Abstract
Endogenous angiogenesis inhibitors have shown promise in preclinical trials, but clinical use has been hindered by low half-life in circulation and high production costs. Here, we describe a strategy that targets the angiostatin receptor angiomotin (Amot) by DNA vaccination. The vaccination procedure generated antibodies that detected Amot on the endothelial cell surface. Purified Ig bound to the endothelial cell membrane and inhibited endothelial cell migration. In vivo, DNA vaccination blocked angiogenesis in the matrigel plug assay and prevented growth of transplanted tumors for up to 150 days. We further demonstrate that a combination of DNA vaccines encoding Amot and the extracellular and transmembrane domains of the human EGF receptor 2 (Her-2)/neu oncogene inhibited breast cancer progression and impaired tumor vascularization in Her-2/neu transgenic mice. No toxicity or impairment of normal blood vessels could be detected. This work shows that DNA vaccination targeting Amot may be used to mimic the effect of angiostatin.
Keywords: cancer vaccines, neoplasia, neovascularization, breast cancer, angiostatin
The expansion of the circulatory system by the mechanism of angiogenesis is a driving force behind diseases such as cancer, macular degeneration, and atherosclerosis (1). Inhibition of the signaling pathways underlying pathological angiogenesis therefore offers a possible way of intervening with the progress of the disease. Indeed, recent evidence has shown that treatment with antibodies targeting VEGF in combination with chemotherapy prolongs life in patients with metastatic colon, breast, and lung cancer (2, 3). At least in the case of tumor growth, inhibition of one signaling pathway may not be sufficient, because compensatory pathways may be activated when VEGF signaling is inhibited (4, 5). Tumor angiogenesis may therefore involve a coordinate expression of a variety of angiogenic factors, such as IL-8, basic fibroblast growth factor (bFGF), and others (6). The implication of these findings is that therapies that target more than one pathway or target endothelial functions downstream of these pathways could improve the efficacy of antiangiogenic therapies.
The induction of tumor angiogenesis is likely regulated by a balance between endogenous proangiogenic and antiangiogenic factors (7). At least 16 endogenous angiogenesis inhibitors have been isolated that exhibit antiangiogenic and tumor suppressive activity (8, 9). One of the earliest inhibitors to be reported, angiostatin, was shown to be specific for endothelial cells and could maintain dormancy of established metastases in vivo (10, 11). However, the serum half-life of angiostatin in patients is ≈3 h, making it necessary to administer high amounts of angiostatin at frequent intervals (12). Thus, the pharmacodynamics of angiostatin and other inhibitors such as endostatin constitute a major obstacle for the use of these agents in cancer patients (13, 14). To design alternative molecules more suited for antiangiogenic therapy, it is of importance to identify the receptors that mediate the antiangiogenic effect. We have previously reported the cloning and characterization of the angiostatin receptor angiomotin (Amot) that is expressed in tumor and placental endothelium (15). Other receptors for angiostatin have also been identified: for example, ATP synthase, the integrin αvβ3, and c-Met (16). Amot is a membrane-associated protein that mediates angiostatin inhibition of endothelial migration and tube formation in vitro (17–20). A role of Amot in cell motility is also indicated by the finding that Amot-deficient mouse embryos exhibit a migratory defect in the anterior visceral endoderm at embryonic day 7.5 (21). High Amot mRNA levels also have been correlated to poor survival in breast cancer patients (22). Therefore, the functional role of Amot as an angiostatin receptor and its expression in angiogenic vessels makes it a possible target for antiangiogenic therapy.
The development of active immunotherapy of cancer has been hampered by limited success in the clinic, related to difficulties of breaking tolerance against weak self-antigens on tumor cells and the genetic variability of tumor cells resulting in immunologic escape variants. This limited clinical efficacy has spurred the development of combination approaches in which tumor vaccines are combined with cancer therapies that target the tumor stroma, which is more genetically stable. Recent evidence has shown the feasibility of targeting molecules that are expressed by angiogenic endothelium [for example, VEGF-R2 (23, 24)] and that synergy between tumor immunotherapy and antiangiogenic therapy can be achieved (25). Here, we have used DNA vaccination to break tolerance and invoke an immune response against Amot. Our approach generates Amot-specific Ig, resulting in inhibition of angiogenesis and tumor growth without detectable toxicity and thus circumventing the problems experienced with endogenous angiogenesis inhibitors.
Results
Amot Is Expressed in Endothelial Cells During Angiogenesis.
