Abstract
In diabetes, the death of insulin-producing β-cells by apoptosis leads to insulin deficiency. The lower prevalence of diabetes in females suggests that female sex steroids protect from β-cell injury. Consistent with this hypothesis, 17β-estradiol (estradiol) manifests antidiabetic actions in humans and rodents. In addition, estradiol has antiapoptotic actions in cells that are mediated by the estrogen receptor-a (ERα), raising the prospect that estradiol antidiabetic function may be due, in part, to a protection of β-cell apoptosis via ERα. To address this question, we have used mice that were rendered estradiol-deficient or estradiol-resistant by targeted disruption of aromatase (ArKO) or ERα (αERKO) respectively. We show here that in both genders, ArKO−/− mice are vulnerable to β-cell apoptosis and prone to insulin-deficient diabetes after exposure to acute oxidative stress with streptozotocin. In these mice, estradiol treatment rescues streptozotocin-induced β-cell apoptosis, helps sustain insulin production, and prevents diabetes. In vitro, in mouse pancreatic islets and β-cells exposed to oxidative stress, estradiol prevents apoptosis and protects insulin secretion. Estradiol protection is partially lost in β-cells and islets treated with an ERα antagonist and in αERKO islets. Accordingly, αERKO mice are no longer protected by estradiol and display a gender nonspecific susceptibility to oxidative injury, precipitating β-cell apoptosis and insulin-deficient diabetes. Finally, the predisposition to insulin deficiency can be mimicked in WT mice by pharmacological inhibition of ERα by using the antagonist tamoxifen. This study demonstrates that estradiol, acting, at least in part, through ERα, protects β-cells from oxidative injury and prevents diabetes in mice of both genders.
Keywords: estradiol, oxidative stress
In diabetes, hyperglycemia arises from the loss of insulin production from pancreatic β-cells. Disregarding the causal events, in type 1 and the late stages of type 2 diabetes, proinflammatory cytokines and/or chronic elevation of blood glucose generate oxidative stress in pancreatic islets, which ultimately provokes β-cell death by apoptosis (1, 2). To date, little progress has been made in understanding how to halt the loss of insulin-producing β-cells.
Recent data from human and animal studies suggest that the ovarian estrogen, 17β-estradiol (estradiol), protects insulin production in diabetic states. First, the prevalence of diabetes is lower in females (3). More specifically, this observed “female protection” is more pronounced in diabetic syndromes with severe insulin deficiency (4, 5). Second, in most rodent models of diabetes, females are protected from β-cell death and hyperglycemia, whereas, conversely, males develop overt insulin-deficient diabetes (reviewed in ref. 6). Furthermore, estradiol, used in pharmacological concentrations, protects human pancreatic islets from apoptosis induced by proinflammatory cytokines in vitro (7). In both sexes, estradiol is biosynthesized by the cytochrome P450 enzyme complex called aromatase (8) and acts predominantly via two distinct nuclear estrogen receptors (ERs), ERα and ERβ (9). ERα is predominant in many biological functions of estradiol (9). In neurons, notably, estradiol suppresses apoptosis in vivo. It is considered that this estrogenic effect is primarily mediated through ERα (10). Together, this information raises the prospect that estradiol antidiabetic function may be due, in part, to a protection of β-cell apoptosis through activation of ERα.
Results
Estradiol Protects Pancreatic Islet Survival and Function in Vivo.
