Abstract
Shigella is a major cause of morbidity, mortality, and growth retardation for children in developing countries. Emergence of antibiotic resistance among Shigellae demands the development of effective medicines. Previous studies found that the endogenous antimicrobial peptide LL-37 is down-regulated in the rectal epithelium of patients during shigellosis and that butyrate up-regulates the expression of LL-37 in colonic epithelial cells in vitro and decreases severity of inflammation in experimental shigellosis. In this study, Shigella-infected dysenteric rabbits were treated with butyrate (0.14 mmol/kg of body weight) twice daily for 3 days, and the expression levels of the rabbit homologue to LL-37, CAP-18, were monitored in the colon. Butyrate treatment resulted in (i) reduced clinical illness, severity of inflammation in the colon, and bacterial load in the stool, (ii) significant up-regulation of CAP-18 in the surface epithelium, and (iii) disappearance of CAP-18-positive cells in lamina propria. The active CAP-18 peptide was released in stool from its proform by butyrate treatment. In healthy controls, CAP-18 expression was localized predominantly to the epithelial surface of the colon. In infected rabbits, CAP-18 expression was localized to immune and inflammatory cells in the colon, whereas the ulcerated epithelium was devoid of CAP-18 expression. The combination of CAP-18 and butyrate was more efficient in killing Shigella in vitro than CAP-18 alone. Our findings indicate that oral butyrate treatment in shigellosis may be of clinical value because of induction of the endogenous cathelicidin CAP-18 in the colonic epithelium, stimulation of the release of the active peptide CAP-18, and promoting elimination of Shigella.
Keywords: antimicrobial peptides, CAP-18, cathelicidin, colon, Shigella
Shigellosis is one of the major causes of morbidity and mortality in many developing countries. It is estimated that, every year, 165 million people are infected with Shigella species, and 1.1 million die from shigellosis (1). Children under the age of five are most susceptible.
Antibiotics are the mainstay of therapy for all cases of shigellosis, because an effective vaccine against shigellosis is not currently available. However, because of excessive use of antibiotics, emergence of multi-drug-resistant species of Shigella has accelerated (2). Shigella is a facultative intracellular pathogen but cannot invade intestinal epithelial cells through the apical surface (3). Initial invasion may take place by Shigella-induced transepithelial migration of polymorphonuclear cells in a basolateral-to-apical direction. This migration destroys cohesion of the epithelial barrier and facilitates invasion by Shigella (4). Shigella may also be taken up by M cells in follicular-associated epithelium (5), phagocytosed by resident tissue macrophages, where it causes macrophage apoptosis (6, 7), and further escape into the local milieu. Thereafter, the bacteria invade the epithelial cells via the basolateral side, multiply intracellularly, and are spread laterally, causing epithelial erosion and ulceration.
Antimicrobial peptides are essential effector molecules of the innate immune system (8, 9) and are of great importance in bacterial host defense (10, 11). There are two major classes of antimicrobial peptides in humans: the defensins (12) and the cathelicidins (13). Defensins are widely distributed in mammalian epithelial cells and phagocytes. Cathelicidins are a family of antimicrobial peptides with a conserved proregion, cathelin, on which a variable antimicrobial domain is connected. Cathelicidins are secreted from neutrophils, are found in the bloodstream, and are also expressed at epithelial surfaces. LL-37 is the only member of this family identified in humans and is an amphipathic α-helical peptide composed of 37 amino acids (14). In addition to its microbicidal activity, LL-37 has been demonstrated to function as a chemoattractant for PMN and CD4 T cells and to be involved in angiogenesis and immune modulation (15).
In 2001, our group showed that expression of the human antimicrobial peptide LL-37 and human β-defensin-1 was down-regulated early in Shigella infections (16). In contrast, we and others have detected up-regulation in colon epithelium of LL-37 expression with short-chain fatty acids (SCFAs), and sodium butyrate was the best inducer (17, 18). Butyrate is a major product of bacterial fermentation in the colon, has been shown to be a crucial energy source for gut epithelial cells, and is readily metabolized by cells of the colonic mucosa (19). Furthermore, butyrate has, indeed, been shown to decrease clinical symptoms of Shigella infections in a rabbit experimental model (20). A connection between the severity of Shigella infections and the mucosal level of cathelicidins seems likely.
We hypothesized that butyrate therapy in shigellosis will induce expression of cathelicidin, which will contribute to elimination of Shigella and, thus, affect recovery. Here, we address the hypothesis in an experimental rabbit model of shigellosis by studying the expression of the rabbit cathelicidin CAP-18 in the colonic mucosa in healthy and infected rabbits that have been treated with butyrate or saline.
Results
Clinical Response of Dysenteric Rabbits and Treatment with Butyrate.
