Abstract
Vibrio cholerae is the causal bacterium of the diarrheal disease cholera, and its growth and survival are thought to be curtailed by bacteriovorous predators, e.g., ciliates and flagellates. We explored Caenorhabditis elegans as a test organism after finding that V. cholerae can cause lethal infection of this nematode. By reverse genetics we identified an extracellular protease, the previously uncharacterized PrtV protein, as being necessary for killing. The killing effect is associated with the colonization of bacteria within the Caenorhabditis elegans intestine. We also show that PrtV is essential for V. cholerae in the bacterial survival from grazing by the flagellate Cafeteria roenbergensis and the ciliate Tetrahymena pyriformis. The PrtV protein appears to have an indirect role in the interaction of V. cholerae with mammalian host cells as judged from tests with tight monolayers of human intestinal epithelial cells. Our results demonstrate a key role for PrtV in V. cholerae interaction with grazing predators, and we establish Caenorhabditis elegans as a convenient organism for identification of V. cholerae factors involved in host interactions and environmental persistence.
Keywords: cholera, host interactions, environmental persistence
Cholera continues to be a major public and individual health problem, especially in those regions of the world where it is endemic. Colwell (1) first hypothesized that coastal waters were an important reservoir of Vibrio cholerae. Huq et al. (2) reported that V. cholerae O1 cells could be observed to be attached to a variety of phytoplankton and zooplankton species. The incidence and severity of epidemics have been linked to salinity, water temperature, turbidity, and plankton blooms (3, 4). Cholera epidemics occur in a regular seasonal pattern. It has been suggested that during interepidemic periods V. cholerae exists in an unexplained ecological association with aquatic organisms (5). During the environmental phase, V. cholerae resides in diverse aquatic environments, often in association with marine plankton (6). The association of V. cholerae with zooplankton has proven to be a key factor in deciphering the global nature of cholera epidemics (7). In such natural bacterioplankton communities V. cholerae and other bacteria are also at the base of the pelagic microbial food web (8). Bacterial growth and survival are subject to constraint by bacteriovorous predators, e.g., protozoa such as ciliates and flagellates (9, 10). Little has been known about mechanisms and adaptations of bacteria to reduce grazing mortality compared with adaptations toward abiotic factors (substrate, temperature, pH, etc.) (11).
V. cholerae expresses well characterized factors to establish and cause disease in the mammalian host, including cholera toxin (CT) and toxin-coregulated pili (Tcp). It has been shown that quorum sensing (QS) plays a role in the regulation of virulence in V. cholerae (12). At least three autoinducer signaling circuits function through the action of LuxO, leading to the repression of the gene encoding the HapR regulatory protein (12–14). The QS regulatory cascade appears to be central to a number of virulence-related phenotypes, including CT production, biofilm formation, and protease secretion. QS may also be important in the environmental phase of the V. cholerae life cycle (12). Extracellular proteases are considered as putative virulence factors in several microbial diseases, including those caused by Pseudomonas aeruginosa (15) and V. cholerae (16, 17). Recently, Matz et al. (18) observed that a QS mutant of P. aeruginosa had a significantly reduced antipredator fitness compared with isogenic WT strains.
Although cholera is commonly considered to be a noninflammatory secretory disease, there are indications of some inflammatory component(s) to the disease (19). In clinical trials most V. cholerae vaccine candidates still exhibit reactogenicity (20). The mechanisms of reactogenicity caused by V. cholerae vaccine candidates lacking e.g., CT gene, however, are still not known. Levine and Noriega (21) suggested that an unidentified enterotoxin could cause reactogenic symptoms in the absence of CT but whose existence had been masked by the presence of CT.
The nematode Caenorhabditis elegans has been used successfully as an invertebrate infection model to screen for virulence factors of several human pathogens, e.g., P. aeruginosa, Salmonella enterica, and Serratia marcescens (22–28).
Here, we establish that Caenorhabditis elegans is a useful model system for identifying and assessing factors other than CT from V. cholerae that may contribute to bacterial survival and persistence in the environment and thereby can be important for pathogenesis and damage to host organisms.