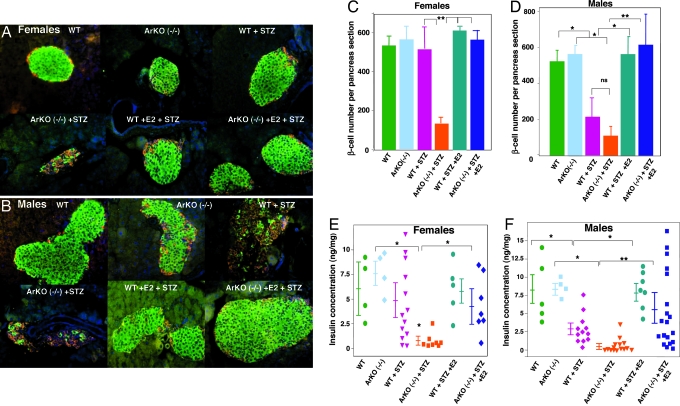
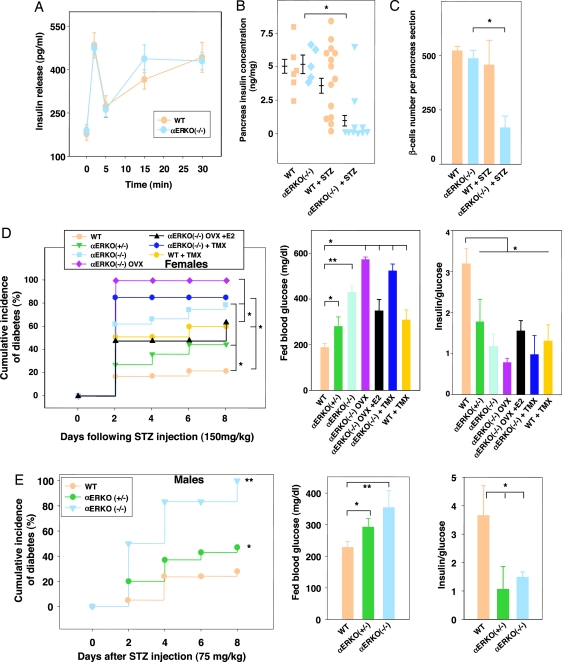
To study the protection of estradiol in vivo, we used estradiol-deficient (αERKO) aromatase-deficient (ArKO−/−) mice. We provoked oxidative stress in β-cells, using a single high-dose injection of streptozotocin (STZ) (150 mg/kg of body weight), which augments the generation of reactive oxygen species in pancreatic islets (11). We determined the STZ dose used from a dose–response study that defined the minimal amount causing diabetes with a gender dimorphism (see Table 1, which is published as supporting information on the PNAS web site). In basal conditions, WT and ArKO−/− mice of both genders displayed a normal islet architecture, with insulin-producing β-cells in a central location, glucagon-producing α-cells at the periphery (Fig. 1 A and B), similar β-cell numbers (Fig. 1 C and D), and pancreatic insulin concentration (Fig. 1 E and F). After exposure to STZ in WT mice, females were protected and retained a normal islet architecture, β-cell numbers, and pancreas insulin concentration (Fig. 1 A, C, and E); conversely, males were vulnerable to STZ and exhibited a loss of β-cells and pancreas insulin concentration (Fig. 1 B, D, and F). We found that exposure to STZ in ArKO−/− mice of both genders provoked severe β-cell destruction and a dramatic decrease in pancreatic insulin concentration (Fig. 1 A–F). Furthermore, in males, the fall in pancreas insulin concentration was more severe in WT than in ArKO−/− mice (Fig. 1F). In WT and mutant mice of both sexes, estradiol treatment at physiological dose prevented the destruction of β-cells (Fig. 1 A–D) and helped retain pancreas insulin concentration in ArKO−/− mice (Fig. 1 E and F).
Fig. 1.
Disruption of islet architecture and function in ArKO−/− mice exposed to STZ. (A and B) Representative pancreatic sections showing immunofluorescent staining for insulin (green) and glucagon (red) was performed in the indicated mice in basal conditions (vehicle) and after STZ injection (day 8). (C and D) β-cell number per pancreas section (n = 4–5) was calculated from pancreatic sections from A and B. (E and F) Pancreas insulin concentration was measured as described (24) in basal conditions and after STZ challenge (day 8). Values are represented as scatter plot and mean ± SE in ng/mg of pancreas (n = 4–19). ∗, P < 0.05; ∗∗, P < 0.01.
Estradiol Prevents β-Cell Apoptosis in Vivo and Protects Mice from Insulin-Deficient Diabetes.