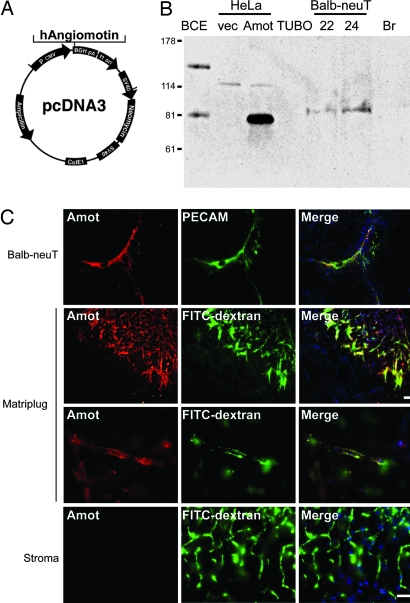
For DNA vaccination, we used cDNA encoding the human p80 isoform of Amot inserted into the pcDNA3 vector (Fig. 1A). Transient transfection of this vector into HeLa cells yielded an expected 80-kDa band similar to that of endogenous p80 Amot in bovine capillary endothelial cells (Fig. 1B). To verify that the target protein was present in endothelial cells during angiogenesis, we analyzed Amot levels in lobular mammary carcinomas that spontaneously arise in BALB/c mice that are transgenic for the human EGF receptor 2 (Her-2)/neu oncogene (BALB-neuT mice) (26). p80 Amot was clearly detectable by Western blot analysis of two individual carcinomas from 22- and 24-week-old BALB-neuT mice. However, Amot was not detectable in normal breast tissue from a virgin female of the same age (Fig. 1B). Immunofluorescence analysis of the cellular localization of Amot expression in these tumors showed that positive staining overlapped with that of the endothelial marker PECAM (platelet endothelial cell adhesion molecule) (Fig. 1C). Furthermore, bFGF-induced vessels in the in vivo angiogenesis matrigel plug assay were positive for Amot, whereas surrounding stromal tissue was negative (Fig. 1C). This finding is in accordance with our previously published data that showed that Amot is primarily expressed in cytotrophoblasts of the placenta as well as in endothelial cells of angiogenic tissues (15). The spatiotemporal restricted expression of Amot by angiogenic vessels is therefore consistent with a candidate target for antiangiogenic vaccination.
Fig. 1.
Expression of Amot in angiogenic vessels. (A) The human p80 isoform of Amot was cloned into the pCDNA3 vector, which was then used for DNA vaccination in mice. (B) Western blot analysis of Amot protein levels. Bovine capillary endothelial cells (BCE) expressed both p80 and p130 isoforms of Amot. Transient transfection with the pcDNA3-Amot construct into HeLa cells yielded a band corresponding to 80 kDa (Amot), whereas vector control was negative. The TUBO mouse breast cancer cell line is derived from BALB-neuT transgenic mice and did not express detectable levels of Amot. In contrast, total lysates from tumors harvested at 22 and 24 weeks after birth from BALB-neuT transgenic mice showed detectable expression of p80 Amot (BALB-neuT). Breast tissue harvested from a normal virgin female mouse was below detection (Br). (C) The top row shows a vessel positive for Amot and for the endothelial marker PECAM in a tumor derived from a BALB-neuT mouse. The two middle rows show angiogenic vessels activated by bFGF in the in vivo matrigel plug assay that were strongly positive for Amot as shown by immunofluorescence analysis using polyclonal anti-Amot antibodies. Functional vessels were visualized by i.v. FITC-dextran injection before euthanization of the animal. The bottom row shows vessels of the normal stroma that did not stain positively for Amot. (Scale bars, 100 μm.)
Amot Vaccination Inhibits Tumor Growth.
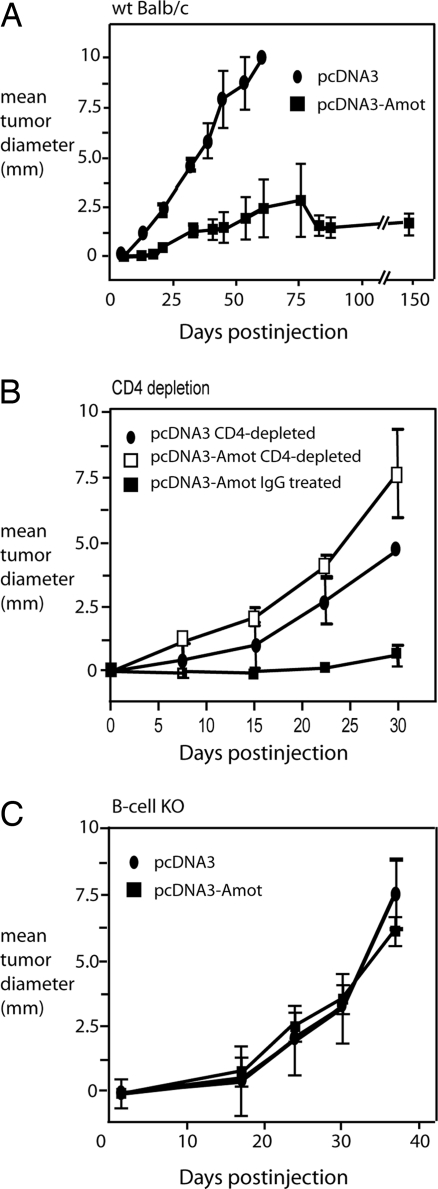
To break tolerance against Amot and consequently activate an Amot-specific immune response, we vaccinated BALB/c mice by intramuscular injection followed by electroporation with pcDNA3-Amot or empty vector as a negative control as described in ref. 27. The use of a human Amot DNA construct was motivated by the ability of xenogeneic proteins to break immunological tolerance against self-antigens (28). The mice were vaccinated twice, with a 2-week interval. To assess the protective response elicited by vaccination, mice were challenged with a lethal dose of TUBO carcinoma cells. TUBO is a cloned cell line established from a mammary carcinoma of BALB-neuT mice (29). TUBO cells display a high cytoplasmic and membrane expression of Her-2/neu but no detectable levels of Amot (Fig. 1B). Injection of TUBO cells in control-vaccinated animals resulted in rapidly growing s.c. tumors in all (n = 13) mice, which were euthanized 40–60 days after