Abstract
The human malaria parasite Plasmodium falciparum relies on the acquisition of host purines for its survival within human erythrocytes. Purine salvage by the parasite requires specialized transporters at the parasite plasma membrane (PPM), but the exact mechanism of purine entry into the infected erythrocyte, and the primary purine source used by the parasite, remain unknown. Here, we report that transgenic parasites lacking the PPM transporter PfNT1 (P. falciparum nucleoside transporter 1) are auxotrophic for hypoxanthine, inosine, and adenosine under physiological conditions and are viable only if these normally essential nutrients are provided at excess concentrations. Transport measurements across the PPM revealed a severe reduction in hypoxanthine uptake in the knockout, whereas adenosine and inosine transport were only partially affected. These data provide compelling evidence for a sequential pathway for exogenous purine conversion into hypoxanthine using host enzymes followed by PfNT1-mediated transport into the parasite. The phenotype of the conditionally lethal mutant establishes PfNT1 as a critical component of purine salvage in P. falciparum and validates PfNT1 as a potential therapeutic target.
Keywords: conditional knockout, sequential pathway, transporter
Malaria is one of the foremost threats to human health in the tropical regions of the world (1). The disease is especially challenging to treat because of the widespread emergence of drug-resistant parasites and the limited number of available antimalarial drugs. Consequently, better antimalarial therapies are urgently needed. Plasmodium falciparum, the causative agent of the most deadly form of human malaria, exhibits a complex life cycle that involves both an invertebrate vector, the anopheline mosquito, and humans. Although the ability of the parasite to invade human RBCs provides it with an ideal refuge from immune attacks, RBCs are deficient in various nutrients required for parasite survival and multiplication. Therefore, the parasite has evolved novel transport machineries and specialized enzymes to acquire and metabolize host nutrients. Perhaps most striking is the nutritional requirement for purines. Unlike mammalian cells that synthesize purines de novo, P. falciparum is incapable of purine biosynthesis and has consequently evolved a unique complement of purine salvage enzymes that enable the parasite to scavenge host purines (2–4). The first step in purine acquisition entails the uptake of purines from the host milieu. Because of the essential function of purine salvage in parasite growth and multiplication, purine transporters are regarded as ideal targets for the development of novel therapeutic strategies to combat malaria (5).
Although it is known that the movement of purine across the parasite plasma membrane (PPM) requires specialized purine transporters (6), the pathways of purine translocation between the intracellular parasite and the host environment are unknown. Uninfected RBCs express a high-affinity human equilibrative nucleoside transporter, hENT1, that mediates the sodium-independent uptake of a broad spectrum of purine and pyrimidine nucleosides (7). RBCs also harbor adenosine deaminase and purine nucleoside phosphorylase enzymes, which convert adenosine and inosine into hypoxanthine; however, the role of host transporters and enzymes in purine salvage by the parasite also remains unknown. Furthermore, it is not known whether intraerythrocytic P. falciparum salvage a full complement of purine nucleosides and nucleobases, or only hypoxanthine, from the RBC cytosol. Recently, the purine transporter PfNT1 (P. falciparum nucleoside transporter 1) was identified and functionally characterized at the molecular level in P. falciparum (8–10). PfNT1 shares sequence and topological similarities with well characterized transporters of the eukaryotic equilibrative nucleoside transporter family. However, the primary sequence of PfNT1, its substrate specificity, kinetic properties, and inhibition profile are sufficiently different from hENT1 to suggest that, if PfNT1 plays an essential function in P. falciparum development and multiplication, then PfNT1 could be an excellent drug target.
