binds exclusively to another receptor, the LTβ receptor (LTβR), which is expressed on nonlymphoid cells (4). Over the past years, a wealth of data has indicated an indispensable role for the LTαβ–LTβR interaction in secondary lymphoid organ structure development and function (5, 6). In addition, some studies have shown a functional impairment in generating secondary antibody responses to certain antigens in LT-deficient mice, although normal numbers of T and B cells are found (7, 8).
Natural killer (NK) T cells recognize glycolipid antigens (9, 10), and they form a unique lymphocyte subset with important immunoregulatory properties (11). They coexpress NK receptors and intermediate levels of T cell receptor (TCR) αβ and have a phenotype reminiscent of activated T cells. Several distinct subsets of NKT cells have been described (12). In mice the most abundant NKT cell subpopulation is characterized by an invariant TCRα rearrangement, Vα14-Jα18, and is reactive with CD1d, a nonclassical class I antigen-presenting molecule (13, 14). We will hereafter refer to these cells as Vα14 invariant (Vα14i) NKT cells. Upon recognition of the synthetic glycolipid α-galactosylceramide (α-GalCer), which is presented by CD1d (10), TCR stimulation results in the rapid production of proinflammatory cytokines that influence other immune cells, including NK cells, dendritic cells, and B and T cells (11).
There is evidence that Vα14i NKT cells form a separate T cell lineage because they are selected in the thymus by a hematopoietic cell type, which contrasts with the selection of conventional T cells by thymic epithelial cells (15–18). With the availability of α-GalCer-loaded CD1d tetramers (19), important new information has been obtained about Vα14i NKT cell ontogeny, showing that NK1.1 receptor expression is modulated during development, meaning that not all Vα14i NKT cells express NK1.1 (20, 21). Although a considerable amount of information exists on their intrathymic differentiation (20–22), the signals that regulate the export of Vα14i NKT cells or conventional T cells from the thymus to peripheral organs are poorly understood. Here we describe that LTαβ and its receptor, LTβR, are instrumental in regulating thymic emigration of Vα14i NKT cells but not of conventional T cells.
Results
Reduction of Vα14i NKT Cells in Peripheral Organs, but Not Thymus, in LT- and LTβR-Deficient Mice.
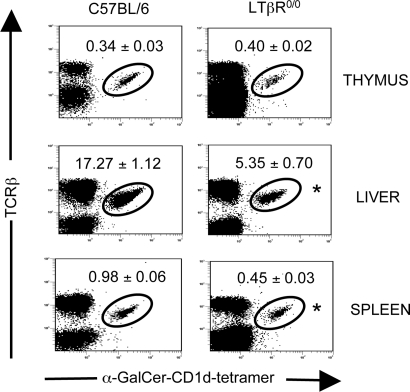
To determine the prevalence of Vα14i NKT cells in LTα0/0, LTβ0/0, and LTβR0/0 mice, we isolated mononuclear cells from the principal sites where Vα14i NKT cells are found, including thymus, spleen, liver, and bone marrow, and we determined the fraction of α-GalCer/CD1d tetramer+ TCRβ+ cells by flow cytometry. The fraction and absolute number of Vα14i NKT cells were greatly and consistently reduced in liver, spleen, and bone marrow of LTα0/0, LTβ0/0, and LTβR0/0 mice (P < 0.05; LT- and LTβR-deficient mice versus respective controls; Student’s t test) (Table 1, Fig. 1, and unpublished data). Peripheral Vα14i NKT cell numbers appeared to be more affected in LTα0/0 mice than in LTβ0/0 and LTβR0/0 mice, suggesting a potential additional role for LTα3 homotrimers in Vα14i NKT cell ontogeny. These data could be confirmed in an additional LTα0/0 mouse strain (8). Curiously, Vα14i NKT cell numbers were much less affected within the thymus, especially in LTβ0/0 and LTβR0/0 mice. The LTβR can bind to another TNF family cytokine, LIGHT (23). Peripheral Vα14i NKT cell numbers in LIGHT0/0 mice were normal (Table 1), indicating that the interaction of LTβR with LTαβ is indispensable for generating normal numbers of Vα14i NKT cells in the periphery, rather than its interaction with LIGHT. Consistent with these findings, cytokine production levels induced by in vivo immunization with the glycolipid α-GalCer were found to be reduced in LTα0/0, LTβ0/0, and LTβR0/0 mice but not in LIGHT0/0 mice (ref. 24 and unpublished data). Nevertheless, the ability of the residual Vα14i NKT cells to produce IFN-γ (Fig. 5, which is published as supporting information on the PNAS web site) and IL-4 (unpublished data) after stimulation with α-GalCer was not impaired in LTβ0/0 mice, indicating that disruption of LTαβ–LTβR signaling does not affect the functional capacity of Vα14i NKT cells.
Table 1.