Results and Discussion
V. cholerae Kills Caenorhabditis elegans upon Colonization of the Intestinal Tract.
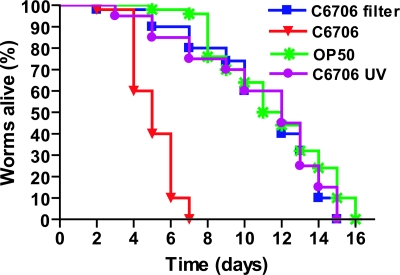
We first tested the ability of V. cholerae Ol El Tor Inaba strain C6706 to kill Caenorhabditis elegans. L4 hermaphrodites raised on OP50, a standard Escherichia coli strain used for cultivating Caenorhabditis elegans (29), were transferred onto the lawns of V. cholerae strain C6706. The nematodes died within ≈5 days, indicating that V. cholerae exerted a slow killing effect (Fig. 1). Routinely the parental worms were moved to a new plate with V. cholerae every 2 days to distinguish between parents and progeny. To examine whether diffusible compounds were responsible for the killing effect, V. cholerae strain C6706 was grown on nitrocellulose filters (pore diameter 0.2 μm) covering the surface of the nematode growth medium plates. After growth, the filters were removed and worms were placed on the plates. After ≈12 h on such a plate the worms were transferred to the plate containing OP50. As seen in Fig. 1, no killing effect was observed in this experiment, suggesting that the killing of Caenorhabditis elegans by V. cholerae requires direct contact with the bacteria.
Fig. 1.
Characterization of the killing of Caenorhabditis elegans by V. cholerae. Kinetics of killing of worms (n = 50) with V. cholerae strain C6706, E. coli OP50 (P < 0.0001), UV-killed C6706 (P < 0.0001), and a plate where C6706 had been grown on a filter and then removed (P < 0.0001).
To investigate whether bacteria needed to be alive to cause killing, L4 worms were placed on C6706 bacteria killed by UV irradiation. In this case, the worms survived as long as those fed on E. coli (Fig. 1). To establish whether a continuous or a transient contact was necessary, worms were transferred to the lawn of V. cholerae C6706, allowed to feed for 24 h, and then, after three washes with sterile M9 buffer to remove surface-located bacteria, placed onto lawns of E. coli OP50. Locomotion, pharyngeal pumping, and egg-laying ability appeared reduced for the first 48–72 h. However, over the next 24–48 h, all of these activities gradually improved. Our results show that the killing effect requires direct and continuous contact with live bacteria, which shows resemblance to the case of P. aeruginosa infection in Caenorhabditis elegans as described (28).
To determine whether the ability to kill Caenorhabditis elegans is a common property of different serotypes of V. cholerae strains, we tested several clinical isolates in the nematode killing assay. Representative strains of the serotypes Classical Inaba, Classical Ogawa, and non-O1 non-0139 were found to kill (Fig. 7, which is published as supporting information on the PNAS web site). V. cholerae does not, however, seem to be inherently toxic to Caenorhabditis elegans because we also found some strains that did not kill the nematodes (Fig. 7).
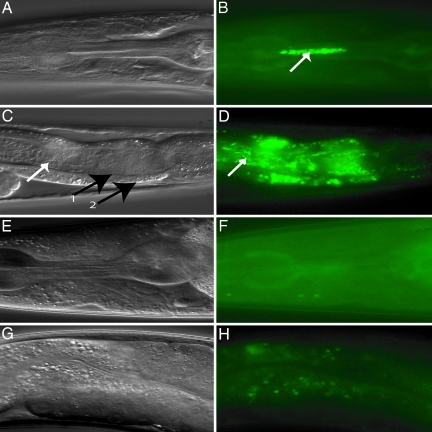
To follow the fate of the bacteria upon ingestion by the worm, we used derivatives of the bacteria (Table 1, which is published as supporting information on the PNAS web site) expressing the GFP. V. cholerae strain C6706/pSMC2 was found to be as virulent as C6706 in the killing assay (data not shown). When L4 worms were placed on the GFP-labeled V. cholerae for 12 h, intact V. cholerae curved rods were seen to accumulate in both the pharynx and the lumen of the intestine (Fig. 2A–D). However, in worms feeding on OP50/pSMC2 we did not observe any fluorescent bacteria (Fig. 2 E–H).
Fig. 2.
Visualization of V. cholerae colonizing the intestinal lumen of Caenorhabditis elegans. Nomarski (A, C, E, and G) and fluorescence (B, D, F, and H) photomicrographs of worms fed for 12 h with V. cholerae C6706/pSMC2 (A–D) or E. coli OP50/pSMC2 (E–H). The white arrows in B–D show V. cholerae bacilli. Black arrows in C (numbered 1 and 2) show the apical membrane and the basal membrane of intestinal tract, respectively. Note that C6706/pSMC2 bacteria were present both in the pharynx (B) and the intestinal lumen (D).
Killing of Caenorhabditis elegans by V. cholerae Does Not Depend on CT or Biofilm Formation.