Expression studies in Xenopus laevis oocytes revealed that PfNT1 has a broad ligand specificity for d- and l-nucleosides and for nucleobases (8, 10). In regard to this heterologous transport system, Carter et al. (8) showed that PfNT1 has a high affinity for adenosine and provided indirect evidence that hypoxanthine was not a high-affinity ligand (N.S.C., unpublished data), whereas Parker et al. (10) found that PfNT1 has a low affinity for both adenosine and hypoxanthine. Subsequent studies with infected RBCs revealed that PfNT1 is localized to the PPM and expressed throughout the intraerythrocytic life cycle, but is maximally expressed before parasite schizogony at the peak of nucleic acid utilization (9). Subsequent to the initial characterization of PfNT1, three other putative equilibrative nucleoside transporters, designated PfNT2, PfNT3, and PfNT4, have been identified within the P. falciparum genome. However, because these transporters have not yet been functionally authenticated, their contribution to parasite purine acquisition remains undetermined. To establish the importance of PfNT1 in parasite purine salvage, the PfNT1 gene was genetically disrupted in P. falciparum by using a positive and negative selection strategy (11). Our data indicated that, unlike WT parasites that could use hypoxanthine, inosine, or adenosine, transgenic parasites lacking PfNT1 were incapable of using physiological concentrations of these purines. The growth capability of pfnt1Δ parasites was restored, however, if hypoxanthine, inosine, or adenosine was provided at very high, nonphysiological concentrations in the culture medium. Furthermore, transport studies with RBC-free pfnt1Δ parasites revealed a severe reduction in transport capability for hypoxanthine, although adenosine and inosine uptake remained near WT levels. These data demonstrate (i) that PfNT1 performs an essential physiological function in purine salvage in P. falciparum, (ii) that the parasite exploits host enzymes for conversion of adenosine and inosine into hypoxanthine before translocation across the PPM, and (iii) that hypoxanthine uptake by PfNT1 is the principal route of purine salvage in P. falciparum. The essential role of PfNT1 in purine salvage validates PfNT1 as a rational target for antimalarial drug development.
Results
Disruption of the PfNT1 Gene in pKEΔPfNT Parasites.
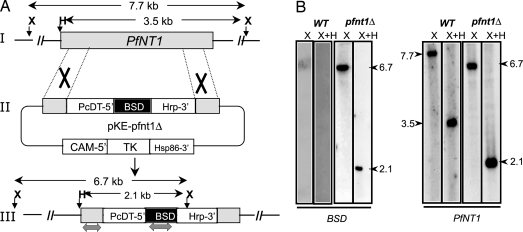
To evaluate the importance of PfNT1 in parasite development and survival, the generation of transgenic parasites lacking PfNT1 was attempted by using a targeted gene replacement approach (11). To achieve this goal, a targeting vector, pKEΔPfNT, was constructed (Fig. 1A). The overall knockout strategy entails a two-step process by which PfNT1 is first interrupted with the blasticidin S deaminase (BSD) (12) cassette after a double-crossover event (Fig. 1A), followed by loss of the episome after selection against thymidine kinase (TK) gene expression. This targeted gene-disruption strategy with double crossover caused a 255-bp truncation in the middle of the PfNT1 ORF. Because loss of PfNT1 was conjectured to be a potentially lethal event (if PfNT1 is the major route of purine salvage in P. falciparum), pfnt1Δ mutants were selected in medium supplemented with 1.5 mM hypoxanthine, a concentration ≈300-fold above that required for optimal growth of WT parasites. Transgenic parasites harboring the pKEΔPfNT targeting vector were first selected on blasticidin and then treated with ganciclovir to eliminate parasites containing the targeting cassette in an episomal form. Parasites were cloned by limiting dilution and genomic DNA from cloned parasites purified for molecular analysis. Diagnostic PCR analysis with various combinations of primer pairs demonstrated the integration of the PfNT1.5′-BSD-PfNT1.3′ cassette into the PfNT1 locus and the absence of intact WT PfNT1 in 59 individual clones (data not shown). Southern blot analyses on genomic DNA by using various restriction digestions and probes specific for PfNT1 or BSD further confirmed disruption of the PfNT1 chromosomal locus by a double crossover event (Fig. 1B).
Fig. 1.