injection. Suppression of tumor growth was detected in 12 of 18 mice electroporated with the pcDNA3-Amot construct in three independent experiments. In Amot-vaccinated animals, only a minority (6 of 18) of the mice developed tumors that grew progressively, albeit at a slower rate than in the control-vaccinated mice. Moreover, in one of three performed experiments, mice were monitored for tumor growth for 200 days after TUBO challenge (Fig. 2A). In this experiment, Amot vaccination induced a state of no growth for >150 days, after which tumors recurred in two of five animals. To determine whether CD4 T cells were responsible for the antitumor activities, we conducted an in vivo T cell depletion assay in DNA-vaccinated mice. Mice were treated with anti-CD4 or control Ab before and during vaccinations with Amot or empty vector. The antitumor effect of vaccination against Amot was abrogated in CD4-depleted mice (Fig. 2B), demonstrating that CD4 T cell help was necessary for the induction of antitumor activity. To examine the role of antibodies in the protection elicited by Amot plasmid vaccination, μ-chain-deficient BALB/c mice lacking B cells [B cell knockout (KO) mice] were vaccinated with pCDNA3-Amot or empty vector. In these mice, Amot vaccination did not inhibit tumor growth (Fig. 2C). Therefore, we conclude that B cells, and probably antibodies, are required for the observed antitumor effect.
Fig. 2.
Amot DNA vaccination inhibits tumor growth. (A) WT BALB/c mice were vaccinated twice with either Amot or control vector pcDNA3 21 and 7 days before s.c. challenge with the TUBO mouse breast cancer cell line. In three independent experiments, Amot vaccination suppressed tumor growth for >150 days in 12 of 18 treated mice; in two mice, tumors recurred 150 days after challenge. (B) To determine whether the induction of the antitumor activities depended on CD4+ T cells, we conducted an in vivo T cell depletion of this subset during DNA vaccination. Mice were treated with either anti-CD4 or control IgG before and during vaccinations with Amot or empty vector. The antitumor effect of vaccination against Amot was abrogated in the CD4-depleted mice. The size of tumors in Amot-vaccinated mice receiving control IgG or anti-CD4 Ig was significantly different between 7 and 35 days after challenge (n = 5; P < 0.0001, unpaired Student t test). (C) Amot plasmid vaccination was also performed in μ-chain-deficient BALB/c mice lacking B cells (B cell KO mice). No significant difference in the growth of TUBO tumor cells could be detected between Amot- and control pcDNA3 plasmid-vaccinated mice.
Transgenic tumor models differ from tumor transplantation models in that the tumor cells are derived from normal cells that progress through multiple stages in the progression to neoplasia. To study the effect of Amot, we used the BALB-neuT transgenic breast cancer model system. These mice are transgenic for the rat transforming Her-2/neu oncogene driven by the mouse mammary tumor virus promoter (which confers expression to the mouse mammary epithelium). Tumors progress through the following stages: at 6–7 weeks, female BALB-neuT mice develop atypical ductal hyperplasia; the angiogenic switch occurs at weeks 8–10, and mammary lesions progress to invasive cancers at weeks 23–30. The mice have to be killed between weeks 27 and 33, when the tumors exceed the size of 1.5 cm in mean diameter (30, 31).
To assess the effect of pcDNA3-Amot vaccination on the angiogenic switch and tumor progression, BALB-neuT mice were vaccinated either before (6–8 weeks) or after (10–12 weeks) the onset of tumor angiogenesis. Although Amot plasmid vaccination had no significant effect on tumor progression (Fig. 3), significant alterations were evident in the vasculature of vaccinated lesions. The percentage of the area occupied by blood vessels was lower in Amot-vaccinated tumors (4.38 ± 1.31%) as compared with control tumors (7.12 ± 0.95%; P < 0.0005).
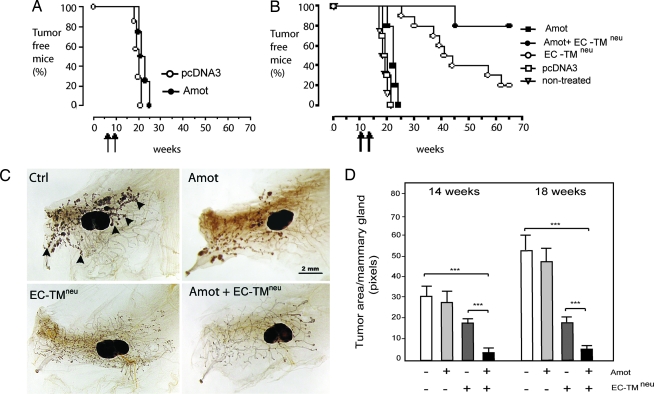
Fig. 3.