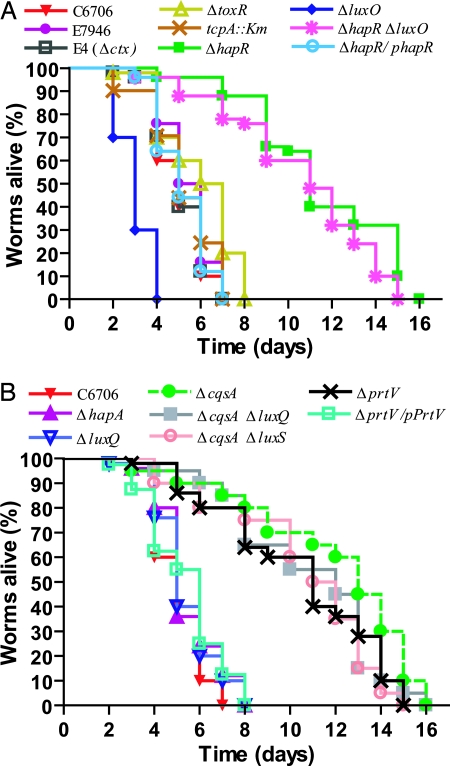
The ctx and tcpA mutant strains were able to kill Caenorhabditis elegans worms as efficiently as the WT bacteria (Fig. 3A), indicating that CT and Tcp are not absolutely required for lethal infection. In the case of the toxR mutant, altered in the regulatory gene affecting both ctx and tcpA expression, there was a small, but significant, reduction in killing.
Fig. 3.
Effect of V. cholerae mutations on nematode killing. (A) The LuxO–HapR regulatory network influences V. cholerae nematode infection. V. cholerae mutants (n = 50) ΔhapR (P < 0.0001), ΔluxO (P < 0.0001), ΔhapRΔluxO (P < 0.0001), tcpA::Km (P = 0.2), ΔtoxR (P = 0.0001), and ΔhapR/phapR (P = 0.7) were compared with the WT strain C6706 (n = 50). The Δctx mutant strain E4 was compared with the otherwise isogenic WT strain E7946 (n = 50, P = 0.3). (B) Kinetics of killing of Caenorhabditis elegans by V. cholerae QS-deficient mutants. The WT V. cholerae strain C6706 (n = 50) was compared with mutant derivatives (n = 50): ΔcqsA (P < 0.0001), ΔluxQ (P = 0.1), ΔcqsAΔluxQ (P < 0.0001), ΔcqsAΔluxS (P < 0.0001), ΔhapA (P = 0.6), ΔprtV (P < 0.0001), and transcomplemented strain ΔprtV/pPrtV (P = 0.06).
The ability to form biofilm has been associated with V. cholerae pathogenicity in some systems (12). We found, however, that some of the biofilm-deficient mutants that we had obtained in a separate study with the Ol El Tor Inaba strain A1552 were able to kill Caenorhabditis elegans with WT efficiency (Fig. 8, which is published as supporting information on the PNAS web site).
V. cholerae Worm Killing Effect Is Mediated by LuxO-Regulated Genes in the QS Pathway.
The expression of known virulence factors of V. cholerae is regulated in a coordinated fashion in response to QS (12). Transcription of the regulatory gene hapR leads to reduced biofilm formation, reduced CT expression, and increased protease expression. Intriguingly, the strain carrying a hapR gene deletion was strongly attenuated in its ability to kill worms (Fig. 3A). The attenuation was not caused by, e.g., a growth defect of hapR because we detected no difference in growth rate when we compared it with WT V. cholerae C6706. Transcomplementation of the hapR deficiency by introducing a plasmid (phapR) carrying a WT allele restored the killing effect (Fig. 3A).
The luxO gene encodes a negative regulator of hapR transcription (12). In the absence of LuxO, hapR is constitutively transcribed, leading to increased production of several secreted proteins. We found that luxO mutant V. cholerae killed worms more quickly (within 3 days) than WT (Fig. 3A). A luxO hapR double mutant was attenuated, indicating that the effect of luxO depends on functional hapR (Fig. 3A). Similarly, we investigated the effect of mutations in the upstream part of the QS regulatory pathways. Mutations abolishing the synthesis of the receptors of the two known QS compounds in V. cholerae, i.e., the Cqs and AI-2 systems were tested (Fig. 3B). The cqsA (gene for synthesis of autoinducer 1) mutant bacteria were attenuated, whereas the luxQ (gene for receptor of autoinducer 2) mutant bacteria could cause lethal infection of worms as efficiently as WT. The result with the cqsA mutant is consistent with the hapR mutant results described above because it is known that a cqsA mutation results in strongly reduced hapR expression (30). When both systems were abolished (i.e., ΔcqsA ΔluxQ and ΔcqsA ΔluxS) the V. cholerae were completely attenuated.