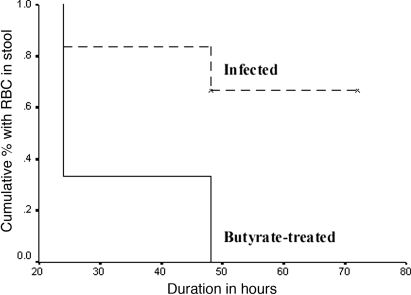
Among the 20 rabbits inoculated with Shigella flexneri 2a, 17 (85%) developed mild to severe diarrhea with thick liquid to bloody stool with occasional mucus. The animals were all lethargic, anorectic, had fever, as judged by increased rectal temperature, and reduction in body weight. Two animals died before any treatment could be started and were excluded from further analysis. Histological evaluation showed evidence of acute colitis with moderate to severe inflammation in the ileum and colon but to a much lesser extent in the rectum. The lesions were characterized by hemorrhagic inflammation in the mucosa, with occasional erosion of the surface epithelium (SE), marked edema, congested blood vessels, and vasculitis. Furthermore, light microscopy showed infiltration of the lamina propria (LP) (21) with neutrophils and macrophages. A marked improvement in the clinical features and physical symptoms was noticeable within 24 h of treatment in the butyrate-treated rabbits compared with the control group. Kaplan–Meier survival curves for the treated rabbits by time showed that treatment with butyrate led to early recovery from clinical symptoms. Compared with infected rabbits, butyrate-treated rabbits had earlier reduction in liquid stool (95% confidence interval of time (h) to recovery, 63–75 vs. 26–43, respectively, log-rank test, 10.22; P = 0.001) and of RBC in stool (45–75 vs. 22–42, respectively; log-rank test, 5.8; P = 0.01) (Fig. 1). No significant differences were obtained in terms of recovery from weight reduction or normalization of body temperature. An additional three rabbits died in the control group within 3–4 days postinfection. Because antibiotics were not given, it was not unusual that the severely infected rabbits would not survive after 3–4 days postinfection because of bacteremia, hypotension, and decreased perfusion of vital organs (22).
Fig. 1.
Kaplan–Meier survival curves of infected rabbits by time after treatment with butyrate or saline. Compared with infected rabbits, butyrate-treated rabbits had earlier reduction of RBCs in stool.
CAP-18 Is Down-Regulated After Shigella Infection.
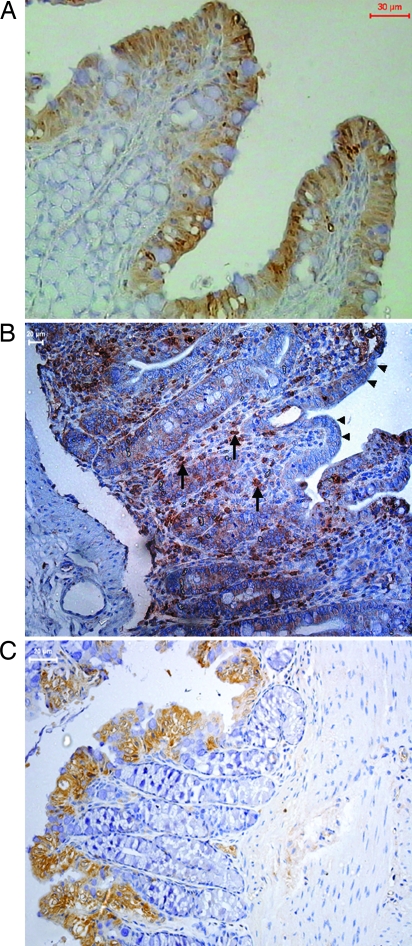
Immunostaining revealed CAP-18 expression specifically localized to the mucosal SE in the proximal and distal colon in healthy rabbits. Expression of CAP-18 occurred predominantly in SE and was barely detectable in the LP (Fig. 2A). A significant reduction in the CAP-18 expression in the rectal SE was apparent in rabbits after infection with Shigella; however, numerous CAP-18-positive cells were visible in the LP of the colon and the rectum (Fig. 2B and Table 1). These results are in agreement with our earlier observation in the rectums of Shigella-infected patients (16). Quantitative image analysis of CAP-18-positive immunostaining showed a significant reduction in the cumulative expression of CAP-18 in the total SE of the large intestines (proximal colon, distal colon, and rectum) of infected compared with healthy rabbits (P < 0.05) (Table 1). Specificity of CAP-18 staining was confirmed by complete abolition of the reactivity by preincubating CAP-18-specific antibody with corresponding synthetic CAP-18 (see Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 2.
Immunohistochemical staining of CAP-18 in paraffin-embedded sections of rabbit colon. All sections were counterstained with hematoxylin. (A) Immunoreactive signals for CAP-18 (brown) in healthy rabbits were almost exclusively located in the SE. (B) In Shigella-infected rabbits, surface and crypt epithelia were almost devoid of CAP-18 staining (arrowheads); abundant CAP-18-expressing inflammatory cells (arrows) were seen in the LP. (C) Reappearance of CAP-18 staining in the SE and disappearance of CAP-18-expressing cells from the LP in infected rabbits treated with butyrate.
Table 1.