Thymic and peripheral Vα14i NKT cell percentages and numbers in C57BL/6 and C57BL/6 × 129 wild-type mice and LTα0/0, LTβ0/0, LTβR0/0, and LIGHT0/0 mice
| Mouse | Thymus |
Liver |
Spleen |
|||
|---|---|---|---|---|---|---|
| Vα14i NKT cells, % | Vα14i NKT cells, absolute cell no. | Vα14i NKT cells, % | Vα14i NKT cells, absolute cell no. | Vα14i NKT cells, % | Vα14i NKT cells, absolute cell no. | |
| C57BL/6 | 0.34 ± 0.03 | 5.7 × 105 ± 0.9 × 105 | 17.27 ± 1.12 | 8.2 × 105 ± 0.9 × 105 | 0.98 ± 0.06 | 8.6 × 105 ± 1.0 × 105 |
| B6129F2 (C57BL/6 × 129) | 0.16 ± 0.07 | 3.4 × 105 ± 1.2 × 105 | 13.05 ± 2.02 | 5.7 × 105 ± 0.9 × 105 | 1.03 ± 0.20 | 6.1 × 105 ± 0.9 × 105 |
| B6.129-LTβR0/0 (C57BL/6) | 0.40 ± 0.02 | 4.9 × 105 ± 1.9 × 105 | 5.35 ± 0.97* | 3.9 × 105 ± 0.8 × 105* | 0.45 ± 0.03* | 2.6 × 105 ± 0.9 × 105* |
| B6.129-LTα0/0 (C57BL/6) | 0.11 ± 0.02* | 1.6 × 105 ± 0.4 × 105* | 2.22 ± 0.44* | 2.0 × 105 ± 0.6 × 105* | 0.10 ± 0.01* | 0.8 × 105 ± 0.2 × 105* |
| B6;129-LTβ0/0 (C57BL/6 × 129) | 0.25 ± 0.04 | 5.1 × 105 ± 0.5 × 105 | 3.68 ± 1.36* | 2.6 × 105 ± 0.7 × 105† | 0.31 ± 0.03† | 1.8 × 105 ± 0.4 × 105* |
| B6.129-LIGHT0/0 (C57BL/6) | 0.47 ± 0.08 | 4.5 × 105 ± 1.8 × 105 | 15.95 ± 1.79 | 9.5 × 105 ± 2.4 × 105 | 0.79 ± 0.11 | 12 × 105 ± 1.9 × 105 |
Thymic, liver, and splenic mononuclear cells from C57BL/6 and C57BL/6 × 129 wild-type mice and from LTα0/0, LTβ0/0, LTβR0/0, and LIGHT0/0 mice were analyzed by FACS (n = 5–10). Vα14i NKT cells were gated as TCRβ+ α-GalCer/CD1d tetramer+ lymphocytes. Data shown are ± SEM. ∗, P < 0.01, knockout versus wild type (Student’s t test). †, P < 0.05, knockout versus wild type (Student’s t test).
Fig. 1.
Vα14i NKT cell defect in LT-deficient mice. Thymic, liver, and splenic mononuclear cells from C57BL/6 wild-type mice and LTβR0/0 mice were analyzed by FACS. Plots were gated on total lymphocytes. Numbers show mean percentage ± SEM of TCRβ+ α-GalCer/CD1d tetramer+ lymphocytes (n = 5–10). ∗, P < 0.01, LTβR0/0 versus C57BL/6 (Student’s t test).
Peripheral Vα14i NKT Cell Dependence on Intact Stromal Cell Function in Vivo.
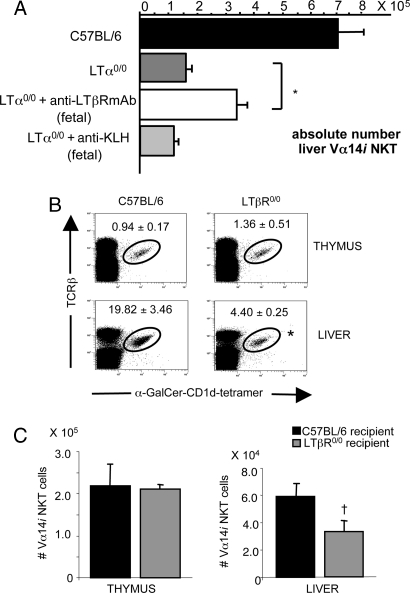
We next evaluated whether the peripheral Vα14i NKT cell deficiency could be restored by in vivo administration of the AF.H6 agonistic anti-LTβR antibody. Previously, this antibody was shown to induce embryonic genesis of lymph nodes in LTα0/0 mice when administered in utero (25). Therefore, we examined the effect of in utero administration of anti-LTβR antibody on Vα14i NKT cell differentiation. Pregnant LTα0/0 mice were injected from fetal day 11 onward every 48 h, and the progeny were examined for Vα14i NKT cell numbers in thymus, liver, and spleen. Controls consisted of pregnant LTα0/0 mice injected with an anti-keyhole limpet hemocyanin (KLH) Ig of the same isotype (isotype control). As shown in Fig. 2A, the absolute Vα14i NKT cell number in livers of LTα0/0 mice was partially restored by in utero treatment. By contrast, administration of isotype control had no effect. Similar findings were made in the spleen (unpublished data). Thymic Vα14i NKT cell numbers, however, were not influenced, indicating that the partial restoration in the periphery could not simply be due to an increase in thymic Vα14i NKT cell levels (unpublished data). Consistent with previous reports (25), lymph node formation could be partially restored with anti-LTβR antibody but not with isotype control (unpublished data). These findings suggest a role for a LTβR-expressing stromal cell during fetal ontogeny for the differentiation of Vα14i NKT cells.
Fig. 2.