V. cholerae Lethal Infection of the Caenorhabditis elegans Depends on a HapR-Regulated Protease.
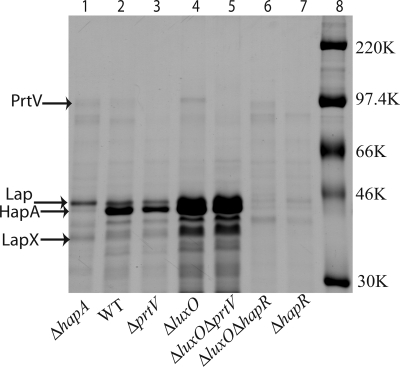
Because two processes known to be regulated by hapR, biofilm formation and CT expression, did not seem to be required for killing of Caenorhabditis elegans, we investigated whether proteases regulated by the LuxO–HapR pathway might be involved. Analysis of supernatants from broth cultures showed that several secreted proteins were more abundant in the case of the luxO mutant than from WT V. cholerae (Fig. 4). The luxO mutant also had clearly higher levels of protease activity (Fig. 9, which is published as supporting information on the PNAS web site). Furthermore, SDS/PAGE analyses indicated that the most abundant protein in the supernatant (from either luxO or WT) was HA/P (Fig. 4).
Fig. 4.
Identification of secreted V. cholerae proteases. Coomassie brilliant blue-stained gel after SDS/PAGE analysis of secreted proteins from different derivatives of the V. cholerae strain C6706: ΔhapA, WT, ΔprtV, ΔluxO, ΔluxO ΔprtV, ΔluxO ΔhapR, and ΔhapR strains. Equal amounts of trichloracetic acid-precipitated samples of supernatants from bacterial cultures at the same OD were tested. Arrows indicate proteins subjected to mass spectrometric analysis. Molecular size markers are shown in lane 8. The identity of the HapA protein was confirmed by immunoblot analysis (data not shown) using anti-HA/P polyclonal antiserum (33).
HA/P (also denoted HapA) is encoded by the hapA gene, is regulated by hapR, and is the major extracellular protease (31). However, when hapA mutant V. cholerae were tested in the Caenorhabditis elegans killing assay we found no attenuation in comparison with WT (Fig. 3B). The culture supernatant from the hapA mutant contained some proteins not found in the supernatants from the hapR mutant (Fig. 4). We identified the three most abundant putative HapR-regulated proteins detected in the supernatant from the hapA mutant V. cholerae by mass spectrometry (see Materials and Methods). This analysis revealed that the protein bands corresponded to proteins encoded by the ORFs VCA0812, VCA0813, and VCA0223 (32). The protein products are a leucine aminopeptidase-related protein, leucine aminopeptidase (Lap) (33), and the PrtV protease (34), respectively. We refer to the ORF VCA0812 as lapX. Recent transcriptome analyses indicated that prtV gene transcription is affected by a hapR mutation (30).
To determine whether any of the identified proteins above played a role in worm killing we tested ΔprtV, Δlap, and ΔlapX mutants in the Caenorhabditis elegans assay. The ΔprtV mutant showed complete attenuation in comparison with the WT (Fig. 3B; P < 0.0001). To assess whether the attenuation effect depended on the prtV mutant locus per se, we performed a transcomplementation test by introducing a plasmid (pKVA232) harboring the cloned WT allele of the prtV gene. The complementation restored the WT killing effect (Fig. 3B), ruling out that the mutation indirectly caused attenuation by polar effects from the prtV deletion mutation. Neither of the Δlap and ΔlapX mutants showed significant attenuation (Fig. 10, which is published as supporting information on the PNAS web site). The ΔprtV mutation was also found to abolish most of the killing effect of the ΔluxO derivative. Furthermore, the cloned prtV gene resulted in an increased killing effect by the WT V. cholerae strain C6706, and it restored a significant killing effect in the case of the hapR mutant derivative (Fig. 10). We conclude that this reverse genetics approach identified the PrtV protein as a factor required for the V. cholerae lethal infection of Caenorhabditis elegans.
This PrtV protein is one of the less abundant of the secreted proteases, and little is known about its activity. The measurements of proteolytic activity against azocasein indicated that 10–20% of total activity in culture supernatants was abolished by the ΔprtV mutation (Fig. 9). In accordance with HapA being most abundant of the secreted proteases, abolishment of HapA reduced the total protease activity to ≈10% of the WT level. It is possible that the different proteases might affect the activity or stability of each other. To test how protease levels might be influenced and to analyze the possible synergistic effect of proteases on worm killing, a series of multiple deletion mutants was constructed. The Δlap ΔlapX and ΔhapA Δlap ΔlapX combinations resulted in a slight, barely significant, attenuation (Fig. 10; P = 0.04, P = 0.004). The ΔhapA Δlap ΔlapX ΔprtV mutant was completely attenuated.