Semiquantitative expression of CAP-18 in the intestinal tissue specimens from saline- and butyrate-treated infected and healthy rabbits
| Anatomic sites | Healthy rabbits (n = 5) |
Shigella-infected rabbits |
|||
|---|---|---|---|---|---|
| Saline treated (n = 4) | P | Butyratetreated (n = 8) | P | ||
| Proximal colon | |||||
| SE | 3.54 ± 0.74 | 3.47 ± 1 | 0.9 | 4.5 ± 2.9 | 0.7 |
| LP | 0.3 ± 0.25 | 1.3 ± 0.4 | 0.16 | 0.8 ± 0.19 | 0.54 |
| Distal colon | |||||
| SE | 7.1 ± 1.1 | 4.22 ± 1.4 | 0.24 | 9.9 ± 1 | 0.039 |
| LP | 1.3 ± 0.69 | 1.6 ± 0.57 | 0.54 | 0.58 ± 0.08 | 0.25 |
| Rectum | |||||
| SE | 6.7 ± 1.5 | 0.3 ± 0.2 | 0.01 | 15 ± 6.5 | 0.18 |
| LP | 0.98 ± 0.2 | 1.7 ± 0.2 | 0.13 | 0.3 ± 0.2 | 0.025 |
| Total | |||||
| SE | 5.8 ± 0.7 | 3.3 ± 0.8 | 0.05 | 10.48 ± 2.7 | 0.008 |
| LP | 0.87 ± 0.26 | 1.51 ± 0.3 | 0.15 | 0.56 ± 0.1 | 0.025 |
Data expressed as mean ± SEM. Healthy rabbits were not infected with Shigella and were not given any treatment. Quantification of immunoreactive area relative to the total tissue section was determined by a computerized image-analyzing technique, and the results were expressed as the total positively stained cell area measured × total mean intensity of the positive area, divided by total cell area measured (see Materials and Methods). Student's t test was used in comparing data between healthy and infected rabbits or between infected rabbits treated with butyrate or saline. The differences were significant when P < 0.05.
Sodium Butyrate Restores Epithelial Production of CAP-18.
When infected rabbits with bloody diarrhea were orally treated with sodium butyrate, CAP-18 staining reappeared in the SE, and the number of CAP-18-positive cells in the LP decreased substantially (Fig. 2C and Table 1). In comparison with infected rabbits, butyrate-treated rabbits had significantly higher expression of CAP-18 in the SE of the distal colon (Fig. 2 B and C and Table 1). The expression of CAP-18 was higher in the SE of butyrate-treated rabbits in the colon and the rectum compared with healthy rabbits, although statistical significance was not achieved (P ≥ 0.07). Notably, butyrate treatment also led to a dramatic decrease of CAP-18-expressing cells in the LP of colon and rectum compared with controls (P < 0.03).
Quantification of CAP-18 Transcripts in Colonic Tissues.
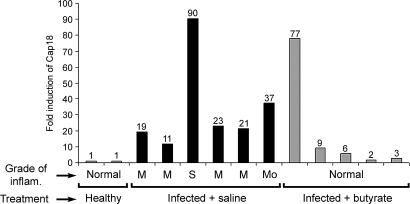
Relative levels of CAP-18 transcripts measured by real-time PCR in colonic biopsy specimens included both LP and SE. In infected rabbits, CAP-18 transcripts were found to be very high compared with healthy and butyrate-treated rabbits (Fig. 3). The high influx into the LP of cells such as macrophages and neutrophils expressing abundant levels of CAP-18 may be responsible for these elevated levels (Fig. 2C). This finding was in accordance with the immunohistochemical data, where CAP-18 expression in the LP in infected rabbits was higher than in butyrate-treated rabbits, and the difference was statistically significant in the rectum (Table 1). Severe inflammation during shigellosis is accompanied by excessive accumulation of immune and inflammatory cells at the site of infection (23). Thus, the highest levels of CAP-18 transcript were seen in colonic tissues, with the most severe histological grade of inflammation (Fig. 3). High levels of CAP-18 transcripts were also observed in the proximal colon from a butyrate-treated rabbit with normal histology (Fig. 3, bar 9) that could reflect prominent epithelial expression.
Fig. 3.
The relative expression of CAP-18 transcript in colonic biopsies from healthy, Shigella-infected, and butyrate-treated rabbits was measured by quantitative real-time PCR. Each bar represents data from one rabbit. For each rabbit, the first bar represents proximal colon, and the second bar represents distal colon. The last bar represents distal colon from only one rabbit. Under each bar, histological grades of inflammation are expressed as mild, 1; moderate, 2; severe, 3; and normal, 0 histology.
Release of Processed CAP-18 Peptide in Stool.
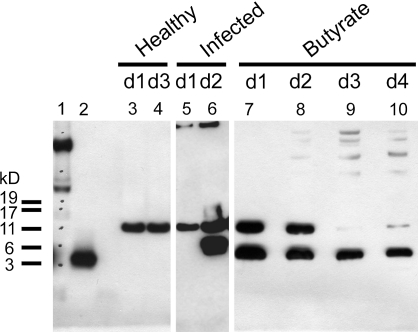
Western blot analysis of CAP-18 in stool extracts of rabbits demonstrated that the proform of CAP-18 was present in the healthy and infected rabbits 1 day postinfection (Fig. 4, lanes 3–5). However, in butyrate-treated rabbits, both the proform and the processed CAP-18 peptide were found in stool extracts on days 1 and 2, whereas, on days 3 and 4, only the processed peptide could be detected (Fig. 4, lanes 7–10). In one infected rabbit, the CAP-18 peptide could also be seen on day 2 postinfection, most likely reflecting infiltrating immune cells harboring CAP-18 (Fig. 4, lane 6). Notably, the size of the processed peptide is different between the samples originating from the infected rabbit and the butyrate-treated rabbit (Fig. 4, lanes 6 and 7–10), an observation that may reflect separate sources of the active peptide from immune cells in infected rabbits versus epithelial cells in butyrate-treated rabbits.