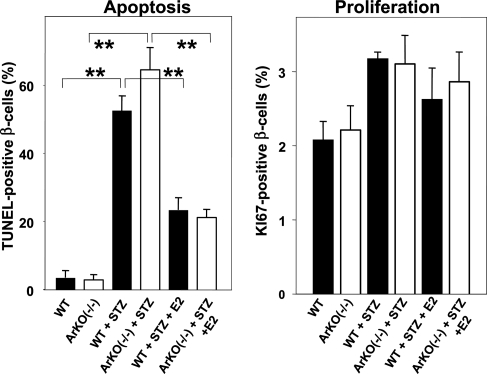
We examined the cellular mechanism of estradiol protection from β-cell death in vivo. β-cell apoptosis (TUNEL staining) and proliferation (KI67 staining) were assessed on pancreas sections from male mice. In STZ-exposed WT and ArKO−/− males, we observed a major increase in apoptotic β-cells (Fig. 2). Conversely, estradiol treatment suppressed apoptosis in both groups, demonstrating the importance of this hormone in preventing acute oxidative injury in vivo (Fig. 2). In contrast, estradiol treatment was not associated with increased β-cell proliferation (Fig. 2) or β-cell hypertrophy (see Fig. 7, which is published as supporting information on the PNAS web site). The glucose analogue STZ requires the β-cell glucose transporter GLUT2 to allow for its apoptotic effect (12). Thus, we studied the effect of estradiol on GLUT2 protein expression in mouse islets, but we observed no effect, ruling out the possibility that estradiol down-regulates GLUT2 expression, thereby preventing STZ entry in β-cells (see Fig. 8, which is published as supporting information on the PNAS web site).
Fig. 2.
Estradiol protects mice from STZ-induced β-cell apoptosis in vivo. Apoptosis (Left) and proliferation (Right) were determined by TUNEL and KI67 immunostaining from pancreas section after vehicle or STZ injection in male mice. ∗∗, P < 0.01.
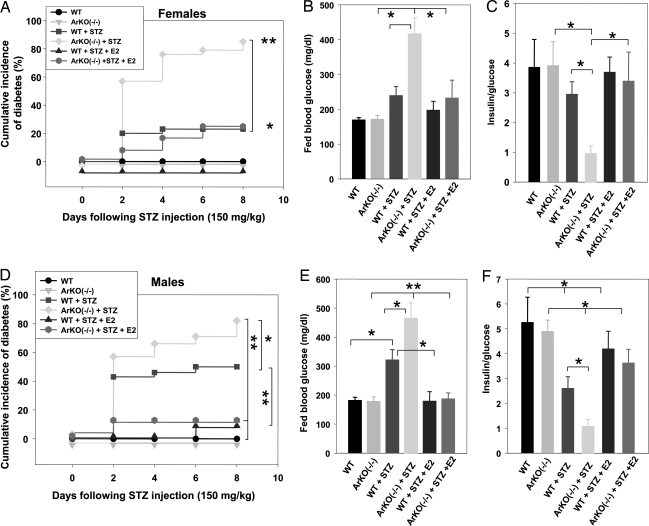
We next determined the consequences of estradiol deficiency and treatment on whole-animal glucose homeostasis. Consistent with the results shown previously, female WT mice were less prone to STZ-induced insulin-deficient diabetes than WT males (Fig. 3, compare A–C with D–F); however, ArKO−/− mice of both genders showed a severe predisposition to insulin-deficient diabetes compared with WT littermates (Fig. 3). Conversely, estradiol treatment prevented the development of insulin-deficient diabetes in WT and ArKO−/− mice regardless of their sex (Fig. 3). In all experiments described above, estradiol treatment was associated with physiological serum hormone concentrations (data not shown).
Fig. 3.