Also the above-mentioned V. cholerae isolates that did not kill Caenorhabditis elegans (Fig. 7) carried the prtV gene locus as determined by PCR (data not shown). However, the preliminary studies of PrtV protein production by Western blot analysis (unpublished data) indicated that the level of secreted PrtV was much reduced in comparison with that of strain C6706 in two of three cases. Whether or not PrtV protease activity might differ among isolates will require further biochemical studies.
PrtV Is Important for V. cholerae Inhibition of Natural Bacteriovorous Predators.
To test whether predator grazing, and thereby V. cholerae persistence/survival, would be influenced by alteration of prtV expression we carried out studies with bacteriovorous unicellular organisms typically found in marine plankton communities. We used a series of mutant bacterial strains in tests with the flagellate Cafeteria roenbergensis and the ciliate Tetrahymena pyriformis. The results clearly indicated that V. cholerae caused a reduction in protozoan activity and survival in a fashion that depended on the QS regulon (Fig. 5 and Table 2, which is published as supporting information on the PNAS web site). WT V. cholerae caused a marked reduction in the number of viable protozoa during prolonged cocultivation, whereas the bacterial density remained virtually unaltered. Furthermore, V. cholerae with mutations abolishing the hapR or prtV genes appeared strongly attenuated in the predator grazing test, and most of the bacteria were consumed by the predators within 3 days. In the case of the luxO mutant bacteria, on the other hand, the effect was more detrimental to the protozoa than that seen with the WT V. cholerae strain (Fig. 5).
Fig. 5.
Survival of ciliates and flagellates grazing on WT and mutant V. cholerae. The ciliate T. pyriformis (A) and flagellates Cafeteria roenbergensis (B) were allowed to graze on different V. cholerae C6706 strains, and the protozoa viability was monitored after 4 days as described in Materials and Methods.
Our findings also provide a feasible explanation for the observation recently reported by Matz et al. (35). Their results suggested that the V. cholerae HapR regulon had a distinctive environmental role for the production of some unidentified antiprotozoal factor(s) inhibiting flagellate plankton activity and thus protecting the bacterial biofilm from grazing. The antiprotozoal factor(s) appeared to be some secreted product because cell-free supernatants of WT V. cholerae significantly reduced the feeding activity of predators. Our results show that the PrtV protein is such a factor.
A recent report by Pukatzki et al. (36) demonstrated that some other secreted, not yet identified, V. cholerae protein(s) may cause cytotoxicity toward the Dictyostelium discoideum amoebae. The VAS secretion system, which is not involved in PrtV secretion, was shown to be required for amoebae killing. Taken together our findings indicate that there are different bacterial factors that contribute to how V. cholerae survives predation by different bacteriovorous organisms.
Lack of PrtV May Cause Enhanced Reactogenicity to V. cholerae.
The PrtV protein did not appear to influence bacterial colonization during infection of a mammalian host as judged by an in vivo colonization assay with the infant mouse model. The results showed that the prtV mutant colonizes infant mice to the same extent as WT (ref. 34 and unpublished data). It has been suggested that there may be some inflammatory component to the disease caused by V. cholerae in humans, and recently it was shown that bacterial supernatants can stimulate production of IL-8 from intestinal epithelial T84 cells in vitro (37). Some IL-8-stimulating factor from the bacteria may thereby play a role in the reactogenicity seen with V. cholerae vaccine candidates. We tested whether the prtV gene product might be involved directly or indirectly as an IL-8 stimulator by using bacterial supernatants added to the apical side of tight monolayers of the T84 cell line. The results showed that the amount of IL-8 released to the basolateral side was significantly higher in the case of supernatants lacking the PrtV protease (Fig. 6). About 2-fold higher IL-8 levels were seen when the supernatants from ΔprtV mutant derivatives were compared with supernatants from otherwise isogenic prtV+ strains. That the effect is PrtV-dependent was verified by the transcomplementation test of the ΔprtV mutation (using the plasmid clone pKVA232) that led to restored lower (i.e., similar to PrtV WT) levels of IL-8. Analyses of IL-8 mRNA levels also revealed an increase in the case of the prtV mutant (Fig. 11, which is published as supporting information on the PNAS web site), and we conclude that PrtV is not an IL-8 stimulator per se. Rather it appeared that PrtV may play some role in modulating/reducing the activity of the as-yet-unidentified component(s) that may be the cause of V. cholerae reactogenicity.
Fig. 6.