Fig. 4.
Western blot analysis of CAP-18 in stool extracts from healthy, Shigella-infected and butyrate-treated rabbits with the CAP-18-specific polyclonal antisera. Lanes 1 and 2 show a marker and the synthetic CAP-18 peptide, respectively. A single band corresponding to the pro-CAP-18 protein appears in the stool of healthy (lanes 3 and 4) and Shigella-infected (lane 5) rabbits 1 day postinfection. Two immunoreactive bands are visualized at day 2 postinfection (lane 6), showing presence of both pro-CAP-18 and active CAP-18 peptides in stool. Butyrate treatment resulted in release of the active CAP-18 peptide in stool (lanes 7–10); day-1 and day-2 stool specimens (lanes 7 and 8) also contained the pro-CAP-18 protein.
Furthermore, in the inhibition-zone assay, we have recorded activity against Escherichia coli strain D21 in the stool extract of a butyrate-treated rabbit. Twenty percent of this activity could be blocked with the CAP-18-specific antibodies but not with an unspecific IgY-antibody, demonstrating that the biologically active peptide is present in this stool extract (data not shown).
Reduction in Bacterial Load in the Intestine After Butyrate Treatment.
Oral treatment of the infected rabbits with sodium butyrate resulted in decreased Shigella load in stool (Table 2) over the study period. Treatment with saline did not result in decrease of bacterial counts in stool.
Table 2.
Shigella counts in stool (cfu per g) from Shigella-infected rabbits before and after butyrate/saline treatment
| Infected rabbits | Hours from inoculation |
||
|---|---|---|---|
| 24 | 48 | 72 | |
| Saline treated, n = 7 | 1 × 1010 ± 8.2 × 109 | 1.1 × 108 ± 8.6 × 107 | 1.1 × 107 ± 2.4 × 106 |
| Butyrate treated, n = 8 | 1 × 1010 ± 3 × 109 | 1 × 106 ± 9.5 × 105 | 6.1 × 103 ± 5 × 103 |
Diarrhea developed within 24 hours of oral administration of the bacterial inoculum. Time of administration of bacterial inoculum was considered as 0 hour. Butyrate or saline treatment was given 24 hours after the time of bacterial inoculation. Three rabbits died in the saline group 72 hours postinfection.
Bactericidal Activity of Synthetic CAP-18.
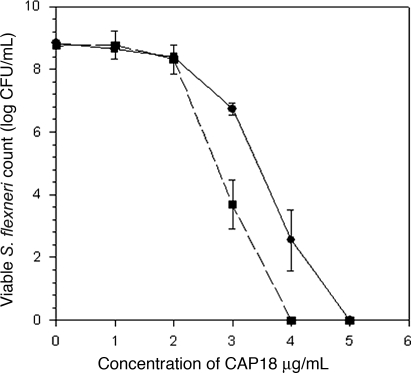
Susceptibility of Shigella to CAP-18 against clinical isolates of Shigella dysenteriae type 1 and Shigella flexneri was measured. After an overnight incubation at 37°C, 97–99% killing of Shigella dysenteriae type I was observed at a concentration of 0.90 μM of CAP-18 (4 μg/ml), whereas, for Shigella flexneri, 1.13 μM (5 μg/ml) was needed (Fig. 5). Thus, in the in vitro assay, Shigella dysenteriae was found to be more susceptible to CAP-18 than Shigella flexneri 2a. The bactericidal activity of CAP-18 was also analyzed against other enteric pathogens, such as Salmonella typhi, Vibrio cholera O1, and enterotoxigenic E. coli and found to be effective in the same range as for the Shigella strains (data not shown).
Fig. 5.
Inhibitory effects of various concentration of CAP-18 in presence (dotted line) or absence (solid line) of 40 mM butyrate (4.4 mg/ml) on the growth of Shigella bacterial colonies.
Bactericidal Effects of CAP-18 in Combination with Sodium Butyrate.
By using synthetic CAP-18, 97–99% killing of Shigella flexneri and Shigella dysenteriae type 1 strains was observed at 1.13 and 0.90 μM (5 and 4 μg/ml), respectively. With butyrate, maximum killing was achieved at 400 mM (44 mg/ml) for both Shigella dysenteriae type 1 strain and Shigella flexneri strain. However, 97–99% killing was observed with CAP-18 in the presence of 40 mM butyrate at 0.90 and 0.68 μM (4 and 3 μg/ml) for Shigella flexneri (Fig. 5) and Shigella dysenteriae type 1, respectively. The trend indicates that butyrate may work with CAP-18 in either an additive or synergistic manner. Similar effects of butyrate and CAP-18 in the killing of various other enteric pathogens such as V. cholera O1, enterotoxigenic E. coli, and Salmonella typhi were also observed (data not shown).
Evaluation of Safety of Oral Butyrate Treatment in Rabbits.
Different concentrations of butyrate were given to healthy rabbits (n = 10) to study the effect on renal and hepatic functions by measuring renal markers and hepatic enzyme levels in serum before and after the final treatment. These results showed that there was no significant increase in the serum levels of the different markers after treatment with various doses of butyrate (see Table 3, which is published as supporting information on the PNAS web site).