Combined vaccination with Amot and EC-TMneu plasmids prevents tumor onset in BALB-neuT transgenic mice. (A) BALB-neuT transgenic mice were vaccinated twice at 6 and 8 weeks (before the angiogenic switch). Anti-Amot vaccination as a single therapy did not significantly delay the onset of tumor formation. (B) A similar result was observed when mice were vaccinated at 10 and 12 weeks (after the angiogenic switch). The EC-TMneu DNA vaccine targeting the Her-2/neu oncogene in the tumor cells delayed tumor progression. However, the combination of the Amot EC-TMneu vaccinations prevented tumor formation in 80% of transgenic mice for >70 weeks. (pcDNA3 vs. pcDNA3-Amot, P < 0.01; EC-TMneu vs. Amot, P <0.0001; EC-TMneu vs. Amot plus EC-TMneu, P < 0.0001; n = 5 animals). (C) Whole-mount analysis of mammary glands from vaccinated mice at 14 and 18 weeks of age. The mice were vaccinated at the time points indicated in A. Mammary glands were stained with ferric hematoxylin to visualize normal mammary structures and neoplastic formation in the whole gland (arrowheads indicate neoplastic foci). No significant reduction in mammary neoplastic lesions, detected as dark masses (the large, oval-shaped dark area is the lymph node, L), was observed in pcDNA3-vaccinated mice. In EC-TMneu-vaccinated mice, neoplastic lesions are reduced, and they are absent in mice vaccinated with both Amot and EC-TMneu plasmids. (Scale bar, 2 mm.) (D) Image analyses of the tumor burden of mammary glands from animals vaccinated with vector control, Amot, EC-TMneu, or a combination as indicated in the graph. Sixteen tumors from four animals in each group were analyzed at 14 weeks, and 20 tumors from five animals were analyzed at 18 weeks (∗∗∗, P < 0.0001 compared with control).
To test the potential synergistic effect of targeting both the endothelial and tumor compartments, we combined vaccination against Amot with a plasmid encoding the extracellular and transmembrane domains of Her-2 (EC-TMneu). Single vaccinations with the EC-TMneu plasmid significantly delayed tumor growth, but, eventually, tumors progressed in 80% of the mice, which is in accordance with our previously published results (Fig. 3B) (27). However, when EC-TMneu was combined with Amot vaccination, 80% of the treated mice were tumor-free for >70 weeks (Fig. 3B). DNA-vaccinated BALB-neuT mice were killed at the age of 14 and 18 weeks. Whole-mount image analysis of mammary glands from pcDNA3-Amot-vaccinated mice revealed no significant inhibition of tumor growth as compared with control animals (Fig. 3 C and D). In the EC-TMneu-vaccinated mice, the number of neoplastic lesions was similar to that in the control mice, but the lesions were markedly reduced in size. Interestingly, the combination of the Amot and EC-TMneu plasmids resulted in mammary glands with an apparent normal phenotype with few neoplastic lesions (Fig. 3 C and D).
Amot Vaccination Inhibits Angiogenesis in Vivo.
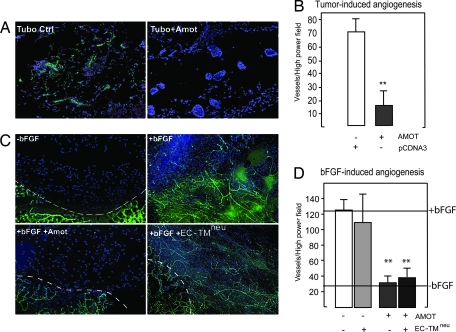
To investigate the antiangiogenic effect of Amot DNA vaccination, we injected Amot- or vector-vaccinated animals with 500 μl of matrigel containing either 75,000 TUBO breast cancer cells or 200 ng/ml bFGF s.c. into the lower abdomen as described in ref. 32. The mice were euthanized 7 days later, and the plugs were harvested and analyzed for neovessel formation. Vessel in-growth was analyzed by isolectin B4 and PECAM staining. Tumor-induced angiogenesis was significantly inhibited in Amot-vaccinated mice (Fig. 4A). Furthermore, the matrigel plugs containing bFGF harvested from Amot-vaccinated animals did not differ significantly from the controls lacking bFGF (Fig. 4 C and D). Animals were also vaccinated with the EC-TMneu construct, which did not affect the bFGF-induced in-growth of vessels into the matrigel (Fig. 4C). The microvascular density of all plugs was also assessed by scoring PECAM-positive cells under high-power magnification with similar results (Fig. 4 B and D). No negative effect on the vasculature of the stromal vessels adjacent to the matrigel plug or on the vessels of retinas isolated from vaccinated mice could be detected (data not shown; see Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 4.