ArKO−/− mice are predisposed to STZ-induced insulin-deficient diabetes. Cumulative incidence of diabetes (random-fed blood glucose >250 mg/dl) was calculated by Kaplan–Meier estimation in female (A) (n = 10–28) and male (D) (n = 10–30) mice after STZ challenge (150 mg/kg of body weight). (B and E) Random-fed blood glucose (day 8), in female (B) and male (E) mice. (C and F) The ratio of random-fed of insulin (pg/ml) and glucose (mg/dl) at day 8 was used as an index of insulin deficiency in female (C) and male (F) mice. ∗, P < 0.05; ∗∗, P < 0.01.
ERα Is Expressed In Pancreatic β-Cells.
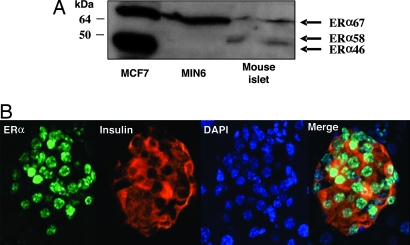
The predominant biological effects of estradiol are mediated through two members of the nuclear receptor family, ERα and ERβ (9). We focused on ERα because no significant role of ERβ has been demonstrated in energy metabolism (13). Using qualitative and real-time quantitative RT-PCR, we confirmed that the full-length ERα mRNA was expressed (see Fig. 9 A and B, which is published as supporting information on the PNAS web site). The study of protein expression by Western blotting demonstrated that the classical full-length ERα 67-kDa isoform was expressed in mouse islets and MIN6 cells (Fig. 4A), thus confirming results obtained by RT-PCR. A smaller 58-kDa isoform was expressed in mouse islets but not in MIN6. The functionality of this isoform is unknown. Neither islets nor β-cells expressed the 46-kDa isoform, which is expressed in human breast cancer cells, MCF7 (Fig. 4A). We next studied ERα localization in mouse pancreatic section by immunofluorescence using the same antibody. ERα was present in β-cells with an exclusive nuclear localization (Fig. 4B). As a control of antibody specificity, we confirmed that the antibody detected ERα in the nuclei of mouse uterine cells. ERα was expressed in both sexes, and its expression was present in vascular endothelium and ductal cells but absent from pancreatic exocrine cells (see Fig. 10, which is published as supporting information on the PNAS web site).
Fig. 4.
ERα is expressed in β-cells. (A) Western blot analysis of ERα expression in MCF7 cells, MIN6, and mouse islets. (B) Female pancreas section showing a single islet with ERα nuclear staining in β-cells (ERα). The insulin (red), nuclear (blue) (DAPI), and triple staining (merge) are shown.
ERα Protects Pancreatic β-Cell Survival and Function in Vitro.
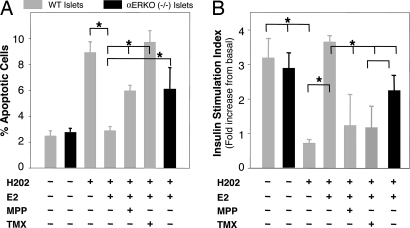
We next asked whether estradiol acts on ERα to suppress apoptosis in mouse islets from WT and ERα-deficient mice (αERKO). We exposed islets to hydrogen peroxide (H2O2), which causes β-cell injury during exposure to cytokines or chronic hyperglycemia in diabetic states (2). In WT islets, exposure to H2O2 provoked a 4-fold increase in apoptotic cells (Fig. 5A) that was suppressed by estradiol treatment. Conversely, treatment with the selective ERα antagonist MPP (14) partially reversed estradiol protection, whereas exposure to the ER antagonist tamoxifen totally reversed estradiol's antiapoptotic property (Fig. 5A). Accordingly, in αERKO−/− islets, as in the case of WT islets treated with MPP, estradiol protection was partially abolished. In addition, estradiol's antiapoptotic property through ERα was also observed in islets treated by STZ (data not shown) and in the β-cell line MIN6 treated by either STZ or H2O2 (see Fig. 11 A and B, which is published as supporting information on the PNAS web site). Furthermore, consistent with its antiapoptotic properties, in WT islets, after exposure to H2O2, estradiol prevented alteration of insulin release, which was reversed by MPP and tamoxifen (Fig. 5B). Accordingly, in αERKO−/− islets, estradiol protection of insulin release was partially eliminated (Fig. 5B).