Discussion
We have demonstrated, in an experimental rabbit model of shigellosis, that oral administration of sodium butyrate led to a substantially improved clinical course and outcome (Fig. 1) and a significant decrease of Shigella in the stool (Table 2). Our data support the hypothesis that the mechanism of this therapeutic effect of sodium butyrate is, in part, through its effect on the expression and activity of the endogenous antibiotic CAP-18.
We have earlier shown that down-regulation of the innate effectors cathelicidin LL-37 and β-defensin-1 takes place in rectal epithelial cells in adult patients with shigellosis (16). The study also indicated that Shigella mediated the immune evasion by its virulence plasmid. The down-regulation of LL-37 in the intestinal surface epithelium may have significance in establishment of intraepithelial infection by Shigella (24). In the present study, a significant reduction of the rabbit cathelicidin CAP-18 in the intestinal SE was evident in experimental shigellosis, according to immunohistochemical findings. Notably, treatment of infected rabbits with butyrate restored CAP-18 expression in the colonic SE that occurred in parallel to clinical recovery from shigellosis. We also observed that treatment with butyrate liberates the active CAP-18 peptide (Fig. 4), which is a potent cathelcidin in killing Shigella bacteria (Fig. 5). It is known that SCFAs are capable of killing bacteria at high doses (20). Interestingly, we found that CAP-18 in combination with butyrate is more efficient in killing bacteria than CAP-18 alone (Fig. 5). Together, the combined effects of butyrate on the expression, cleavage, and activity of CAP-18 most likely contributed to the elimination of Shigella bacteria in the intestinal mucosa.
SCFAs are important end products of bacterial fermentation in the colon, and are vital for the commensal microflora of the gut (25). Butyrate has been used in therapeutic treatment of different inflammatory bowel diseases, such as ulcerative colitis and Crohn's disease, with positive outcomes in several clinical trials (21, 25). We have demonstrated that SCFAs, especially butyrate, were capable of inducing LL-37 in human colonic epithelial cells (18). Having had this work as a background, we attempted to examine whether the cathelicidin can be induced in vivo by butyrate treatment and whether the induction of the cathelicidin could be used to block the down-regulation by Shigella.
Upon butyrate treatment, the active form of CAP-18 is released into the intestinal mucosa. Hence, butyrate may activate some proteases with the capacity to process the precursor of CAP-18 to the mature peptide into the gut lumen. In this study, we have observed two different forms of the CAP-18 peptide, as detected by Western blot with the specific CAP-18 polyclonal antibody. Because these two forms migrate differently in the SDS/PAGE (see Fig. 4), we may speculate that one form originates from immune cells and the other from epithelial cells; however, this speculation will require further studies to substantiate. It has recently been reported that several processing forms of the human cathelicidin are generated in sweat, resulting in several active peptides with a different spectrum of antibacterial activity (26).
The quantification of the transcripts of CAP-18 in rabbit colon specimens revealed that the highest levels were observed in the infected rabbits. Transcript levels of CAP-18 in healthy and butyrate-treated rabbit colon samples were similar but lower in comparison with saline-treated rabbits (Fig. 3). The biopsy specimens included both the SE and the LP, reflecting the total picture of cells expressing CAP-18 in the colon. During acute shigellosis, a massive influx of various immune and inflammatory cells takes place in the LP (23), and a majority of these cell types express cathelicidin. We found that the transcript levels matched the degree of inflammation in the colon, with the highest level detected in the most severe grade of inflammation. Butyrate treatment resulted in healing of the colonic mucosa, leading to reduction in numbers of infiltrating inflammatory cells, as has also been reported by Rabbani et al. (20) in their experimental model of shigellosis. In addition, there was regeneration of the SE expressing similar levels of CAP-18, as seen in the healthy mucosa. Butyrate is known to induce colonic epithelial cell differentiation, and it has been claimed that butyrate-induced cell differentiation is a determinant for the up-regulation of LL-37 in colonic epithelial cells (17). However, we have shown that pathways other than those involved in cell differentiation trigger the up-regulation of LL-37 with butyrate, indicating that differentiation and LL-37 expression can be separated (18).
After butyrate treatment, CAP-18-expressing inflammatory cells were hardly detectable, showing that resident as well as accumulated immune cells did not harbor CAP-18 peptide, whereas the expression in SE was very prominent. This finding may indicate that butyrate down-regulates the expression of rabbit cathelicidin in these infiltrating cells, which may be similar to the observation of down-regulation of the human cathelicidin LL-37 by butyrate in peripheral blood mononuclear cells (G.H.G. and B.A., unpublished data).
The mechanism of down-regulation of cathelicidins by Shigella has not been determined. We may speculate that Shigella blocks some critical protein in the signaling pathway for the expression of cathelicidins that is then circumvented by butyrate. We have earlier shown that up-regulation of LL-37 by butyrate in colonic epithelial cells involves the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway (18). Neisseria gonorrhoeae is a pathogen that also down-regulates the expression of cathelicidin in cervical epithelial cells (27). However, butyrate does not counteract this down-regulation, although it can induce the expression of LL-37 in this cell line (27). Therefore, it seems likely that these two pathogens interact with signal pathways linked to the expression of cathelicidins by different mechanisms.