Amot vaccination inhibits angiogenesis in vivo. Tumor-induced angiogenesis was analyzed by the matrigel plug assay. Matrigel is a solubilized basement membrane preparation from Engelbreth–Holm–Swarm mouse sarcoma that polymerizes at 37°C. (A) Angiogenesis was induced by suspending 75,000 TUBO cells into 500 μl of matrigel and injecting s.c. in vaccinated mice as indicated. Seven days later, matrigel plugs were extracted, sectioned, and stained with PECAM antibodies to visualize vessels, and cell nuclei were visualized by DAPI staining. (B) Bar diagram shows vascular density of matrigel plugs stained for the endothelial marker PECAM (∗∗, P < 0.001 compared with control). (C) bFGF (200 ng/ml) was added to the matrigel before injection into nonvaccinated or mice vaccinated with Amot or EC-TMneu plasmids as indicated. (D) Bar diagram shows vascular density of matrigel plugs stained for the endothelial marker PECAM (∗∗, P < 0.001 compared with control). (Scale bars, 100 μm.)
Vaccination Generates Amot-Reactive Antibodies That Inhibit Endothelial Cell Migration.
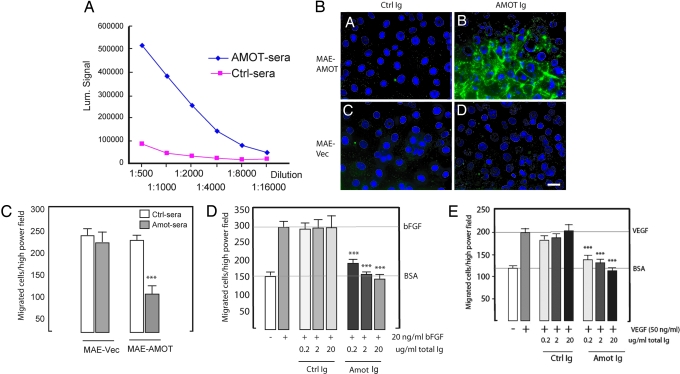
Because the antitumor effect was lost in B cell KO animals, we investigated whether our DNA vaccination strategy generated antibodies that reacted with Amot. Sera were harvested from BALB/c mice vaccinated two times (with a 2-week interval) with the Amot construct. Binding to recombinant mouse Amot was analyzed by ELISA (Fig. 5A). The results showed that vaccination with human Amot cDNA resulted in the generation of Ig that cross-reacted with the mouse orthologue. Sera from all control mice were negative. Cell surface binding of purified Ig fractions was analyzed by incubation with live Amot-transfected mouse aortic endothelial (MAE) cells. A distinct binding could be detected at intercellular junctions with Ig from Amot vaccinated animals, whereas no reactivity was detected with control Ig (Fig. 5B). This reactivity was specific for Amot; MAE vector-transfected cells lacking Amot expression did not exhibit any positive staining with Amot or control Ig.
Fig. 5.
DNA vaccination generates Amot-specific antibodies that inhibit endothelial migration. (A) Sera from vaccinated mice were analyzed for the presence of antibodies that are reactive to mouse Amot as analyzed by ELISA. (B) Purified Ig from sera of Amot-vaccinated animals bound to the surface of live MAE cells transfected with p80 Amot. (Scale bar, 10 μm.) (C) Sera from Amot-vaccinated animals inhibited specifically the migration of MAE cells transfected with Amot. (D and E) Purified Ig abolishes migration stimulated by bFGF (D) or VEGF (E) in the Boyden chamber assay (∗∗∗, P < 0.001 compared with control).
Angiostatin specifically inhibits migration of Amot-transfected cells in the modified Boyden chamber assay (15, 20). We therefore tested whether the Amot-reactive Ig generated by DNA vaccination could mimic the effect of angiostatin. We first tested whole sera and could show that dilution up to 1,000 times could completely block basal migration of Amot-expressing MAE cells (Fig. 5C). We then used purified Ig to test the inhibition of bFGF- and VEGF-stimulated endothelial migration (Fig. 5 D and E). Significant inhibition of migration could be achieved with as little as 0.2 μg/ml total Ig. These results thus show that DNA vaccination generates antibodies that mimic the effect of angiostatin in vitro.
Discussion
In this report, we describe a strategy that circumvents the problems of low half-life in circulation and expensive production of the endogenous angiogenesis inhibitor angiostatin. We report that DNA vaccination targeting the angiostatin receptor Amot mimics the effect of angiostatin and inhibits angiogenesis and tumor growth in mice.
Recent evidence has shown that breaking immune tolerance against angiogenic-associated molecules through active immunotherapy that targets endothelial cell-specific proteins provides an innovative strategy to block tumor angiogenesis (33). One major advantage of this strategy, compared with targeting tumor antigens directly, is that the target cells are readily accessible to the bloodstream. Therefore, vaccination approaches that break immune tolerance may deliver cytotoxic T lymphocytes and antibodies directly to the activated endothelial cells in the tumor. We hypothesized that a DNA vaccination approach targeting Amot could mimic the effect of angiostatin. First, we show that Amot expression is up-regulated in angiogenic vessels in the tumors of BALB-neuT mice. Second, Amot is highly expressed during bFGF-driven angiogenesis in the matrigel assay. Importantly, the surrounding nonangiogenic stroma did not express detectable levels of Amot. These data argue that Amot is up-regulated during angiogenesis and therefore is a target for antiangiogenic vaccination.