A stromal defect is responsible for the Vα14i NKT cell deficiency in LT- and LTβR-deficient mice. (A) In vivo restoration of Vα14i NKT cell defect in LTα0/0 mice by administration of an agonistic anti-LTβR mAb in utero. Pregnant LTα0/0 mice were injected i.p. with agonistic anti-LTβR mAb or anti-KLH as isotype control on days 11, 13, 15, and 17 of gestation. Progeny were analyzed 6 weeks after birth by FACS. Bar graphs show mean absolute numbers ± SEM of Vα14i NKT cells (TCRβ+ α-GalCer/CD1d tetramer+) in the liver of C57BL/6 wild-type control mice, untreated control LTα0/0 mice, LTα0/0 mice treated with anti-LTβR mAb, or LTα0/0 mice injected with anti-KLH (n = 3–4). ∗, P = 0.005; anti-LTβR mAb-treated LTα0/0 mice versus control LTα0/0 mice (Student’s t test). (B) Sublethally γ-irradiated (600 rad) 3-week-old C57BL/6 or LTβR0/0 mice recipient mice were i.v. injected with 107 TCRβ-depleted bone marrow cells from CD90.1 congenic C57BL/6 mice. After 8 weeks, mononuclear cells from thymus and liver were analyzed by FACS. Numbers represent mean percentage ± SEM of Vα14i NKT cells (TCRβ+ α-GalCer/CD1d tetramer+) within the CD90.2− TCRβ+ fraction of lymphocytes in thymus and liver of C57BL/6 or LTβR0/0 recipient mice (n = 3–5). ∗, P = 0.037, LTβR0/0 versus C57BL/6 recipients (Student’s t test). (C) Bar graphs represent mean absolute numbers ± SEM of CD90.2− Vα14i NKT cells (TCRβ+ α-GalCer/CD1d tetramer+) in thymus and liver of C57BL/6 and LTβR0/0 recipient mice. †, P = 0.047, LTβR0/0 versus C57BL/6 recipients (Student’s t test).
To test whether the Vα14i NKT cell defect in LTβR0/0 mice could be restored by transfer of wild-type bone marrow precursors, we created bone marrow chimeras in which we transferred T cell-depleted bone marrow cells from CD90.1 congenic C57BL/6 mice into sublethally irradiated CD90.2+ C57BL/6 or LTβR0/0 mice. Eight weeks later, the fraction of CD90.2− Vα14i NKT cells was determined in thymus, liver, and spleen of the recipient mice. Comparable percentages (Fig. 2B Upper) and absolute numbers (Fig. 2C Left) of Vα14i NKT cells were found within the CD90.2− compartment in the thymus of C57BL/6 and LTβR0/0 recipient mice. By contrast, the fraction (Fig. 2B Lower) and absolute numbers (Fig. 2C Right) of Vα14i NKT cells in the donor-derived compartment in liver and spleen (unpublished data) of LTβR0/0 recipient mice were markedly reduced compared with C57BL/6 recipient mice. These results indicate that a LTβR-dependent stromal cell defect rather than a bone marrow precursor defect affects peripheral Vα14i NKT cell numbers in LT- and LTβR-deficient mice.
In Vivo Dependence of NKT Cells on LTαβ During Fetal Ontogeny and Postnatally.
We next assessed at which time points LTαβ–LTβR interaction would be required during development. Therefore, we evaluated the effects of in vivo administration of LTβR–Fc fusion protein, which acts as a soluble decoy receptor for LTαβ and LIGHT (25), versus control Ig into C57BL/6 mice during different phases of ontogeny and determined the effect on Vα14i NKT cell numbers and function. Previously, the LTβR–Fc fusion protein was shown to block lymph node genesis when given in utero and to disrupt splenic architecture as well as lamina propria B cells after administration to adult mice (26). We tested several regimens of administration (Fig. 6A, which is published as supporting information on the PNAS web site). LTβR–Fc was given to the first two groups of mice in utero on day 11 and day 15 of gestation. After birth, the mice were given either one injection at day 7 or continuous injections once a week until week 6 (continuous). All mice were killed at week 6 after birth, and the frequency and function of Vα14i NKT cells were determined in liver, spleen, and thymus. Consistent with previous reports (26), mice given in utero injections lacked peripheral lymph nodes, and their splenic architecture was found to be disrupted (unpublished data). A third group was composed of adult mice treated once a week with 100 μg of LTβR–Fc for 4 weeks (adult) (Fig. 6A), after which the mice were killed for analysis of Vα14i NKT cells. As previously demonstrated, administration of LTβR–Fc after birth affected splenic architecture (unpublished data).
As indicated in Fig. 6B, Vα14i NKT cell percentages and absolute numbers (unpublished data) were markedly reduced in liver and spleen (unpublished data) when given in utero on day 11 and day 15 of gestation and until week 6 after birth, but the reduction was much less when in utero treatment was followed with only a single postnatal injection on day 7, indicating that LTαβ was required postnatally. Vα14i NKT cells were much less affected in mice treated from day 18 of gestation on (unpublished data) or in adult mice treated with LTβR–Fc (Fig. 6B), which illustrates an additional requirement for LTαβ during fetal ontogeny. By contrast, Vα14i NKT cell frequency in the thymus was only marginally reduced in all treatment schedules. Furthermore, regardless of the treatment regimen, the majority of the thymic Vα14i NKT cells appeared phenotypically mature because they were CD44+NK1.1+ (Fig. 6C). In addition, the maturity of liver Vα14i NKT cells was not influenced by treatment with LTβR–Fc, as judged by their expression of NK1.1 and other NK cell markers such as NKG2, independent of the treatment schedule (unpublished data). Altogether, these results suggest that, in addition to its role during fetal ontogeny, LTαβ interaction is also required during the weeks after birth.