Fig. 5.
ERα is important for β-cell survival and function after exposure to oxidative stress. (A) WT or αERKO mouse islets were incubated with E2 (10−8 M), MPP (10−7 M), and TMX (10−7 M) for 72 h, followed by H2O2 (100 μM) for the last 6 h. Percent of apoptotic cells was assessed by flow cytometry by using TUNEL/PI double labeling. Values represent five independent experiments. (B) Insulin secretion in response to glucose was assessed in static incubations from WT or αERKO−/− mouse islets cultured with E2 (10−8 M), MPP (10−7 M), and TMX (10−7 M) for 72 h, followed by H2O2 (100 μM) for the last hour. The stimulation index represents the ratio of insulin released at 16.7 mM glucose to insulin released at 2.8 mM glucose in four to seven replicate experiments). ∗, P < 0.05.
Elimination of ERα in Mice Predisposes to Insulin-Deficient Diabetes.
We determined the physiological relevance of the results presented above in αERKO mice. At basal levels, young female WT and αERKO−/− mice showed similar insulin secretion in response to glucose (Fig. 6A), pancreas insulin concentration, and islet β-cell numbers (Fig. 6 B and C). On the contrary, exposure to STZ caused severe islet β-cell destruction in αERKO−/− compared with WT female mice (Fig. 6C). We further studied the effect of ERα deletion on the occurrence of STZ-induced diabetes in WT and mutant mice of both genders. In female αERKO−/− mice, exposure to STZ caused a rise in the incidence of insulin-deficient diabetes compared with WT mice (Fig. 6D). This phenotypic abnormality was dominant, because it was also observed in αERKO+/− mice. In addition, treatment with the antagonist tamoxifen did not protect female αERKO−/− mice from STZ toxicity. Rather, as observed in islets, tamoxifen reversed the protection of endogenous estradiol in WT female mice, resulting in an increased incidence of insulin-deficient diabetes (Fig. 6D). These results demonstrate that pharmacological inhibition of ERα also exacerbates STZ-induced diabetes. Furthermore, in αERKO−/− mice, suppression of estradiol production by ovariectomy aggravated insulin-deficient diabetes. Accordingly, in ovariectomized αERKO−/−mice, treatment by estradiol partially reversed diabetes (Fig. 6D), suggesting that, in the absence of ERα, as observed in islets, estradiol partially protects β-cells via an alternate signaling pathway. Finally, male WT and αERKO−/− mice were exposed to a nondiabetogenic single low dose of STZ (75 mg/kg of body weight; Table 1). Such a low dose of STZ caused only a moderate increase in diabetes incidence in WT mice, whereas it provoked an early and severe insulin-deficient diabetes in αERKO−/− and αERKO+/− mice (Fig. 6E).
Fig. 6.
αERKO mice are predisposed to STZ-induced insulin-deficient diabetes. (A) Acute-phase insulin secretion after i.p. glucose injection (3 g/kg of body weight) in female mice (n = 8–10) was measured as described (24). (B) Pancreas insulin concentration was measured in female mice at basal levels or after 150 mg/kg STZ injection (day 8). Values are represented as scatter plot and mean ± SE (n = 5–14). (C) β-cell number per pancreas section at basal levels and after STZ injection in female mice at day 8 (n = 4). (D and E) (Left) Cumulative incidence of diabetes in mice after STZ injection. (Center) Random-fed blood glucose in mice (day 8). (Right) Ratio of random-fed insulin (pg/ml) to glucose (mg/dl) in female and male mice after STZ injection (day 8). ∗, P < 0.05; ∗∗, P < 0.01.
Discussion
This study establishes that estradiol, acting at least in part through ERα, protects pancreatic β-cells from apoptosis induced by oxidative stress, thus maintaining insulin production and preventing diabetes in mice of both genders. The analysis of human and rodent models of aromatase or ERα deficiency has challenged the traditional belief of gender-specificity in sex steroid actions and has revealed critical functions of the estradiol–ERα axis in the prevention of obesity and insulin resistance in mammals of both sexes (9, 13, 15–18).