Strategy for PfNT1 disruption by double crossover. (A) Gene disruption strategy. I, schematic representation of WT PfNT1 chromosomal locus; II, the plasmid vector pKEΔPfNT1 used for stable transformation of P. falciparum; III, the proposed model of integration of the plasmid by double crossover into the PfNT1 chromosomal locus. (B) Southern blot analysis of XbaI (X) and XbaI plus HindIII (X+H) digested genomic DNA from WT and pfnt1Δ parasites by using BSD-specific and PfNT1-specific probes (as indicated by the gray double-headed arrows in part III of A).
Loss of Expression of PfNT1 in the pfnt1Δ Knockout.
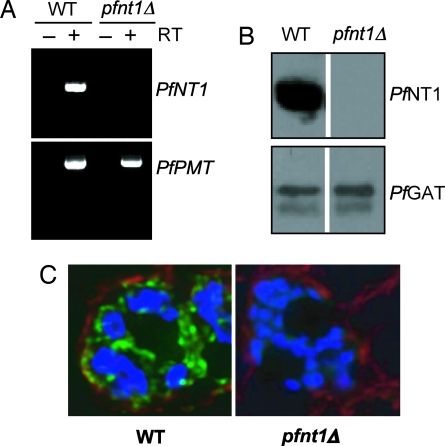
To demonstrate the loss of PfNT1 expression in a pfnt1Δ clone, RT-PCR analysis was performed on RNA purified from WT and knockout parasites by using primers within the PfNT1 ORF. PfNT1 cDNA was amplified from WT but not pfnt1Δ RNA (Fig. 2A). Loss of PfNT1 expression was further analyzed by immunoblotting with affinity-purified anti-PfNT1 antibodies (9). Whereas a 46-kDa PfNT1 band was detected in WT P. falciparum, no signal was identified in the pfnt1Δ parasites (Fig. 2B). As a loading control, antibodies raised against the parasite endoplasmic reticulum membrane protein P. falciparum glycerol-3-phosphate acyltransferase (PfGat) (13) were used, and the expected 64-kDa PfGat band was observed in both the WT and pfnt1Δ lysates (Fig. 2B). Immunofluorescence analysis corroborated the loss of PfNT1 expression in the pfnt1Δ clone and verified its localization to the PPM in WT P. falciparum (Fig. 2C) (9).
Fig. 2.
Altered expression of PfNT1 in the pfnt1Δ strain. (A) RT-PCR analysis of RNA isolated from WT and pfnt1Δ strains by using specific primers within the PfNT1 ORF (Upper). RT-PCR of phosphoethanolamine methyltransferase (PfPMT) cDNA is used as control (Lower). (B) Western blot analysis was performed by using protein extracts from asynchronous cultures of WT and pfnt1Δ strains with PfNT1 antibodies (Upper). PfGat was used as an internal positive control (Lower). (C) Immunofluorescence microscopy of WT-infected (Left) and pfnt1Δ-infected (Right) RBCs at the schizont stage. DNA was counterstained with Hoechst dye (blue). PfNT1 conjugated to the FITC-conjugated goat anti-rabbit secondary antibody appears in green; band 3 conjugated to Texas red-conjugated anti-mouse secondary antibody appears in red.
Purine Requirements for pfnt1Δ Parasites.