IL-8 secretion in tight monolayers of intestinal epithelial T84 cells after exposure to V. cholerae culture supernatants. Tight monolayers of T84 cells (transepithelial resistance >2,000 ohm/cm2) were tested with sterile filtered supernatants from different V. cholerae derivatives for 18 h at which time the tissue culture medium at the basolateral side was collected and analyzed for the concentration of IL-8 as described in Materials and Methods. The transepithelial resistance was decreased to 1,672 and 609 ohm/cm2 in the case of the WT and ΔprtV strain, respectively.
We conclude that the QS regulon has an important role in the encounter between V. cholerae and the grazing predators in bacterioplankton communities. Our findings demonstrate that in particular the prtV gene product should be considered as a factor contributing to the bacterial viability and persistence in such competitive environmental niches. How the PrtV protease evolved in V. cholerae to allow for survival in predators is not known, but evidently it is part of the HapR regulon that coordinates expression of several genes involved in environmental adaptation. It is an intriguing, albeit speculative, possibility that selection for PrtV in the environmental niche by virtue of its role in preventing predator grazing is matched by a role in keeping reactogenicity low in mammalian infections.
Materials and Methods
Bacterial Strains, Culture Conditions, and Plasmids.
The bacterial strains and plasmids used are listed in Table 1. Bacteria were grown overnight at 37°C in LB broth supplemented, as appropriate, with kanamycin (30 μg/ml) or ampicillin (50 μg/ml). Mutagenesis of the V. cholerae strain C6706 in the luxO, hapR, hapA, prtV, lap, and lapX loci were constructed by making in-frame deletions of the entire reading frame in each case by using procedures as described (12, 38). Oligonucleotide primers used are listed in Table 3, which is published as supporting information on the PNAS web site.
Caenorhabditis elegans Maintenance.
Caenorhabditis elegans WT strain Bristol N2 was routinely maintained at 20°C on nematode growth medium agar plates seeded with E. coli OP50 by standard methods (29). Antibiotics were included for bacterial strains carrying plasmids.
Caenorhabditis elegans Killing Assays.
An overnight LB broth culture (100 μl) of the test bacterial strain was spread on a 5-cm-diameter nematode growth medium agar plate and incubated at 30°C for 24 h before the bacterial lawn was seeded with 50 L4 stage worms of Caenorhabditis elegans WT strain Bristol N2. Plates were incubated at room temperature (23°C) and scored for live worms every day. E. coli OP50 was used as a negative control. A worm was considered dead when it no longer responded to touch. Any worms that died as a result of getting stuck to the wall of the plate were excluded from the analysis. Data were subjected to statistical analyses and plotted according to a Kaplan–Meier survival graph by using the program prism, version 4.0 (GraphPad, San Diego). Multiple experiments (three to four worm-killing assays per bacterial strain) were done, and the data presented are from a representative experiment. Survival curves are considered significantly different from a V. cholerae strain C6706 when P < 0.05.
Microscopy.
Nematode intestinal tracts and the presence of bacteria were examined by Nomarski differential interference contrast microscopy and fluorescence microscopy with a Zeiss Axioplan microscope.
SDS/PAGE and Western Blot Analyses.
To monitor proteases, supernatants from V. cholerae were concentrated by trichloracetic acid precipitation and separated by SDS/PAGE (39). Western blot analyses were performed as described (40), and detection was done by using the ECL+ chemiluminescence system (Amersham Pharmacia Biotech).
Human Intestinal Epithelial Cell Tight Monolayer Assay.
Tight monolayers of the human colon carcinoma cell line T84 were established in a transwell system by using permeable polycarbonate membrane supports with 0.4-μm-diameter pores (Costar) and a 1:1 mixture of Dulbecco/Vogt modified Eagle’s medium and Ham’s F12 medium supplemented with 8% FCS, 15 mM Hepes buffer, and antibiotics. Cells were cultured in a humidified incubator at 37°C in 5% CO2 until a confluent monolayer with a transepithelial electrical resistance of >2,000 ohm/cm2 was obtained as measured by the Millicell Electrical Resistance System (Millipore) (41). Then the tissue culture medium in the upper chamber was replaced by a 500-μl culture supernatant sample from the tested bacterial strain, and cells were incubated for an additional 5 or 18 h at which time the culture medium from the lower chamber was collected. Thereafter cells were collected for RNA extraction.
Analysis of Secreted IL-8.
Tissue culture medium from the lower compartment of the transwell cultures, i.e., the basolateral side of the T84 monolayers, was collected and thereafter the concentration of IL-8 was determined in duplicates of serial dilutions of tissue culture medium by using a commercially available ELISA (Endogen Human IL-8 ELISA Kit; Pierce).
Grazing Experiments.