Several approaches have been used to vaccinate against targets on tumor endothelial cells. One study has used cross-immunization with xenogeneic endothelial cells as a vaccine to induce endothelial-specific immune responses against tumor vasculature (34). Others have immunized against VEGF-R2 with in vitro-generated dendritic cells pulsed with soluble VEGF-R2 or with a vaccine consisting of attenuated Salmonella typhimurium expressing VEGFR-2 cDNA (23, 24). These immunization strategies generated VEGF-R2-specific neutralizing antibody and/or CD8+ cytotoxic T cell responses, thereby demonstrating that tolerance to self-VEGF-R2 antigen was broken.
We used the method of DNA vaccination where intramuscular injections of pDNA were followed by electroporation. This approach protected a majority of the vaccinated mice from challenge with the TUBO cell line and showed a dramatic synergistic effect when used together with a vaccine targeting the Her-2 oncogene in BALB-neuT transgenic mice. This two-compartment therapy could prevent tumor formation for >70 weeks in 80% of the mice, which is in line with the findings of Nair et al. (25), who reported an improved antitumor effect by combining endothelial and tumor antigens. Furthermore, an early phase clinical study recently showed the added effect of combining herceptin (which targets Her-2) with antibodies targeting VEGF in Her-2/neu-positive breast cancer.‖ Amot vaccination used as a single therapy affected the vascular density of the mammary lesions but did not show any inhibitory effect on tumor growth in the BALB-neuT mice. The explanation for this finding is not yet clear. It is possible that these tumors also depend on other ways to expand their microcirculatory network (e.g., intussusception, vessel cooption, or vascular mimicry). Another explanation is that we could not generate high enough antibody titers in these mice to efficiently suppress tumor growth.
We argue that the observed antiangiogenic and antitumor effect is antibody-dependent because (i) B cell KO mice were not protected against tumor challenge and (ii) antibodies generated by DNA vaccination bound Amot and could inhibit endothelial cell migration in vitro. In addition, the importance of CD4 T cell help for the induction of tumor protection is compatible with the antibody dependency. These findings provide the rationale for generating antibodies that bind to Amot and thereby could inhibit angiogenesis. The use of therapeutic antibodies would potentially be a marked advantage, because generation of an efficient antibody titer would not depend on breaking tolerance against Amot, and the dosage of antibody could therefore be more easily controlled. It should be noted, however, that although antibodies appear as the major component of the observed antiangiogenic effect, it does not exclude an involvement of CD4 or CD8 T cells.
A major concern when activating the immune system to recognize self-antigens is possible toxicity. We have analyzed the normal vasculature of the retinas of animals vaccinated against Amot, and we could find no significant changes in animals at 16 weeks or 70 weeks after vaccination. In addition, adjacent normal stroma in the matrigel plug assay did not express Amot and remained functional as analyzed by FITC-dextran injection. Others have also reported that antiangiogenic vaccination targeting VEGF-R2 inhibits tumor angiogenesis without affecting the wound-healing process (23).
Angiostatin was originally identified by its ability to maintain dormancy of lung metastasis in the Lewis lung carcinoma mouse model system (10, 11). Our observations that some tumors recur after 150 days of latency show that, similar to angiostatin, Amot DNA vaccination can maintain dormancy of established tumors. The escape from dormancy may be explained by the fact that either the tumor has generated resistance to Amot therapy or that a continuous vaccination regimen is required for maintaining inhibition of tumor angiogenesis. In conclusion, our report provides the rationale for targeting Amot to inhibit tumor angiogenesis, which may be achieved through either DNA vaccination or the use of therapeutic mAbs (35).
Materials and Methods
Cell Lines.
TUBO is a cloned cell line established in vitro from lobular carcinomas that arose in a BALB-neuT mouse. TUBO and HeLa cells were cultured in DMEM (BioWhittaker) with 20% FBS (Life Technologies, Paisley, Scotland). MAE cells stably expressing mouse p80 Amot (15) were cultured in DMEM (Sigma) and 10% FCS (GIBCO).
Tumor Experiments in Mice.
WT BALB/cnAnCr (BALB/c) (Charles River Breeding Laboratories) and BALB/c mice KO for the Ig μ-chain (B cell KO mice) were kindly provided by T. Blankenstein (Institute of Immunology, Charite Campus Benjamin Franklin, Berlin). BALB-neuT mice transgenic for transforming rat Her-2/neu (Charles River Breeding Laboratories) were generated as described in ref. 30. Mice were treated according to European Community guidelines. Additional information can be found in Supporting Text, which is published as supporting information on the PNAS web site.