Homing, Expansion, and/or Survival in the Periphery of Vα14i NKT Cells Is Not Impaired in LTβR0/0 Mice.
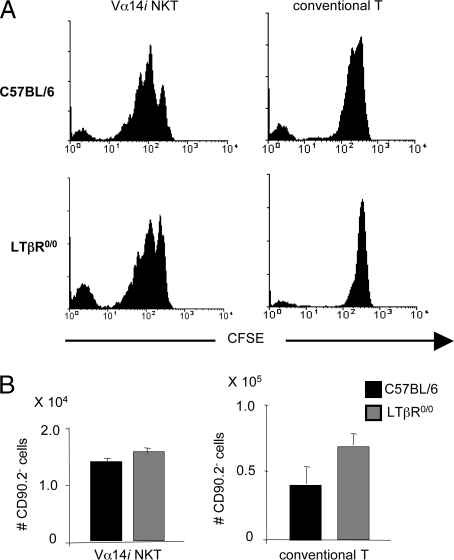
To determine whether the reduced peripheral numbers of Vα14i NKT cells was due to an impaired migration to or homeostatic proliferation in peripheral organs, we transferred carboxyfluorescein succinimidyl ester (CFSE)-labeled CD8α-depleted thymocytes from CD90.1 congenic C57BL/6 mice to irradiated C57BL/6 or LTβR0/0 recipient mice. One week later, thymus, liver, and lung of recipient mice were isolated and analyzed for the presence of CD90.2− Vα14i NKT cells and conventional T cells, and their CFSE intensity was measured. The number of cell divisions for both CD90.2− Vα14i NKT cells and conventional T cells was similar in C57BL/6 and LTβR0/0 recipient mice (Fig. 3A). Comparable numbers of CD90.2− Vα14i NKT cells and conventional T cells were found in the liver and lung (unpublished data) of C57BL/6 or LTβR0/0 recipients (Fig. 3B), whereas CD90.2− Vα14i NKT cells were virtually absent from the thymus of both C57BL/6 and LTβR0/0 recipients (unpublished data). The numbers of CD90.2− conventional T cells were comparable in the thymus of C57BL/6 or LTβR0/0 recipients, and they were remarkably lower than those found in the liver (unpublished data). To formally exclude that a reentry of the transferred thymocytes into the thymus of the recipient mice would affect the peripheral homing, homeostatic expansion, or survival of Vα14i NKT cells, we transferred CFSE-labeled, CD8α-depleted thymocytes from CD90.1 congenic C57BL/6 mice into irradiated thymectomized C57BL/6 and LTβR0/0 recipient mice and determined the homeostasis of donor-derived Vα14i NKT cells. Results were similar to those obtained in euthymic mice (Fig. 7, which is published as supporting information on the PNAS web site). These results unambiguously indicate that peripheral Vα14i NKT cell homing and homeostasis do not depend on LTαβ–LTβR signaling.
Fig. 3.
Homing, expansion, and/or survival in the periphery of Vα14i NKT cells is not impaired in LTβR0/0 mice. Six-week-old γ-irradiated (300 rad) C57BL/6 and LTβR0/0 recipient mice (n = 4) received an i.v. injection with CFSE-labeled, CD8-depleted thymocytes from CD90.1 congenic C57BL/6 mice. After 1 week, liver mononuclear cells of recipient mice were analyzed by FACS. (A) Analysis of the fluorescence intensity of the CFSE signal from Vα14i NKT cells and conventional T cells in the CD90.2− fraction of C57BL/6 versus LTβR0/0 recipients. (Left) Histograms gated on CD90.2− Vα14i NKT cells (TCRβ+ α-GalCer/CD1d tetramer+) lymphocytes. (Right) Histograms gated on CD90.2− TCRβ+ α-GalCer/CD1d tetramer− lymphocytes. (B) Bar graphs show mean absolute numbers ± SEM of CD90.2− Vα14i NKT cells (Left) and CD90.2− conventional T cells (Right) in the liver of C57BL/6 and LTβR0/0 recipient mice.
Regulation of Thymic Export of Vα14i NKT Cells by LTαβ.
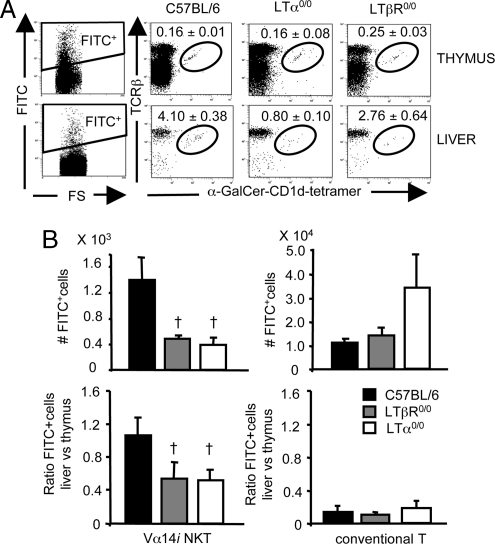
We next evaluated whether the decrease of Vα14i NKT cells observed in liver and spleen of LTα0/0, LTβ0/0, and LTβR0/0 mice could be indicative of a defect in the emigration from the thymus. To directly examine thymic emigration, we injected FITC into each thymic lobe of anesthetized LTα0/0 and LTβR0/0 or control mice and examined the fraction of FITC+ Vα14i NKT cells or conventional T cells in thymus, liver, and spleen 36 h later. The fraction of FITC+ Vα14i NKT cells in LTα0/0 and LTβR0/0 mice in liver (Fig. 4A) and spleen (unpublished data) was significantly reduced compared with wild-type controls. Similar results were obtained in C57BL/6 mice that were injected with LTβR–Fc in utero on day 11 and day 15 of gestation and until week 6 after birth (unpublished data). Remarkably, whereas the absolute numbers of Vα14i NKT cells that emigrated from the thymus were substantially reduced in LTα0/0 or LTβR0/0 animals, the number of FITC+ conventional T cell emigrants was slightly enhanced in LTα0/0 mice (Fig. 4B Upper), whereas the absolute number of both FITC+ Vα14i NKT cells and conventional T cells was not reduced in the thymus of LTα0/0 and LTβR0/0 mice versus controls (unpublished data). Whereas the relative fraction of FITC+ Vα14i NKT cells compared to the number of NKT cells labeled with FITC in the thymus was reduced in liver and spleen in LTα0/0 and LTβR0/0 mice versus controls, the relative fraction of FITC+ conventional T cells did not differ between either of the groups (Fig. 4B Lower). These findings together indicate that LTαβ–LTβR signaling is indispensable for the regulation of thymic Vα14i NKT cell emigration.