The first finding of this study is the identification of circulating estradiol as a protective hormone for β-cell survival in mice. Mice of both sexes develop a vulnerability to STZ-induced insulin deficiency when estradiol production is suppressed. We show that estradiol treatment, at physiological concentrations, prevents β-cell apoptosis in these mice.
The second observation is the authentication of ERα function in β-cell survival. After exposure to oxidative stress, elimination of ERα impairs the ability of circulating estradiol to prevent β-cell apoptosis, resulting in insulin-deficient diabetes. This finding has implications for genetic predisposition to diabetes. Young mice of both sexes lacking ERα show no detectable alteration in insulin secretion or insulin sensitivity. They are vulnerable, however, to oxidative injury in β-cells. Moreover, although only one copy of ERα is sufficient for reproduction (19), haploinsufficiency for ERα in mice aggravates the β-cell vulnerability to STZ toxicity. This finding suggests that, in mice, both ERα alleles are required to guard β-cells from oxidative stress. Thus, ERα gene variations may participate in the genetic predisposition to human diabetes.
Another significance of this study is therapeutic. Estradiol is an ERα agonist, and estrogen replacement therapy (ERT) reduces the incidence of diabetes in postmenopausal women (20, 21). However, estradiol has undesirable side effects, such as breast cancer, and ERT is no longer suitable. Rather, selective ER modulators are compounds that act as ER agonists or antagonists, depending on the tissue considered. Tamoxifen, for example, acts as an ERα antagonist in breast and as an ERα agonist in bone. Accordingly, tamoxifen is used in the treatment of breast cancer while preventing osteoporosis. In our study, tamoxifen reverses estradiol protection of β-cell survival in vitro and exacerbates the predisposition to insulin-deficient diabetes. Together, these data suggest that tamoxifen acts as an ERα antagonist in β-cells and may precipitate insulin dependence in diabetic women treated for breast cancer. This critical issue merits investigation.
The last observation of this study is that estradiol partially prevents β-cell apoptosis in the absence of ERα, suggesting the existence of an alternate estradiol signaling pathway in β-cells. One possibility is that estradiol functions via ERβ. Alternatively, estradiol may activate nongenomic pathways via a membrane receptor unrelated to ERs and mediating estradiol survival signals (22).
Materials and Methods
Creation of Mutant Mice, Exogenous Substance Infusion, and Induction of Experimental Diabetes.
ArKO−/− and αERKO−/− were generated as described (19, 23). Estradiol (0.48 mg), 4-hydroxytamoxifen (0.48 mg), and vehicle (cholesterol) were administered by s.c. pellets (Innovative Research of America, Sarasota, FL). One week after pellet insertion, diabetes was induced in 8-week-old mice by a single i.p. injection of either 75 or 150 mg/kg of body weight STZ prepared in 50 mM citrate buffer (pH 4.5). Blood glucose was measured every 48 h after STZ injection with a glucose monitor (OneTouch Ultra; Lifescan, Mountain View, CA).
Islet Isolation.
Mouse islets were isolated by collagenase digestion. Briefly, the pancreas was injected through the pancreatic duct with 3 ml of 2 mg/ml collagenase (Sigma) in Hanks' buffered saline solution (HBSS), dissected out, incubated at 37°C for 12 min, and passed through a 400-mm wire mesh. The digested pancreas was rinsed with HBSS, and islets were separated by density gradient in Histopaque (Sigma). After several washes with HBSS, islets were hand picked under a dissection microscope.
Islet Culture and Compound Stimulation.
Islets were cultured in phenol-red-free DMEM and 5 mM glucose containing 10% charcoal-stripped FBS. Islets were incubated with estradiol (10−8 M; Steraloids, Newport, RI), Methyl–Piperidino–Pyrazole (MPP, 10−7 M; Tocris Cookson, Ellisville, MO), and 4-hydroxytamoxifen (10−7 M; Sigma) for 72 h. After ER ligand treatment, islets were exposed to H2O2 (100 μM; Sigma) for the last 6 h before assessment of apoptosis. For additional information, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Insulin Secretion in Static Incubations.