Plasmids and in Vivo Electroporation.
pcDNA3 and Amot plasmid and EC-TMneu plasmids were produced as previously described. Twenty-five micrograms of plasmid in 20 μl of 0.9% NaCl with 6 mg/ml polyglutamate was injected bilaterally into the tibial muscle of the hind legs of anesthetized mice. Two 25-ms transcutaneous electric pulses were generated by a T820 electroporator (BTX, San Diego) with a field strength of 375 V/cm, as described in detail in refs. 27 and 36. In BALB/c and B cell KO BALB/c mice, plasmids were electroporated 21 and 7 days before TUBO cell challenge, whereas transgenic BALB-neuT mice were electroporated with plasmids at 10 and 12 weeks of age.
Whole-Mount Image Analyses.
A detailed description of the staining procedure of tumor imaging and vessel quantification can be found in Supporting Text.
In Vivo Matrigel Plug Angiogenesis Assay.
Five hundred microliters of matrigel (BioSite, Täby, Sweden) mixed with either 75,000 TUBO cells or 200 ng/ml human bFGF (PeproTech, Rocky Hill, NJ) were injected s.c. in the midventral abdominal region of BALB/c mice as described by Passaniti et al. (32). Migration assays were performed in a modified Boyden chamber as described in ref. 15. A more detailed description of this assay can be found in Supporting Text.
Supplementary Material
Acknowledgments
The authors dedicate this paper to the memory of Carl Tullus (1979–2006). We thank Kristina Berggren (BioInvent International, Lund, Sweden) for expert help with the Amot ELISA. This work was supported by grants from the Italian Association for Cancer Research, the Italian Ministries for the Universities and Health, and the Center of Excellence on Aging (University of Chieti, Chieti, Italy); grants from the Swedish Cancer Society, the Cancer Society of Stockholm, the Swedish Research Council, BioInvent International, and the Karolinska Institutet (to L.H.); a postdoctoral stipend from the Wenner–Gren Foundation (to O.B.); and grants from the Swedish Cancer Society, the Cancer Society of Stockholm/King Gustaf V Jubilee Fund, the European Community, the Swedish Medical Research Council, BioInvent International, and the National Institutes of Health (CA102280) (to R.K.).
Abbreviations
- Amot
angiomotin
- bFGF
basic fibroblast growth factor
- Her-2
human EGF receptor 2
- KO
knockout
- MAE
mouse aortic endothelial cell
- PECAM
platelet endothelial cell adhesion molecule.
Footnotes
Conflict of interest statement: L.H. and R.K. are supported by grants from BioInvent International (Lund, Sweden).
Pegram, M. D., Yeon, C., Ku, N. C. Gaudreault, J., Durna, L. & D. J., Slamon (2004) Breast Cancer Res. Treat. 88, Suppl. 1, S124 (abstr.).
References
- 1.Folkman J. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E., et al. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 3.Marx J. Science. 2005;308:1248–1249. doi: 10.1126/science.308.5726.1248. [DOI] [PubMed] [Google Scholar]
- 4.Mizukami Y., Jo W. S., Duerr E. M., Gala M., Li J., Zhang X., Zimmer M. A., Iliopoulos O., Zukerberg L. R., Kohgo Y., et al. Nat. Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 5.Casanovas O., Hicklin D. J., Bergers G., Hanahan D. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Relf M., LeJeune S., Scott P. A., Fox S., Smith K., Leek R., Moghaddam A., Whitehouse R., Bicknell R., Harris A. L. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 7.Hanahan D., Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. Cancer Biol. Ther. 2003;2:S127–S133. [PubMed] [Google Scholar]
- 9.Nyberg P., Xie L., Kalluri R. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 10.O’Reilly M. S., Holmgren L., Shing Y., Chen C., Rosenthal R. A., Moses M., Lane W. S., Cao Y., Sage E. H., Folkman J. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 11.Holmgren L., O’Reilly M. S., Folkman J. Nat. Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 12.Beerepoot L. V., Witteveen E. O., Groenewegen G., Fogler W. E., Sim B. K., Sidor C., Zonnenberg B. A., Schramel F., Gebbink M. F., Voest E. E. Clin. Cancer Res. 2003;9:4025–4033. [PubMed] [Google Scholar]
- 13.Herbst R. S., Hess K. R., Tran H. T., Tseng J. E., Mullani N. A., Charnsangavej C., Madden T., Davis D. W., McConkey D. J., O’Reilly M. S., et al. J. Clin. Oncol. 2002;20:3792–3803. doi: 10.1200/JCO.2002.11.061. [DOI] [PubMed] [Google Scholar]