In conclusion, we have shown that butyrate, a natural product of digestion, can be used to stimulate production of endogenous antimicrobials in the gut that, in turn, can promote the clearance of Shigella and ease the symptoms of bacillary dysentery. These findings may provide the basis for therapeutic manipulation of endogenous antibiotics, including CAP-18 production in vivo, by using dietary substances and SCFA to strengthen the epithelial defense barrier. This strategy for treatment of shigellosis has a potential to be used in patients, either as an alternative treatment or in conjunction with classical antibiotics.
Materials and Methods
The study was approved by the Animal Ethics Committee of the ICDDR,B: Centre for Health and Population Research.
Antimicrobial Peptide CAP-18 and the Corresponding Specific Antibody.
The bioactive synthetic peptide CAP-18 (GLRKRLRKFRNKIKEKLKKIGQKIQGLLPKLAPRTDY) was synthesized (Innovagen, Lund, Sweden) and is the rabbit homologue of LL-37. Polyclonal antiserum against CAP-18 was made in chicken (Innovagen), and enriched IgY was purified by affinity chromatography, where the CAP-18 peptide was crosslinked to the gel matrix (Innovagen).
Animal Model.
Inbred New Zealand White rabbits of either gender weighing 1.8–2 kg were maintained in the animal resource facilities of the ICDDR,B. Health status of the rabbits was determined by physical examination, culture of stool specimens, and fecal parasitic examination. Healthy rabbits that were free of enteric pathogens (e.g., Salmonella, Shigella, and Vibrio cholera) and coccidia were studied. Twenty rabbits were infected and divided into two groups; one group was treated orally with sodium butyrate and the other with saline. Expression of the CAP-18 peptide, its proform, and mRNA in colonic and rectal tissue specimens were analyzed in these infected rabbits and in five healthy rabbits. Ten healthy rabbits were used for testing the toxicity effects of sodium butyrate.
Bacterial Strain.
Shigella flexneri 2a strain was isolated from stool of one patient attending the hospital of ICDDR,B. The strain was positive for the Serény test and Congo red binding, reflecting invasive properties (28).
Infection Procedure.
A nonsurgical rabbit model of shigellosis was used in this study as described in ref. 22. A 7-ml dose of the bacterial suspension [109 cfu in 7 ml of normal saline (0.9% wt/vol, pH 7.2)] was given via sterile feeding tube to each rabbit. Usually, rabbits developed dysentery within 24 h of bacterial inoculation. After development of dysenteric symptoms, rabbits were given sodium butyrate (0.14 mmol/kg of body weight) in 20 mM sodium chloride or 20 mM saline (0.07 mmol/kg of body weight, pH 7.2) orally twice daily at 12-hour interval for 3 days. Four days after inoculation, rabbits were killed with an overdose of i.v. sodium pentobarbitol (66 mg/kg of body weight; Sigma).
Specimen Collection.
Stool samples were collected before inoculating bacterial suspension, 24 h after inoculation, and daily after saline or butyrate treatment for 3 days. Rectal swabs were plated onto MacConkey and trypticase soy agar plates and incubated overnight at 37°C. After the rabbits were killed, the abdomen was opened and 2-in-long sections of proximal, mid, and distal colon and rectum were collected from each rabbit in 4% buffered formalin for immunohistochemical and histological evaluation. For quantitative real-time PCR, tissue specimens were collected in Trizol (GIBCO/BRL).
Clinical Efficacy and Biosafety of Butyrate Treatment.
Clinical recovery of the rabbits from shigellosis was established by disappearance of blood from stool, reappearance of formed stool, normalization of weight and temperature, and return of normal appetite and playful activity. For biosafety evaluation, 10 healthy rabbits were given variable doses of oral sodium butyrate twice daily for 3 days, and serum was collected before and after the final treatment for measuring the concentration of hepatic enzymes (alkaline phosphatase, aspartate transaminase, alanine transaminase, and γ-glutamyl transferase) and renal markers (urea and creatinine).
Bacterial Counts in Stool.
Bacterial load in stool was quantified by plating serial dilutions of stool onto MacConkey agar, and colonies were counted after an overnight incubation at 37°C. The results were expressed as cfu per ml.
In Vitro Bacterial Killing.
Shigella flexneri or Shigella dysenteriae type 1 from trypticase soy agar plates were inoculated in 20 ml of Mueller–Hinton broth (MHB; Becton Dickinson) and incubated for 4 h at 37°C with shaking. After washing in PBS, optical density was adjusted to 0.4 at 600 nm, which equals 108 cfu per ml. Bacterial suspension (2 × 102 cfu), different dilutions of CAP-18, and sodium butyrate at a final concentration of 40 mM (pH 7.2) were added to each well in a microtiter plate (Nunc). Control wells contained MHB alone, bacteria in MHB, MHB containing CAP-18, and, finally, butyrate with and without bacteria. The plate was incubated at 37°C in a shaker incubator overnight. From individual wells, the mixture was plated on MacConkey agar for bacterial colonies.
Stool Extraction and Enrichment of Peptides/Proteins.
Feces collected from rabbits were diluted 1:5 in PBS (pH 7.2) containing soy bean trypsin inhibitor (100 μg/ml; Sigma) and phenylmethylsulfonyl fluoride (1 mg/ml; Sigma) and centrifuged. Supernatants were collected, passed through a 0.45-μm filter, and kept frozen at −70°C. To enrich peptides/proteins in stool extract, trifluoroacetic acid (TFA) was added to supernatants and were applied onto acetonitrile (AcN)-activated OASIS cartridges (Waters) equilibrated in aqueous 0.1% TFA. Bound peptides/proteins were eluted with 80% aqueous AcN in 0.1% TFA and lyophilized. These peptide/protein concentrates were used for further analyses.