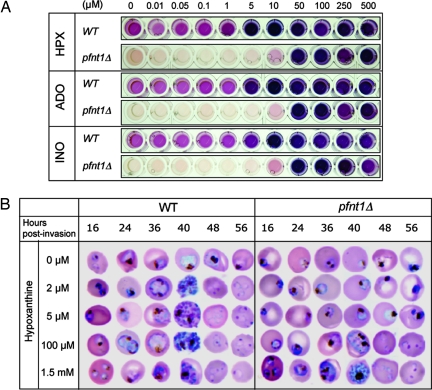
To assess the role of PfNT1 in purine acquisition during the parasite intraerythrocytic life cycle, WT and pfnt1Δ P. falciparum were grown in the presence of increasing concentrations of hypoxanthine, adenosine, or inosine, and parasite growth was monitored by measuring parasite lactate dehydrogenase production. Whereas optimal growth was observed in WT parasites at purine concentrations of 5 μM and above, pfnt1Δ parasites were not viable in adenosine, inosine, or hypoxanthine at concentrations below 50 μM (Fig. 3A). To measure the impact of the pfnt1Δ lesion on parasite intraerythrocytic development, WT and pfnt1Δ cells were synchronized and cultured in the absence or presence of increasing concentrations of hypoxanthine. Parasite progression through the life cycle (ring → trophozoite → schizont → ring) was monitored at various times after erythrocyte invasion. For both strains, lack of purine resulted in blockage of growth at the ring stage (Fig. 3B). However, although WT P. falciparum completed their entire intraerythrocytic cycle at concentrations of hypoxanthine as low as 2 μM (Fig. 3B), pfnt1Δ mutants required higher concentrations of hypoxanthine to progress beyond the ring stage of development (Fig. 3B). Similar results were obtained with adenosine and inosine (data not shown). Concentrations of hypoxanthine between 100 μM and 1.5 mM restored parasite progression throughout the cell cycle, although the growth of pfnt1Δ was slower than that of the WT strain at concentrations below 500 μM (data not shown). These results demonstrate that at physiological purine concentrations, between 0.4 and 6 μM (14), PfNT1 is essential for the intraerythrocytic development of the parasite, and that at higher concentrations, hypoxanthine, adenosine, and inosine could be transported by a secondary mechanism. The parasite could utilize adenosine and inosine either by direct transport of these substrates or by conversion of the nucleosides into hypoxanthine through the sequential actions of adenosine deaminase and purine nucleoside phosphorylase, respectively, before hypoxanthine uptake via PfNT1 (see below).
Fig. 3.
Growth of pfnt1Δ parasites in the presence of increasing concentrations of hypoxanthine (HPX), adenosine (ADO), or inosine (INO). (A) Parasite lactate dehydrogenase assay to detect the growth of the parasites. (B) Synchronized WT and pfnt1Δ parasites grown at different concentrations (0, 2, 5, 100, and 1,500 μM) of hypoxanthine were collected at different stages, analyzed by Giemsa stain, and evaluated by light microscopy.
Transport of Purines by pfnt1Δ Parasites.
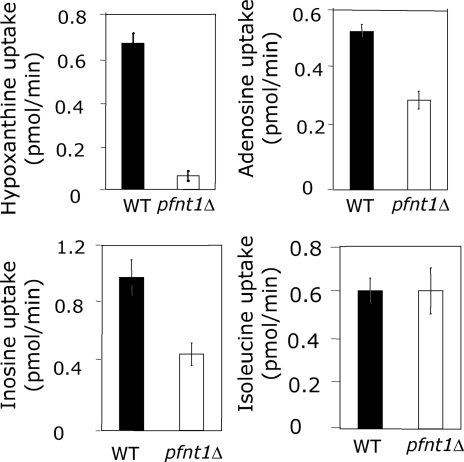
The inability of pfnt1Δ to grow at physiological concentrations of purine suggests that PfNT1 is the major route of purine translocation across the PPM. To test this supposition, both WT and pfnt1Δ parasites were purified from erythrocytes, and the parasites’ ability to transport radiolabeled hypoxanthine, adenosine, and inosine was measured. The transport of adenosine and inosine was diminished only slightly in the pfnt1Δ strain; however, the transport of hypoxanthine was reduced dramatically when compared with its WT parent (Fig. 4). These differences in transport between WT and pfnt1Δ parasites did not arise from general discrepancies in translocation across the PPM because transport of isoleucine was identical in both strains (Fig. 4). The ability of erythrocyte-liberated pfnt1Δ parasites to effectively transport adenosine and inosine demonstrates that the PPM harbors one or more additional permeases capable of transporting these nucleosides.
Fig. 4.
Purine uptake in WT and pfnt1Δ free parasites. Uptake of hypoxanthine, adenosine, and inosine in free WT and pfnt1Δ trophozoites was performed as described in Materials and Methods. Isoleucine uptake is used as a control. These experiments were performed at least three times, and each value is the mean ± SD of at least triplicate experiments.
PfNT1 Is Essential for Intraerythrocytic Development but Not for Erythrocyte Invasion.