Fig. 4.
Defective thymic export in LT-deficient mice. Four- to 5-week-old wild-type C57BL/6 mice and LTα0/0 and LTβR0/0 mice were intrathymically injected with FITC. Thymic and liver mononuclear cells were analyzed by FACS 36 h later. (A) Plots were gated on FITC-positive lymphocytes as indicated in the left dot plots. Numbers represent mean percentage ± SEM of FITC-positive Vα14i NKT cells in thymi and livers of wild-type control C57BL/6 mice and LTα0/0 and LTβR0/0 mice (n = 5–6). (B Upper) Absolute numbers ± SEM of FITC-positive Vα14i NKT cells and FITC-positive conventional T cells in livers of wild-type control C57BL/6 mice and LTα0/0 and LTβR0/0 mice. (B Lower) Ratios of the number of FITC-positive liver Vα14i NKT cells and conventional T cells relative to the number of FITC-positive thymic Vα14i NKT cells and conventional T cells, respectively. †, P < 0.05, LTα0/0 or LTβR0/0 versus wild type (Student’s t test).
Discussion
Thymic emigration of T cells is an essential step in immune homeostasis. However, surprisingly little is known about the mechanisms that regulate the export of either conventional T cells or Vα14i NKT cells from the thymus. Over the past years, the molecular signals governing the ontogeny of T cells and the central role of the thymus have been unraveled in significant detail. There is considerable information on the regulation of the different steps of TCR rearrangement, as well as on positive and negative selection (27, 28). Much less described, however, are the events that immediately precede the export from the thymus to the periphery. In mice, mature thymocytes congregate near the lymphatics and blood vessels of the thymic medulla and are exported at a consistent daily rate of 1–2% of the total thymocytes (29, 30). It has been suggested that, for conventional T cells, functional maturity determines when their export from the thymus occurs, largely because of the mature status of most medullary thymocytes (30), and it has been reported that sphingosine 1 phosphate receptors play an important role in thymic export of conventional T cells (31). However, the emigration requirements of Vα14i NKT cells are yet unclear. The development of α-GalCer-loaded CD1d tetramers has provided a powerful tool to examine the developmental biology of Vα14i NKT cells (19). By using these tetramers, the expression of NK receptors such as NK1.1 has been shown to be much more flexible than previously anticipated. For example, NK1.1 expression is developmentally regulated, because immature Vα14i NKT cells lack NK1.1 expression (20, 21). Moreover, analysis of recent thymic emigrants by intrathymic injection of FITC has indicated that most Vα14i NKT cells leave the thymus as NK1.1− cells (20, 21). However, it was unclear whether the same signals that regulate emigration of conventional T cells also apply to Vα14i NKT cells or other unconventional T cells. The results presented here indicate that LTαβ is indispensable for the normal regulation of thymic emigration of Vα14i NKT cells but not conventional T cells.
To explore the role of LTβR-expressing stromal cells in Vα14i NKT cell development, we treated LTα0/0 mice with an agonistic anti-LTβR antibody in utero and during adult life. In addition, we treated C57BL/6 mice with a LTβR–Fc fusion protein during fetal development and postnatally or at adult age. In vivo administration of agonistic anti-LTβR antibodies in utero, but not in adult LTα0/0 mice, partially restored Vα14i NKT cell numbers, illustrating the importance of intact LTαβ–LTβR signaling during early Vα14i NKT ontogeny. In addition, transfer of wild-type bone marrow cells to LTβR0/0 mice could not restore the reduced peripheral Vα14i NKT cell numbers. These findings indicate that peripheral Vα14i NKT cells depend on LTβR-expressing stromal cells during fetal development. Several studies have highlighted the role of stromal cells, particularly those in the thymic medulla, as gatekeepers in the thymic export, and different models have been suggested by which the medulla may regulate this process (32). Interestingly, mice lacking a thymic medulla such as RelB0/0 and aly/aly mice, which carry a point mutation in the NF-κB-inducing kinase, were shown to have a profound deficiency in Vα14i NKT cells (33, 34). This finding suggests a critical role for the medulla in Vα14i NKT cell ontogeny. Likewise, thymic medullary epithelial cell differentiation was found to be disturbed in LTβR0/0 animals (35), although the defects were less pronounced than in aly/aly mice. In addition, in both aly/aly and RelB0/0 mice, a reduction in thymic Vα14i NKT cells was also observed (34), which is in contrast to the defects described here in LTβ0/0 and LTβR0/0 mice, where a reduction in only peripheral Vα14i NKT cells and not thymic Vα14i NKT cells is observed. This finding suggests that other upstream signals may regulate NF-κB-inducing kinase-dependent activation of RelB, which may have additional effects on Vα14i NKT cells, e.g., by influencing Vα14i NKT cell survival.