Insulin release from islets was measured by static incubation. Batches of 10 islets were cultured for 72 h in phenol-red-free DMEM. Islets were washed twice for 30 min in KRB buffer containing 0.1% BSA and 2.8 mM glucose and estradiol or ER antagonists. Oxidative stress was induced by addition of 100 μM H2O2 during the second wash. Islets were then incubated for 30 min in the presence of 2.8 or 16.7 mM glucose. Insulin was measured by using a sensitive rat insulin radioimmunoassay (RIA) kit (Linco Research, St. Charles, MO).
Pancreas and Islet Immunostaining.
Tissues were fixed by cardiac perfusion with 4% paraformaldehyde, dissected out, further fixed overnight in 10% neutral formalin, and embedded in paraffin. Sections were cut at 5 μm. After dewaxing, slides were washed, microwave antigen retrieval was performed, and the slides were permeabilized with 0.2% Triton X-100 and blocked in 10% serum. Primary antibodies were incubated overnight at 4°C with the following dilutions: guinea pig anti-insulin (1:1,000; Linco), rabbit anti-glucagon (1:1,000; DiaSorin, Sallugia, Italy), and rabbit anti-ERα (E1644, 1:200; Spring Biosciences, Fremont, CA). Slides were then incubated with the appropriate secondary antibodies: FITC-donkey anti-guinea pig, (1:400; Jackson ImmunoResearch) and Cy3-donkey anti-rabbit (1:800; Jackson ImmunoResearch). For ERα, tyramide signal amplification (Molecular Probes) was performed. For TUNEL assay, sections were incubated with terminal deoxytransferase enzyme in the presence of Biotin-dUTP (Roche). TUNEL-positive cells were revealed with Alexa Fluor 488-Streptavidin (Molecular Probes).
Measurement of Apoptosis and Proliferation in Vivo.
Pancreata were fixed 12 h after STZ injection. Quantification of apoptosis and proliferation were performed by TUNEL and KI67 staining, respectively. Percent apoptosis or proliferation was calculated by dividing the number of TUNEL- or KI67-positive β-cells by the total number of β-cell stained nuclei. At least four mice per condition and 50 islets per mouse were counted.
Calculation of β-Cell Number per Pancreas Section.
We counted the β-cell number from merged pictures of pancreas sections immunostained with insulin and DAPI in all islets present in each section. β-Cell number per section was quantified by using five sections per pancreas and five mice per condition.
Measurement of Apoptosis by Flow Cytometry.
Apoptosis was measured by a DNA fragmentation assay (FlowTACS; R & D Systems) according to the manufacturer's instructions, and cells were analyzed by flow cytometry by using a Beckman Coulter EPICS XL-MCL flow cytometer. Double staining was performed in duplicate by incubating FITC-labeled cells with RNaseA/propidium iodide to stain total DNA. Gating of nonapoptotic populations was used as a reference.
Statistical Analysis.
Results are presented as mean ± SE unless otherwise stated. All statistical analyses were performed by using the unpaired Student t test. Cumulative incidence of diabetes was determined by Kaplan–Meier estimates, and statistical analysis of differences was determined by log-rank test. A value of P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Rachel E. Webb for editorial expertise. This work was supported by National Institutes of Health Grant R21 DK069362-01 (to F.M.-J.), and a Carolyn Weiss Law Award in Molecular Medicine (to F.M.-J.). C.L.M. is the recipient of a Juvenile Diabetes Research Foundation Postdoctoral Fellowship.