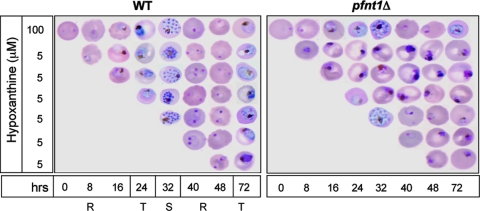
To determine whether the pfnt1 lesion is deleterious to P. falciparum parasites at all stages of intraerythrocytic development, WT and pfnt1Δ strains were synchronized and transferred at the ring, trophozoite, or schizont stage from medium containing 100 μM hypoxanthine to medium containing 5 μΜ hypoxanthine, and their continued development was monitored. Although WT parasites exhibited normal progression from stage to stage throughout their entire intraerythrocytic development, pfnt1Δ parasites failed to transform from ring to trophozoite or from trophozoite to schizont stage (Fig. 5). However, the reduction in exogenous hypoxanthine concentration did not affect the ability of pfnt1Δ merozoites to invade new erythrocytes after the rupture of infected erythrocytes during schizogony (Fig. 5).
Fig. 5.
PfNT1 is required for parasite intraerythrocytic development but is not essential for rupture of schizont-infected erythrocytes or invasion. WT and pfnt1Δ strains were synchronized and transferred at different stages [ring (R), trophozoite (T), and schizont (S)] from medium containing 100 μM hypoxanthine to medium containing 5 μM hypoxanthine. Parasite development was then monitored, by Giemsa staining and microscopy, every 8 hr after starvation.
Discussion
The ability to create a conditionally lethal pfnt1Δ mutant in P. falciparum establishes that PfNT1 is absolutely indispensable for purine acquisition by P. falciparum under physiological circumstances. P. falciparum cannot synthesize purine nucleotides de novo and obligatorily depend on purine salvage from the host for their synthesis of nucleotides and nucleic acids, and consequently for their ability to proliferate and cause disease. The loss of PfNT1 activity results in inhibition of parasite growth under physiological conditions under which concentrations of salvageable purines have been reported to be between 0.4 and 6 μM (14). This growth defect can be suppressed by the addition of excessive, nonphysiological levels of hypoxanthine, adenosine, or inosine, thus enabling purine acquisition by some additional but unknown mechanism and pharmacologically circumventing the genetic consequences of the conditionally lethal mutation.
The Role of PfNT1 in Hypoxanthine Uptake Across the PPM.
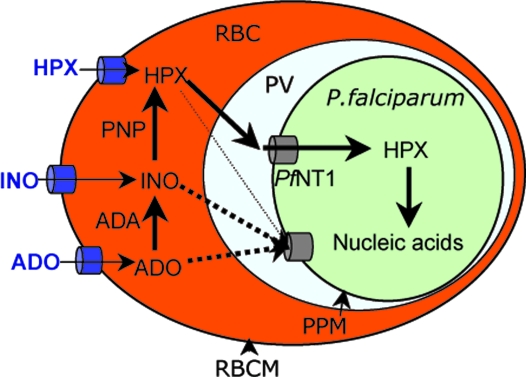
Purine transporters have been identified in several parasitic protozoa, and their biochemical properties and sensitivities to inhibitors have been determined in a number of heterologous expression systems within null backgrounds (15). By using a parallel approach, PfNT1 was shown to have a relatively high affinity for adenosine and a lower affinity for inosine and hypoxanthine (8, 10). Interestingly, in the present study pfnt1Δ parasites were inviable at physiological concentrations of hypoxanthine, adenosine, or inosine and exhibited relatively normal intraerythrocytic cycle only at higher concentrations of these substrates. These results demonstrated that, under physiological conditions, PfNT1 is essential for hypoxanthine, adenosine, and inosine uptake into the parasite, and that at higher concentrations, these substrates could be transported by means of a secondary mechanism. The ability of RBC-liberated pfnt1Δ parasites to effectively transport adenosine and inosine demonstrates that the PPM harbors one or more additional permeases capable of transporting these nucleosides. Because nutrient limitation studies verified that adenosine and inosine do not support growth of the pfnt1Δ mutant at physiological concentrations, these data imply that these nucleosides do not reach the PPM membrane in their native form. Rather, adenosine and inosine are converted in the RBC cytoplasm into a PfNT1 substrate (Fig. 6). Hypoxanthine is the most likely candidate for this ligand, because RBCs are known to express high levels of both adenosine deaminase and purine nucleoside phosphorylase activities (16, 17), which sequentially metabolize adenosine to inosine and then hypoxanthine. This hypothesis is supported by the fact that P. falciparum-infected RBCs are capable of converting radiolabeled adenosine into hypoxanthine in the RBC cytoplasm and, subsequently, radiolabeled hypoxanthine can be detected in the parasite cytoplasm (2, 18).