Because of the reported role of the medulla in controlling thymic emigration of conventional T cells (30) and the medullary defects in LTβR0/0 mice (35), we directly examined thymic emigration of Vα14i NKT cells compared with conventional T cells by analyzing intrathymic injections with FITC. Consistent with previous reports, ≈5% of FITC+ cells stained with α-GalCer/CD1d tetramers in the livers of C57BL/6 wild-type mice (20). These cells were strongly reduced in LTα0/0 and LTβR0/0 mice. By contrast, thymic emigration of conventional T cells was not decreased in LTβR0/0 mice and was even slightly enhanced in LTα0/0 mice. These findings corroborate earlier reports in which recently emigrated splenic CD8+ T cell numbers were found to be increased in LTα0/0 mice versus controls (36). Our data strongly support a model in which an LTβR-expressing thymic stromal cell type controls the emigration of Vα14i NKT cells from the thymus to the peripheral organs. In contrast, i.v. transfer of wild-type thymocytes to LTβR0/0 mice showed that LTαβ–LTβR interaction was not required for regulation of peripheral homing, homeostatic proliferation, or survival of Vα14i NKT cells.
The combined approach of administering agonistic anti-LTβR antibodies to LT-deficient mice and treatment of pregnant C57BL/6 mice using LTβR–Fc has also provided new information on the contribution of the thymic stromal compartment to the development of Vα14i NKT cells in relation to age. LTβR-dependent stromal function is required from day 11 of fetal ontogeny onward, similar to the requirement reported for lymphoid organ development (26). Little effect on Vα14i NKT cells was observed when an agonistic anti-LTβR antibody was given to pregnant LTα0/0 mice at fetal day 18 or, conversely, when wild-type mice were treated with LTβR–Fc at the same time point. This finding indicates a narrow time frame for the effect of LTαβ–LTβR interaction on Vα14i NKT cells early in fetal development. Presumably, intact LTβR signaling is required for proper thymic medulla formation, as previously reported (35). Our results indicate only a marginal effect of LTβR-dependent stromal cell function in adult mice, at least for Vα14i NKT cell development, although it was suggested that organization of medullary epithelial cells also depends on continuous signaling through LTβR. The precise details of these interactions, however, remain to be determined.
In summary, the results presented here indicate a pivotal role for LTαβ–LTβR interaction in the regulation of thymic emigration of Vα14i NKT cells by influencing thymic stroma function. These results underscore the importance of TNF cytokines in the ontogeny of innate immune lymphocytes.
Materials and Methods
Reagents and Antibodies.
α-GalCer was synthesized at the Pharmaceutical Research Laboratories of Kirin Brewery (Gunma, Japan). Agonistic anti-LTβR-mAb, anti-mouse LTβR–hIgG1 fusion protein, and control human IgG were provided by Jeff Browning (Biogen Idec, Cambridge, MA). Anti-KLH isotype control was purchased from BD Pharmingen. The following mAbs from BD Pharmingen were used in this study: anti-CD4, anti-TCRβ, anti-NK1.1, anti-CD8α, anti-IFN-γ, and anti-FcγR mAb. Anti-CD90.2 was obtained from eBioscience (San Diego). Production of α-GalCer/CD1d tetramers was described in ref. 19.
Mice.
C57BL/6, B6129F2 (C57BL/6J × 129S1/SvImJ, F2 hybrid) mice and B6;129-Ltbtm1Flv/J mice (LTβ0/0; mixed C57BL/6J × 129 background) were originally purchased from The Jackson Laboratory. Mice with disrupted LTα, B6.129-Ltatm1Tab (LTα0/0, backcrossed eight times onto C57BL/6 background) were described in ref. 37. LTβR, B6.129Ltbrtm1Kpf (LTβR0/0, backcrossed six times onto C57BL/6 background) or LIGHT-deficient mice, B6.129-Tnfsf14tm1Kpf (LIGHT0/0, backcrossed six times onto the C57BL/6 background) were described in refs. 23 and 38. All mice were housed and bred according to the guidelines of the Ghent University vivarium. CD90.1 congenic C57BL/6 mice, B6.Cg-Igha Thy1a Gpila/Crl, were purchased from Charles River/Iffa Credo. Unless stated otherwise, experiments were done with 8- to 10-week-old mice. LTβR0/0 mice were 5–6 weeks old. All animal procedures were approved by the Institutional Animal Care and Ethics Committee of Ghent University.
In Vivo Administration of Antibodies and Fusion Proteins.
Pregnant LTα0/0 mice were injected i.p. with 250 μg of agonistic anti-LTβR mAb or anti-KLH as isotype control on days 11, 13, 15, and 17 of gestation. Progeny were analyzed 6 weeks after birth. Pregnant C57BL/6 mice were injected simultaneously i.v. and i.p. with 50 μg of LTβR fusion protein (LTβRFc) on day 11 and day 15 of gestation. Progeny were injected i.p. with 25 μg of LTβRFc on day 7 after birth or received weekly injections with 25 μg of LTβRFc (continuous) until they were killed at the age of 6 weeks. Controls consisted of C57BL/6 mice treated with control IgG. In addition, adult C57BL/6 mice were injected i.p. weekly with 100 μg of LTβRFc or control IgG for 4 weeks and were killed 1 day after the last injection.