Abbreviations
- ArKO
aromatase-deficient
- αERKO
αER-deficient
- ER
estrogen receptor
- STZ
streptozotocin
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Mathis D., Vence L., Benoist C. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 2.Robertson R. P., Harmon J., Tran P. O., Tanaka Y., Takahashi H. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 3.Wild S., Roglic G., Green A., Sicree R., King H. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Gale E. A., Gillespie K. M. Diabetologia. 2001;44:3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- 5.Mauvais-Jarvis F., Sobngwi E., Porcher R., Riveline J. P., Kevorkian J. P., Vaisse C., Charpentier G., Guillausseau P. J., Vexiau P., Gautier J. F. Diabetes. 2004;53:645–653. doi: 10.2337/diabetes.53.3.645. [DOI] [PubMed] [Google Scholar]
- 6.Louet J. F., Le May C., Mauvais-Jarvis F. Curr. Atheroscler. Rep. 2004;6:180–185. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]
- 7.Contreras J. L., Smyth C. A., Bilbao G., Young C. J., Thompson J. A., Eckhoff D. E. Transplantation. 2002;74:1252–1259. doi: 10.1097/00007890-200211150-00010. [DOI] [PubMed] [Google Scholar]
- 8.Simpson E. R., Misso M., Hewitt K. N., Hill R. A., Boon W. C., Jones M. E., Kovacic A., Zhou J., Clyne C. D. Endocr. Rev. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 9.Couse J. F., Korach K. S. Endocr. Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 10.Behl C. Nat. Rev. Neurosci. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- 11.Friesen N. T., Buchau A. S., Schott-Ohly P., Lgssiar A., Gleichmann H. Diabetologia. 2004;47:676–685. doi: 10.1007/s00125-004-1367-x. [DOI] [PubMed] [Google Scholar]
- 12.Schnedl W. J., Ferber S., Johnson J. H., Newgard C. B. Diabetes. 1994;43:1326–1333. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- 13.Ohlsson C., Hellberg N., Parini P., Vidal O., Bohlooly M., Rudling M., Lindberg M. K., Warner M., Angelin B., Gustafsson J. A. Biochem. Biophys. Res. Commun. 2000;278:640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 14.Harrington W. R., Sheng S., Barnett D. H., Petz L. N., Katzenellenbogen J. A., Katzenellenbogen B. S. Mol. Cell. Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 15.Morishima A., Grumbach M. M., Simpson E. R., Fisher C., Qin K. J. Clin. Endocrinol. Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 16.Smith E. P., Boyd J., Frank G. R., Takahashi H., Cohen R. M., Specker B., Williams T. C., Lubahn D. B., Korach K. S. N. Engl. J. Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 17.Heine P. A., Taylor J. A., Iwamoto G. A., Lubahn D. B., Cooke P. S. Proc. Natl. Acad. Sci. USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones M. E., Thorburn A. W., Britt K. L., Hewitt K. N., Wreford N. G., Proietto J., Oz O. K., Leury B. J., Robertson K. M., Yao S., Simpson E. R. Proc. Natl. Acad. Sci. USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubahn D. B., Moyer J. S., Golding T. S., Couse J. F., Korach K. S., Smithies O. Proc. Natl. Acad. Sci. USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolis K. L., Bonds D. E., Rodabough R. J., Tinker L., Phillips L. S., Allen C., Bassford T., Burke G., Torrens J., Howard B. V. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 21.Kanaya A. M., Herrington D., Vittinghoff E., Lin F., Grady D., Bittner V., Cauley J. A., Barrett-Connor E. Ann. Intern. Med. 2003;138:1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 22.Nadal A., Rovira J. M., Laribi O., Leon-Quinto T., Andreu E., Ripoll C., Soria B. FASEB J. 1998;12:1341–1348. doi: 10.1096/fasebj.12.13.1341. [DOI] [PubMed] [Google Scholar]
- 23.Fisher C. R., Graves K. H., Parlow A. F., Simpson E. R. Proc. Natl. Acad. Sci. USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauvais-Jarvis F., Virkamaki A., Michael M. D., Winnay J. N., Zisman A., Kulkarni R. N., Kahn C. R. Diabetes. 2000;49:2126–2134. doi: 10.2337/diabetes.49.12.2126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.