Fig. 6.
Model for sequential purine uptake pathways in P. falciparum-infected erythrocytes. Hypoxanthine (HPX), adenosine (ADO), and inosine (INO) are transported into the RBC cytoplasm by means of endogenous purine transporters. Inside the erythrocyte cytoplasm, adenosine and inosine are first converted into hypoxanthine before being transported, along with transported hypoxanthine, across the PPM into the parasite cytoplasm. Alternative routes for entry of adenosine and inosine are represented by dotted lines. These additional transporters are unlikely to play an important role under physiological conditions. See Discussion for details. PNP, purine nucleoside phosphorylase; ADA, adenosine deaminase; PV, parasitophorous vacuole; RBCM, RBC membrane.
Nutrient Uptake into P. falciparum Is Sequential.
In the past 30 years, three models have been proposed to account for the movement of nutrients between the host and the intracellular parasite (19). In the first model, referred to as the “sequential pathway,” nutrients are first transported across the RBC membrane into the erythrocyte cytoplasm and then translocated into the parasite after crossing the parasitophorous vacuolar membrane and PPM by means of specialized PPM transporters. In the second model, referred to as the “endocytosis model,” nutrients are first transported across the RBC membrane into the erythrocyte cytoplasm and then progress into the parasite by endocytosis. The third model involves specialized entry mechanisms, referred to as “parallel pathways,” that allow direct movement of nutrients between the host medium and the parasite without entering the erythrocyte cytoplasm. Our results strongly support a sequential pathway mechanism for entry of exogenous purines into P. falciparum (Fig. 6), because the parasite harbors additional pathways capable of transporting adenosine and inosine, although at physiological concentrations these purines are not capable of supporting the growth of knockout parasites. This finding supports the conjecture that these nucleosides do not gain direct access to the parasite and that they require prior conversion into hypoxanthine, reactions that occur within the RBC cytoplasm.
The Role of Other Permeases in Malarial Purine Uptake.
Growth of pfnt1Δ could be maintained only at high concentrations of hypoxanthine, adenosine, or inosine. Furthermore, transport studies in free parasites revealed that the PPM harbors additional mechanisms capable of transporting adenosine or inosine. The completed genome sequence of P. falciparum revealed three nucleoside permease candidates, all members of the equilibrative nucleoside transporter family, for purine permeases (20). Whether these putative permeases are capable of recognizing and transporting nucleosides, and whether they are expressed at the PPM, are issues that await further investigation. However, our results suggest that additional PPM permeases are unlikely to play an important role in parasite intraerythrocytic development and that PfNT1 is the major route of purine uptake under physiological situations and is essential for intraerythrocytic parasite development.
In conclusion, our results validate the purine salvage pathway as a viable drug target in P. falciparum. The conditional lethality of the pfnt1Δ mutation in P. falciparum establishes that PfNT1 is absolutely indispensable for purine acquisition by P. falciparum under physiological purine conditions and that PfNT1 is the major route for purine salvage. This study also substantiates that erythrocyte enzymes funnel exogenous adenosine and inosine through hypoxanthine before translocation into the parasite by PfNT1. Finally, our results promote PfNT1 as a drug target. Although PfNT1 shares sequence and topological similarities with hENT1, the major nucleoside transporter in human cells, including erythrocytes, the ligand specificity, kinetic parameters, and inhibition profile of PfNT1 are all sufficiently different from hENT1 to make PfNT1 an attractive target for selective therapeutic design.