Cell Preparation.
Liver mononuclear cells were isolated by using a Percoll gradient as described in ref. 39 followed by osmotic lysis to remove the remaining red blood cells. Cell suspensions from thymus, spleen, and bone marrow were prepared by conventional methods. To analyze the expression of surface markers on α-GalCer/CD1d tetramer-binding thymocytes, CD8+ T cells were depleted with anti-CD8α Dynabeads according to the manufacturer’s instructions (Dynal).
Flow Cytometry.
Cell-surface staining and tetramer staining were performed as described in ref. 34. Unloaded tetramers were used as a control. Intracellular cytokine stainings were performed after fixation and permeabilization of the cells by using Cytofix/Cytoperm and Perm/Wash buffer according to the manufacturer’s protocol (BD Pharmingen). Cells were analyzed on a FACSort (Becton Dickinson) or FC500 (Beckman Coulter) flow cytometer. Data were analyzed by using cytomics cxp software (Beckman Coulter).
Intrathymic Injections.
Four- to 5-week-old mice were anesthetized by i.p. injection of a mixture of ketamin, xylazin, and PBS. After the chest was opened, each lobe was injected with 10 μl of FITC (Molecular Probes) dissolved in PBS (1 mg/ml). The chest and skin were closed. Mice were killed 36 h after injection, and thymus, liver, and spleen were removed.
Thymectomy.
Anesthetized C57BL/6 and LTβR0/0 mice underwent a small sternotomy to assure optimal access to the thymus. The thymus was gently removed, and the chest and skin were closed. The mice were allowed to recover for 1 week, after which the homeostasis experiments were initiated. When the mice were killed, the mediastinum was carefully inspected to ensure that the thymus had been removed entirely.
Bone Marrow Transfers.
Bone marrow transfers were performed as described in ref. 34. Bone marrow was isolated from the femur and tibia of CD90.1 congenic C57BL/6 mice. T cell-depleted bone marrow cells (107, purity was >98–99%) were i.v. transferred into sublethally γ-irradiated (600 rad) 3-week-old C57BL/6 and LTβR0/0 recipient mice. Thymus, liver, and spleen of recipients were analyzed 8 weeks after bone marrow transfer.
i.v. Transfer of CFSE-Labeled Thymocytes.
Thymocytes were isolated from CD90.1 congenic C57BL/6 mice and depleted from CD8+ cells with anti-CD8α Dynabeads (Dynal). CD8− cells were labeled with 1 μM CFSE (Molecular Probes). A total of 5–10 × 106 labeled cells were i.v. injected in 6-week-old γ-irradiated (300 rad) C57BL/6 and LTβR0/0 recipient mice. Liver and lung of recipient mice were analyzed 1 week later.
Supplementary Material
Acknowledgments
We gratefully acknowledge Dr. Carl Ware (La Jolla Institute for Allergy and Immunology), Dr. Jeff Browning, and Kirin Brewery for supplying reagents; Dr. Theresa Banks (La Jolla Institute for Allergy and Immunology) for providing LTα0/0 mice; and Dr. Klaus Pfeffer (Technical Institute of Munich, Munich) for providing LTβR0/0 and LIGHT0/0 mice. We are thankful to Dr. Tom Boterberg for irradiation of the mice. This work was supported by grants from the Fund for Scientific Research–Flanders (Belgium) and from the Research Fund of Ghent University. A.S.F., S.L., and P.D. are research assistants of the Fund for Scientific Research–Flanders (Belgium). K.V.B. is a postdoctoral fellow supported by the Research Fund of Ghent University. K.J.L.H. is supported by a C. J. Martin Postdoctoral Fellowship from the Australian National Health and Medical Research Council (ID no. 237029).
Abbreviations
- NK
natural killer
- Vα14i
Vα14 invariant
- TCR
T cell receptor
- LT
lymphotoxin
- LTβR
LTβ receptor
- α-GalCer
α-galactosylceramide
- KLH
keyhole limpet hemocyanin
- CFSE
carboxyfluorescein succinimidyl ester.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS ofice. S.J. is a guest editor invited by the Editorial Board.