Antibacterial Assay.
An inhibition-zone assay with E. coli strain D21 and Medium E included in the agarose plate was performed as described (14).
Depletion of Antibacterial Activity.
Synthetic CAP-18 peptide (0.063 μg/μl) and stool extract from a butyrate-treated rabbit (0.25 μg/μl) were mixed with excess of anti-CAP-18 (2 mg/ml) or normal IgY (2 mg/ml Santa Cruz Biotechnology) and incubated at room temperature for 4 h. The mixtures were then analyzed in the inhibition-zone assay against E. coli D21 in agarose plates supplemented with Medium E.
Western Blot Analysis.
The presence of CAP-18 (80 or 100 μg) originating from the different stool extracts was determined with Western blot analysis using the CAP-18-specific chicken polyclonal antiserum. Separation was done with SDS/PAGE using 4–12% NuPAGE Ready Gels (Invitrogen). Polyclonal CAP-18 antiserum (Innovagen) (0.09 μg/ml in PBS) was used as the primary antibody and donkey anti-chicken IgY conjugated with horseradish peroxidase (Jackson ImmunoResearch) as the secondary antibody. An ECL Western blot detection system (GE Healthcare, Uppsala, Sweden) was used to visualize the results.
Cloning of CAP-18.
Total RNA was isolated from rabbit spleen, and cDNA was synthesized by using reverse transcriptase. The cDNA was then used as a template in a PCR with the primer pairs: 5′-TCCCTGGCCTGGTGGTCACT-3′ and 5′-GCGCAGCCCAGTAGGTTCTG-3′. The annealing temperature was 50°C, and the PCR was performed at 35 cycles. A DNA fragment of the expected size that was purified (theoretically 387 bp) was obtained, blunt ends created, and the fragment was inserted into a pPCR-ScriptAmp Sk(+) vector by using the PCR-Script Amp cloning kit (Stratagene). Transformation was done in E. coli strain DH5α by electroporation, and the clones were color screened. Five clones were sequenced by MWG Biotech (Ebersberg, Germany), and two of them were found to contain the sequence of proCAP-18.
RT-PCR and TaqMan Real-Time RT-PCR.
Total RNA was prepared from rabbit colonic specimens according to the manufacturer's instructions (Qiagen, Valencia, CA). Reverse-transcription of RNA into cDNA was performed essentially as described in ref. 29. For quantification, the relative expression of CAP-18 and the housekeeping gene 18S RNA were measured in triplicate from the different RNA extractions by real-time quantitative RT-PCR, using an Applied Biosystems PRISM model 7700 sequence detection instrument and the 18S RNA-Housekeeping kit. The sequences of forward and reverse primers for CAP-18 mRNA were 5′-GGAAGATGGGCTGGTGAAGC-3′ and 5′-GCGCAGCCCAGTAGGTTCTG-3′, respectively (Primer Express; Applied Biosystems). The results were analyzed with the use of the comparative threshold cycle (Ct) method. The results are expressed as fold-induction relative to the healthy control samples (expression levels = 1) (27).
Histology.
After fixation in buffered formalin, tissue pieces were embedded in paraffin. Paraffin sections were deparaffinized and stained with hematoxylin and eosin, and coded sections were read by using criteria described in ref. 20.
Immunohistochemistry.
Immunohistochemical staining for CAP-18 was performed by using the chicken polyclonal antibody specific to CAP-18 (Innovagen) as described in ref.16. As a control, specific antibodies were replaced by irrelevant isotype-matched antibodies. To control for specific staining, synthetic CAP-18 was incubated at 10-fold-higher concentration with the CAP-18 antibody overnight at 4°C, and the mixture was used as above for immunostaining.
Computerized Image Analysis for Detection of Immunostaining.
Immunohistochemical staining of CAP-18 in the intestinal tissue specimens was analyzed by using a DMLB microscope (Leica) and the image analysis system Quantimet Q550 (Lieca). For each tissue section at ×40 magnification, at least 80 0.4 × 105-μm2 fields were investigated, and the average was used for quantification of CAP-18 staining in each tissue section. The data acquired were imported to Microsoft excel. The results were expressed as total positively stained area measured times total mean intensity (1–256 levels per pixel) of the positive area divided by total cell area measured.
Statistical Analyses.
Statistical analyses were performed by using Sigma stat for Windows version 3.1, (Jandel) and spss. Data were expressed as mean with standard error. Probabilities were regarded as significant if they reached a 95% level of confidence (P < 0.05). Differences in characteristics between the two treatment groups of rabbits were tested for statistical significance by Student's t test or Fisher's exact test and χ2 test when appropriate. Clinical recovery was compared between the treatment groups by Kaplan–Meier survival curves, which were calculated for each clinical parameter (fever, watery stool, RBC in stool, and weight loss) and were compared by using the log-rank (Mantel–Cox) test.