Materials and Methods
Construction of Transfection Plasmids.
To construct the targeting vector pRZ/TK/PfNT1.5′-BSD-PfNT1.3′ for PfNT1 disruption, a 482-bp fragment of PfNT1 (nucleotides 50–532 of the ORF) was amplified by PCR and subcloned into the HindIII/BlpI site upstream of the PcDT promoter in the pRZ-TK-BSD2 vector. This plasmid encompasses the positive selectable marker BSD (12) from Aspergillus terreus that confers resistance to blasticidin and whose expression in P. falciparum is under the regulatory control of the Plasmodium chabaudi dihydrofolate reductase/thymidylate synthase promoter and the negative marker TK from Herpes simplex that confers sensitivity to ganciclovir and whose expression is under the regulatory control of the P. falciparum cell adhesion molecule (CAM) promoter. A second PCR was used to amplify a 482-bp fragment of PfNT1 (nucleotides 772-1255 of the ORF) for directional cloning at the EcoRI site downstream of the HrpII terminator in the pRZ-TK-BSD2 vector.
Disruption of the PfNT1 Locus.
Packed P. falciparum strain 3D7-infected human RBCs (100 μl) were mixed with ≈100 μg of plasmid DNA in 400 μl of cytomix and electroporated at 0.31 kV and 950 μF in a 0.2-cm cuvette by using a Gene Pulser II (Bio-Rad). After 48 hr, 2.5 μg/ml blasticidin was added. Blasticidin-resistant parasites were observed after 3 weeks. To eliminate parasites containing episomes, ganciclovir, a subversive substrate of the TK enzyme, was applied for an additional 3 weeks. Surviving parasites were cloned by limiting dilution. Note that parasites were cultured as described in ref. 21, except that 1.5 mM hypoxanthine was added to the medium for transfectants.
Southern Blot Hybridization of the Disrupted PfNT1 Locus.
Ten micrograms of genomic DNA from WT and pfnt1Δ parasites was digested with XbaI and HindIII, separated on a 0.8% agarose gel, and transferred to positively charged nylon membrane (Roche Molecular Biochemicals). Membranes were probed with [α-32P]dCTP-labeled PfNT1 and BSD probes.
Western Blot Analysis.
Western blot analysis was performed, as described in refs. 9 and 13, on protein extracts from asynchronous cultures of WT and pfnt1Δ strains. Blots were probed with affinity-purified antibodies to PfNT1 (1:1,000) and PfGat (1:1,000), a marker that served as a loading control.
Microscopy.
To determine the parasite life cycle stage, infected RBCs were Giemsa-stained and analyzed by bright-field microscopy. For immunofluorescence microscopy, asynchronous cultures of P. falciparum were prepared essentially as described in ref. 9. Coverslips were incubated at room temperature with gentle shaking for 1 hr with both affinity-purified PfNT1 antibodies at a concentration of 1:500 and mouse monoclonal antibodies to erythrocyte band 3 protein from Sigma, which were used at a concentration of 1:500. The coverslips were washed to remove excess antibody and incubated with anti-rabbit antibody conjugated to FITC and anti-mouse secondary antibody conjugated to Texas red dye (Molecular Probes) for 1 hr at room temperature to visualize the PfNT1 and band 3 antibodies. Nuclei were stained by incubating the coverslips in PBS containing 3 μg/ml Hoechst 33258 stain (Molecular Probes) for 5 min at room temperature. The coverslips were washed and then mounted on slides with Antifade (Molecular Probes), and images were analyzed by high-resolution fluorescence using deconvolution protocols. Microscopy was performed with an inverted microscope (Eclipse TE2000-E; Nikon) and filter 96320/HYQ (excitation 480–440 nm/emission 440 nm) for FITC, filter 96312/G2EC (excitation 540–525 nm/emission 620–660 nm) for rhodamine, and filter 96310/UV2EC (excitation 360–340 nm/emission 460–450 nm) for DAPI.