Experiments investigating the viability of the ciliate protozoan T. pyriformis CCAP 1630/1W (obtained from Culture Collection of Algae and Protozoa Scottish Association for Marine Science Research Services, Argyll, Scotland) and the flagellate Cafeteria roenbergensis (purchased from Culture Collection of Algae and Protozoa Scottish Association for Marine Science Research Services) were performed in 24-well tissue culture plates. The axenic protozoan culture was grown and maintained in CCAP protease peptone yeast extract medium (room temperature without shaking). To make them bacteria-free, the ciliate and flagellate cultures were treated with antibiotics (50 μg/ml ampicillin; 30 μg/ml kanamycin; 30 μg/ml polymyxin; 30 μg/ml streptomycin; 25 μg/ml chloramphenicol), and tests with LB medium and agar plates confirmed that they were axenic.
Overnight cultures of the V. cholerae strains were diluted in 0.9% NaCl, and 2.5 × 107/ml bacteria were transferred into wells in tissue culture plates. Subsequently, T. pyriformis or Cafeteria roenbergensis was added to the wells at a final concentration of 6 × 103/ml. Numbers of ciliates and flagellates were analyzed by direct inspection with an inverted light microscope over 4 days. Generally each treatment was performed in replicate wells of four. Protozoa cell numbers were counted by using an inverted light microscope (Zeiss). Samples (50 or 10 μl) were taken from each well into counting chambers, and the numbers of motile protozoa were recorded. To obtain the total number of protozoa per sample, formaldehyde was added to a final concentration of 2% (42), and all fixed cells were counted. The survival (percentage of input) after 4 days was calculated from the direct counts of actively motile organisms.
Protein Identification.
Mass spectrometry, protein identification, and database searches were carried out at the Wallenberg Consortium North Expression Proteomics Facility (Institutionen för Medicinsk Biokemi och Mikrobiologi, Uppsala University, Uppsala, Sweden).
Supplementary Material
Acknowledgments
We thank Dr. Bernt Eric Uhlin for helpful suggestions and critical reading of the manuscript and Daw Hla Hla Nyunt for encouragement. This work was supported by grants from the Swedish Research Council, the Swedish Foundation for International Cooperation in Research and Higher Education, Cancerfonden, The Wallenberg Foundation, and the Faculty of Medicine, Umeå University.
Abbreviations
- CT
cholera toxin
- Tcp
toxin-coregulated pili
- QS
quorum sensing.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Colwell R. R. J. Ind. Microbiol. Biotechnol. 1997;18:302–307. doi: 10.1038/sj.jim.2900390. [DOI] [PubMed] [Google Scholar]
- 2.Huq A., Colwell R. R., Rahman R., Ali A., Chowdhury M. A., Parveen S., Sack D. A., Russek-Cohen E. Appl. Environ. Microbiol. 1990;56:2370–2373. doi: 10.1128/aem.56.8.2370-2373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huq A., Sack R. B., Nizam A., Longini I. M., Nair G. B., Ali A., Morris J. G., Jr., Khan M. N., Siddique A. K., Yunus M., et al. Appl. Environ. Microbiol. 2005;71:4645–4654. doi: 10.1128/AEM.71.8.4645-4654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis V. R., Russek-Cohen E., Choopun N., Rivera I. N., Gangle B., Jiang S. C., Rubin A., Patz J. A., Huq A., Colwell R. R. Appl. Environ. Microbiol. 2003;69:2773–2785. doi: 10.1128/AEM.69.5.2773-2785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colwell R. R., Huq A. Ann. N. Y. Acad. Sci. 1994;740:44–54. doi: 10.1111/j.1749-6632.1994.tb19852.x. [DOI] [PubMed] [Google Scholar]
- 6.Huq A., Small E. B., West P. A., Huq M. I., Rahman R., Colwell R. R. Appl. Environ. Microbiol. 1983;45:275–283. doi: 10.1128/aem.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colwell R. R. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 8.Fenchel T. Annu. Rev. Ecol. Syst. 1988;19:19–38. [Google Scholar]
- 9.Sherr E. B., Sherr B. F. Antonie Van Leeuwenhoek. 2002;81:293–308. doi: 10.1023/a:1020591307260. [DOI] [PubMed] [Google Scholar]
- 10.Matz C., Kjelleberg S. Trends Microbiol. 2005;7:302–307. doi: 10.1016/j.tim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Roszak D. B., Colwell R. R. Microbiol. Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J., Miller M. B., Vance R. E., Dziejman M., Bassler B. L., Mekalanos J. J. Proc. Natl. Acad. Sci. USA. 2002;99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacikova G., Skorupski K. Mol. Microbiol. 2002;46:1135–1147. doi: 10.1046/j.1365-2958.2002.03229.x. [DOI] [PubMed] [Google Scholar]