Supplementary Material
Acknowledgments
We thank Dr. Dinesh Mondal for his help with the Kaplan–Meier graph. This study was supported by Swedish Agency for Research Cooperation with Developing Countries (Sida/SAREC) Grant 2001-3970, the Swedish Research Council (project no. 16x-11217), the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), the Ruth and Richard Julin′s Foundation, the Prof. Nanna Svartz′ Foundation, and the Torsten and Ragnar Söderbergs Foundations.
Abbreviations
- cfu
colony-forming unit
- LP
lamina propria
- MHB
Mueller–Hinton broth
- SCFA
short-chain fatty acid
- SE
surface epithelium.
Conflict of interest statement: No conflicts declared.
See Commentary on page 8913.
References
- 1.Kotloff K. L., Winickoff J. P., Ivanoff B., Clemens J. D., Swerdlow D. L., Sansonetti P. J., Adak G. K., Levine M. M. Bull. W. H. O. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 2.Dutta S., Dutta S., Dutta P., Matsushita S., Bhattacharya S. K., Yoshida S. Emerg. Infect. Dis. 2003;9:1471–1474. doi: 10.3201/eid0911.020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mounier J., Vasselon T., Hellio R., Lesourd M., Sansonetti P. J. Infect. Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perdomo J. J., Gounon P., Sansonetti P. J. J. Clin. Invest. 1994;93:633–643. doi: 10.1172/JCI117015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wassef J. S., Keren D. F., Mailloux J. L. Infect. Immun. 1989;57:858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sansonetti P. J., Arondel J., Cantey J. R., Prevost M. C., Huerre M. Infect. Immun. 1996;64:2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zychlinsky A., Prevost M. C., Sansonetti P. J. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 8.Boman H. G. Annu. Rev. Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 9.Zasloff M. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 10.Nizet V., Ohtake T., Lauth X., Trowbridge J., Rudisill J., Dorschner R. A., Pestonjamasp V., Piraino J., Huttner K., Gallo R. L. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 11.Salzman N. H., Ghosh D., Huttner K. M., Paterson Y., Bevins C. L. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 12.Ganz T. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 13.Zanetti M. J. Leukoc. Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 14.Gudmundsson G. H., Agerberth B., Odeberg J., Bergman T., Olsson B., Salcedo R. Eur. J. Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 15.Gudmundsson G. H., Agerberth B. In: Mammalian Host Defence Peptides. Devine D. A., Hancock R. E. W., editors. Cambridge, U.K.: Cambridge Univ. Press; 2004. pp. 139–160. [Google Scholar]
- 16.Islam D., Bandholtz L., Nilsson J., Wigzell H., Christensson B., Agerberth B., Gudmundsson G. Nat. Med. 2001;7:180–185. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 17.Hase K., Eckmann L., Leopard J. D., Varki N., Kagnoff M. F. Infect. Immun. 2002;70:953–963. doi: 10.1128/iai.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauber J., Svanholm C., Termen S., Iffland K., Menzel T., Scheppach W., Melcher R., Agerberth B., Luhrs H., Gudmundsson G. H. Gut. 2003;52:735–741. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruppin H., Bar-Meir S., Soergel K. H., Wood C. M., Schmitt M. G., Jr. Gastroenterology. 1980;78:1500–1507. [PubMed] [Google Scholar]
- 20.Rabbani G. H., Albert M. J., Hamidur Rahman A. S., Moyenul Isalm M., Nasirul Islam K. M., Alam K. J. Infect. Dis. 1999;179:390–397. doi: 10.1086/314584. [DOI] [PubMed] [Google Scholar]
- 21.Vernia P., Annese V., Bresci G., d'Albasio G., D'Inca R., Giaccari S., Ingrosso M., Mansi C., Riegler G., Valpiani D., Caprilli R. Eur. J. Clin. Invest. 2003;33:244–248. doi: 10.1046/j.1365-2362.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- 22.Etheridge M. E., Hoque A. T., Sack D. A. Lab. Anim. Sci. 1996;46:61–66. [PubMed] [Google Scholar]
- 23.Raqib R., Reinholt F. P., Bardhan P. K., Karnell A., Lindberg A. A. Apmis. 1994;102:371–380. doi: 10.1111/j.1699-0463.1994.tb04886.x. [DOI] [PubMed] [Google Scholar]
- 24.Sansonetti P. J. Folia Microbiol. (Prague) 1998;43:239–246. doi: 10.1007/BF02818608. [DOI] [PubMed] [Google Scholar]
- 25.Scheppach W., Weiler F. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:563–567. doi: 10.1097/00075197-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Murakami M., Lopez-Garcia B., Braff M., Dorschner R. A., Gallo R. L. J. Immunol. 2004;172:3070–3077. doi: 10.4049/jimmunol.172.5.3070. [DOI] [PubMed] [Google Scholar]
- 27.Bergman P., Johansson L., Asp V., Plant L., Gudmundsson G. H., Jonsson A. B., Agerberth B. Cell Microbiol. 2005;7:1009–1017. doi: 10.1111/j.1462-5822.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 28.Berkhoff H. A., Vinal A. C. Avian Dis. 1986;30:117–121. [PubMed] [Google Scholar]
- 29.Agerberth B., Grunewald J., Castanos-Velez E., Olsson B., Jornvall H., Wigzell H., Eklund A., Gudmundsson G. H. Am. J. Respir. Crit. Care Med. 1999;160:283–290. doi: 10.1164/ajrccm.160.1.9807041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.