References
- 1.Vandenabeele P., Declercq W., Beyaert R., Fiers W. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 2.Browning J. L., Sizing I. D., Lawton P., Bourdon P. R., Rennert P. D., Majeau G. R., Ambrose C. M., Hession C., Miatkowski K., Griffiths D. A., et al. J. Immunol. 1997;159:3288–3298. [PubMed] [Google Scholar]
- 3.Browning J. L., Ngam-ek A., Lawton P., DeMarinis J., Tizard R., Chow E. P., Hession C., O’Brine-Greco B., Foley S. F., Ware C. F. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 4.Crowe P. D., VanArsdale T. L., Walter B. N., Ware C. F., Hession C., Ehrenfels B., Browning J. L., Din W. S., Goodwin R. G., Smith C. A. Science. 1994;264:707–710. [PubMed] [Google Scholar]
- 5.Mebius R. E. Nat. Rev. Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 6.Gommerman J. L., Browning J. L. Nat. Rev. Immunol. 2003;3:642–655. doi: 10.1038/nri1151. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto M., Lo S. F., Carruthers C. J., Min J., Mariathasan S., Huang G., Plas D. R., Martin S. M., Geha R. S., Nahm M. H., Chaplin D. D. Nature. 1996;382:462–466. doi: 10.1038/382462a0. [DOI] [PubMed] [Google Scholar]
- 8.De Togni P., Goellner J., Ruddle N. H., Streeter P. R., Fick A., Mariathasan S., Smith S. C., Carlson R., Shornick L. P., Strauss-Schoenberger J., et al. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 9.Zhou D., Mattner J., Cantu C., III, Schrantz N., Yin N., Gao Y., Sagiv Y., Hudspeth K., Wu Y. P., Yamashita T., et al. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 10.Burdin N., Brossay L., Koezuka Y., Smiley S. T., Grusby M. J., Gui M., Taniguchi M., Hayakawa K., Kronenberg M. J. Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 11.Godfrey D. I., Hammond K. J., Poulton L. D., Smyth M. J., Baxter A. G. Immunol. Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald H. R. Curr. Opin. Immunol. 2002;14:250–254. doi: 10.1016/s0952-7915(02)00329-1. [DOI] [PubMed] [Google Scholar]
- 13.Lantz O., Bendelac A. J. Exp. Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberl G., Lees R., Smiley S. T., Taniguchi M., Grusby M. J., MacDonald H. R. J. Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- 15.Coles M. C., Raulet D. H. J. Immunol. 2000;164:2412–2418. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- 16.Bix M., Coles M., Raulet D. J. Exp. Med. 1993;178:901–908. doi: 10.1084/jem.178.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bendelac A., Killeen N., Littman D. R., Schwartz R. H. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 18.Bendelac A. J. Exp. Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidobre S., Kronenberg M. J. Immunol. Methods. 2002;268:107–121. doi: 10.1016/s0022-1759(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 20.Pellicci D. G., Hammond K. J., Uldrich A. P., Baxter A. G., Smyth M. J., Godfrey D. I. J. Exp. Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benlagha K., Kyin T., Beavis A., Teyton L., Bendelac A. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 22.Gadue P., Stein P. L. J. Immunol. 2002;169:2397–2406. doi: 10.4049/jimmunol.169.5.2397. [DOI] [PubMed] [Google Scholar]
- 23.Scheu S., Alferink J., Potzel T., Barchet W., Kalinke U., Pfeffer K. J. Exp. Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elewaut D., Brossay L., Santee S. M., Naidenko O. V., Burdin N., De Winter H., Matsuda J., Ware C. F., Cheroutre H., Kronenberg M. J. Immunol. 2000;165:671–679. doi: 10.4049/jimmunol.165.2.671. [DOI] [PubMed] [Google Scholar]
- 25.Rennert P. D., James D., Mackay F., Browning J. L., Hochman P. S. Immunity. 1998;9:71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 26.Rennert P. D., Browning J. L., Mebius R., Mackay F., Hochman P. S. J. Exp. Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starr T. K., Jameson S. C., Hogquist K. A. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 28.Sebzda E., Mariathasan S., Ohteki T., Jones R., Bachmann M. F., Ohashi P. S. Annu. Rev. Immunol. 1999;17:829–874. doi: 10.1146/annurev.immunol.17.1.829. [DOI] [PubMed] [Google Scholar]
- 29.Scollay R., Godfrey D. I. Immunol. Today. 1995;16:268–273. doi: 10.1016/0167-5699(95)80179-0. discussion 273–274. [DOI] [PubMed] [Google Scholar]
- 30.Berzins S. P., Uldrich A. P., Sutherland J. S., Gill J., Miller J. F., Godfrey D. I., Boyd R. L. Trends Mol. Med. 2002;8:469–476. doi: 10.1016/s1471-4914(02)02415-2. [DOI] [PubMed] [Google Scholar]
- 31.Matloubian M., Lo C. G., Cinamon G., Lesneski M. J., Xu Y., Brinkmann V., Allende M. L., Proia R. L., Cyster J. G. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 32.Anderson G., Jenkinson E. J. Nat. Rev. Immunol. 2001;1:31–40. doi: 10.1038/35095500. [DOI] [PubMed] [Google Scholar]
- 33.Sivakumar V., Hammond K. J., Howells N., Pfeffer K., Weih F. J. Exp. Med. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elewaut D., Shaikh R. B., Hammond K. J., De Winter H., Leishman A. J., Sidobre S., Turovskaya O., Prigozy T. I., Ma L., Banks T. A., et al. J. Exp. Med. 2003;197:1623–1633. doi: 10.1084/jem.20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boehm T., Scheu S., Pfeffer K., Bleul C. C. J. Exp. Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabor M. J., Sedgwick J. D., Lemckert F. A., Godfrey D. I., Korner H. Immunol. Cell Biol. 2001;79:323–331. doi: 10.1046/j.1440-1711.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- 37.Banks T. A., Rouse B. T., Kerley M. K., Blair P. J., Godfrey V. L., Kuklin N. A., Bouley D. M., Thomas J., Kanangat S., Mucenski M. L. J. Immunol. 1995;155:1685–1693. [Google Scholar]
- 38.Futterer A., Mink K., Luz A., Kosco-Vilbois M. H., Pfeffer K. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 39.Robbins S. H., Nguyen K. B., Takahashi N., Mikayama T., Biron C. A., Brossay L. J. Immunol. 2002;168:2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.