- 14.Miller M. B., Skorupski K., Lenz D. H., Taylor R. K., Bassler B. L. Cell. 2002;110:303–314. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 15.Malloy J. L., Veldhuizen R. A., Thibodeaux B. A., O’Callaghan R. J., Wright J. R. Am. J. Physiol. 2005;288:L409–L418. doi: 10.1152/ajplung.00322.2004. [DOI] [PubMed] [Google Scholar]
- 16.Silva A. J., Benitez J. A. J. Bacterial. 2004;186:6374–6382. doi: 10.1128/JB.186.19.6374-6382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart-Tull D. E., Bleakley C. R., Galloway T. S. Vaccine. 2004;22:3026–3034. doi: 10.1016/j.vaccine.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Matz C., Bergfeld T., Rice S., Kjelleberg S. Environ. Microbiol. 2004;6:218–226. doi: 10.1111/j.1462-2920.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaper J. B., Morris J. G., Levine M. M. Clin. Microbiol. Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacket C. O., Losonsky G., Nataro J. P., Comstock L., Michalski J., Edelman R., Kaper J. B., Levine M. M. J. Infect. Dis. 1995;172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 21.Levine M. M., Noriega F. PNG Med. J. 1995;38:325–331. [PubMed] [Google Scholar]
- 22.Aballay A., Yorgey P., Ausubel F. M. Curr. Biol. 2000;10:1539–1542. doi: 10.1016/s0960-9822(00)00830-7. [DOI] [PubMed] [Google Scholar]
- 23.Alegado R. A., Campbell M. C., Chen W. C., Slutz S. S., Tan M. W. Cell Microbiol. 2003;5:435–444. doi: 10.1046/j.1462-5822.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 24.Darby C., Cosma C. L., Thomas J. H., Manoil C. Proc. Natl. Acad. Sci. USA. 1999;96:15202–15207. doi: 10.1073/pnas.96.26.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewbank J. J. Microbes Infect. 2002;4:247–256. doi: 10.1016/s1286-4579(01)01531-3. [DOI] [PubMed] [Google Scholar]
- 26.Labrousse A., Chavet S., Couillault C., Kurz C. L., Ewbank J. J. Curr. Biol. 2000;10:1543–1545. doi: 10.1016/s0960-9822(00)00833-2. [DOI] [PubMed] [Google Scholar]
- 27.Kurz C. L., Chauvet S., Andres E., Aurouze M., Vallet I., Michel G. P. F., Uh M., Celli J., Filloux A., de Betnzmann S., et al. EMBO J. 2003;22:1451–1460. doi: 10.1093/emboj/cdg159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan M. W., Rahme L. G., Sternberg J. A., Tompkins R. G., Ausubel F. M. Proc. Natl. Acad. Sci. USA. 1999;96:2408–2413. doi: 10.1073/pnas.96.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J., Mekalanos J. J. Dev. Cell. 2003;5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 31.Benitez J. A., Silva A. J., Finkelstein R. A. Infect. Immun. 2001;69:6549–6553. doi: 10.1128/IAI.69.10.6549-6553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson J. D., Umayam L. A., Dickinson T., Hickey E. K., White O. Nucleic Acids Res. 2001;29:123–125. doi: 10.1093/nar/29.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toma C., Honma Y. Infect. Immun. 1996;64:4495–4500. doi: 10.1128/iai.64.11.4495-4500.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogierman M. A., Fallarino A., Riess T., Williams S. G., Attridge S. R., Manning P. A. J. Bacteriol. 1997;179:7072–7080. doi: 10.1128/jb.179.22.7072-7080.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matz C., McDougald D., Moreno A. M., Yung P. Y., Yildiz F. H., Kjelleberg S. Proc. Natl. Acad. Sci. USA. 2005;102:16819–16824. doi: 10.1073/pnas.0505350102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pukatzki S., Ma A. T., Sturtevant D., Krastins B., Sarracino D., Nelson W. C., Heidelberg J. F., Mekalanos J. J. Proc. Natl. Acad. Sci. USA. 2006;103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X., Gao D. Q., Michalski J., Benitez J. A., Kaper J. B. Infect. Immun. 2004;72:389–397. doi: 10.1128/IAI.72.1.389-397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skorupski K., Taylor R. K. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 39.Laemmli U. K. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 40.Towbin H., Staehelin T., Gordon J. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shieh J. T., Bergelson J. M. J. Virol. 2002;76:9474–9480. doi: 10.1128/JVI.76.18.9474-9480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matz C., Jurgens K. Appl. Environ. Microbiol. 2001;67:814–820. doi: 10.1128/AEM.67.2.814-820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.