- 14.Haviv F., Bradley M. F., Kalvin D. M., Schneider A. J., Davidson D. J., Majest S. M., McKay L. M., Haskell C. J., Bell R. L., Nguyen B., et al. J. Med. Chem. 2005;48:2838–2846. doi: 10.1021/jm0401560. [DOI] [PubMed] [Google Scholar]
- 15.Troyanovsky B., Levchenko T., Mansson G., Matvijenko O., Holmgren L. J. Cell Biol. 2001;152:1247–1254. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahl M. L., Kenan D. J., Gonzalez-Gronow M., Pizzo S. V. J. Cell. Biochem. 2005;96:242–261. doi: 10.1002/jcb.20480. [DOI] [PubMed] [Google Scholar]
- 17.Bratt A., Wilson W. J., Troyanovsky B., Aase K., Kessler R., Van Meir E. G., Holmgren L. Gene. 2002;298:69–77. doi: 10.1016/s0378-1119(02)00928-9. [DOI] [PubMed] [Google Scholar]
- 18.Levchenko T., Aase K., Troyanovsky B., Bratt A., Holmgren L. J. Cell Sci. 2003;116:3803–3810. doi: 10.1242/jcs.00694. [DOI] [PubMed] [Google Scholar]
- 19.Levchenko T., Bratt A., Arbiser J. L., Holmgren L. Oncogene. 2004;23:1469–1473. doi: 10.1038/sj.onc.1207264. [DOI] [PubMed] [Google Scholar]
- 20.Bratt A., Birot O., Sinha I., Veitonmaki N., Aase K., Ernkvist M., Holmgren L. J. Biol. Chem. 2005;280:34859–34869. doi: 10.1074/jbc.M503915200. [DOI] [PubMed] [Google Scholar]
- 21.Shimono A., Behringer R. R. Curr. Biol. 2003;13:613–617. doi: 10.1016/s0960-9822(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W. G., Watkins G., Douglas-Jones A., Holmgren L., Mansel R. E. BMC Cancer. 2006;6:16. doi: 10.1186/1471-2407-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Wang M. N., Li H., King K. D., Bassi R., Sun H., Santiago A., Hooper A. T., Bohlen P., Hicklin D. J. J. Exp. Med. 2002;195:1575–1584. doi: 10.1084/jem.20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niethammer A. G., Xiang R., Becker J. C., Wodrich H., Pertl U., Karsten G., Eliceiri B. P., Reisfeld R. A. Nat. Med. 2002;8:1369–1375. doi: 10.1038/nm1202-794. [DOI] [PubMed] [Google Scholar]
- 25.Nair S., Boczkowski D., Moeller B., Dewhirst M., Vieweg J., Gilboa E. Blood. 2003;102:964–971. doi: 10.1182/blood-2002-12-3738. [DOI] [PubMed] [Google Scholar]
- 26.Di Carlo E., Diodoro M. G., Boggio K., Modesti A., Modesti M., Nanni P., Forni G., Musiani P. Lab. Invest. 1999;79:1261–1269. [PubMed] [Google Scholar]
- 27.Quaglino E., Iezzi M., Mastini C., Amici A., Pericle F., Di Carlo E., Pupa S. M., De Giovanni C., Spadaro M., Curcio C., et al. Cancer Res. 2004;64:2858–2864. doi: 10.1158/0008-5472.can-03-2962. [DOI] [PubMed] [Google Scholar]
- 28.Liu J. Y., Wei Y. Q., Yang L., Zhao X., Tian L., Hou J. M., Niu T., Liu F., Jiang Y., Hu B., et al. Blood. 2003;102:1815–1823. doi: 10.1182/blood-2002-12-3772. [DOI] [PubMed] [Google Scholar]
- 29.Rovero S., Amici A., Carlo E. D., Bei R., Nanni P., Quaglino E., Porcedda P., Boggio K., Smorlesi A., Lollini P. L., et al. J. Immunol. 2000;165:5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 30.Boggio K., Nicoletti G., Di Carlo E., Cavallo F., Landuzzi L., Melani C., Giovarelli M., Rossi I., Nanni P., De Giovanni C., et al. J. Exp. Med. 1998;188:589–596. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boggio K., Di Carlo E., Rovero S., Cavallo F., Quaglino E., Lollini P. L., Nanni P., Nicoletti G., Wolf S., Musiani P., Forni G. Cancer Res. 2000;60:359–364. [PubMed] [Google Scholar]
- 32.Passaniti A., Taylor R. M., Pili R., Guo Y., Long P. V., Haney J. A., Pauly R. R., Grant D. S., Martin G. R. Lab. Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- 33.Rafii S. Cancer Cell. 2002;2:429–431. doi: 10.1016/s1535-6108(02)00208-8. [DOI] [PubMed] [Google Scholar]
- 34.Wei Y. Q., Wang Q. R., Zhao X., Yang L., Tian L., Lu Y., Kang B., Lu C. J., Huang M. J., Lou Y. Y., et al. Nat. Med. 2000;6:1160–1166. doi: 10.1038/80506. [DOI] [PubMed] [Google Scholar]
- 35.Andreasson P., Carlsson R. IDrugs. 2005;8:730–733. [PubMed] [Google Scholar]
- 36.Spadaro M., Ambrosino E., Iezzi M., Di Carlo E., Sacchetti P., Curcio C., Amici A., Wei W. Z., Musiani P., Lollini P. L., et al. Clin. Cancer Res. 2005;11:1941–1952. doi: 10.1158/1078-0432.CCR-04-1873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.