Parasite Lactate Dehydrogenase Assay for Detecting Parasites.
The reagents for the parasite lactate dehydrogenase assay, 3-acetylpyridine adenine dinucleotide (APAD), nitroblue tetrozolium (NBT), and diaphorase, were obtained from Sigma-Aldrich. The malstat reagent was prepared by mixing 13 mg/ml Tris·Cl (pH 9.0), 20 mg/ml lithium l-lactate, 0.66 mg/ml APAD, and 0.2% Triton X-100. To perform the parasite lactate dehydrogenase assay, 20 μl of infected RBCs were mixed with 10 μl of 1 mg/ml diaphorase, 10 μl of 1 mg/ml NBT, and 100 μl of malstat reagent in each well of the 96-well plate. Absorbance was measured at 650 nm by using a plate reader and was always proportional to the parasitemia detected by Giemsa-stained blood smears.
Uptake Assays of Nucleosides and Nucleobases on Free Trophozoites.
WT and pfnt1Δ-infected RBCs were synchronized by using sorbitol (22), and trophozoites were harvested by centrifugation at 720 × g for 5 min at 4°C. The cell pellet was gently resuspended in cold PBS containing 0.005% saponin and again centrifuged as described above. The pellet was washed three times in cold PBS, resuspended in cold PBS supplemented with 20 mM glucose, and used immediately for transport assays. The cells were incubated with 1 μM [3H]adenosine (40.3 Ci/mmol; 1 Ci = 37 GBq), [3H]inosine (50 Ci/mmol), [3H]hypoxanthine (30 Ci/mmol), or [3H]isoleucine (92 Ci/mmol) at 37°C or 4°C for 1.5 min (linear phase of uptake), after which they were rapidly applied to glass fiber filters and washed twice with 5 ml of cold PBS. The filters were then dried, and radioactivity was measured by scintillation spectrometry.
Acknowledgments
We thank Jill Zimmerman and Harriett Zawistowski (General Clinical Research Center, University of Connecticut Health Center) for their technical help. This work was supported by National Institute of Allergy and Infectious Disease Grant AI51507 (to C.B.M. and B.U.) and by the Department of Defense (C.B.M.). This work was also supported in part by National Institutes of Health General Clinical Research Center Grant M01RR06192 (to the University of Connecticut Health Center).
Abbreviations
- hENT1
human equilibrative nucleoside transporter 1
- PfNT1
Plasmodium falciparum nucleoside transporter 1
- PPM
parasite plasma membrane
- BSD
blasticidin S deaminase
- TK
thymidine kinase
- PfGat
P. falciparum glycerol-3-phosphate acyltransferase.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.World Health Organization. WHO Tech. Rep. Ser. 2000;892:1–74. [PubMed] [Google Scholar]
- 2.Gero A. M., O’Sullivan W. J. Blood Cells. 1990;16:467–484. discussion 485–498. [PubMed] [Google Scholar]
- 3.Sherman I. W. In: Malaria: Parasite Biology, Pathogenesis, and Protection. Sherman I. W., editor. Washington, DC: Am. Soc. Microbiol.; 1998. pp. 177–184. [Google Scholar]
- 4.Sherman I. W. Microbiol. Rev. 1979;43:453–495. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gero A. M., Upston J. M. Adv. Exp. Med. Biol. 1994;370:493–498. doi: 10.1007/978-1-4615-2584-4_104. [DOI] [PubMed] [Google Scholar]
- 6.Upston J. M., Gero A. M. Biochim. Biophys. Acta. 1995;1236:249–258. doi: 10.1016/0005-2736(95)00055-8. [DOI] [PubMed] [Google Scholar]
- 7.Boleti H., Coe I. R., Baldwin S. A., Young J. D., Cass C. E. Neuropharmacology. 1997;36:1167–1179. doi: 10.1016/s0028-3908(97)00136-6. [DOI] [PubMed] [Google Scholar]
- 8.Carter N. S., Ben Mamoun C., Liu W., Silva E. O., Landfear S. M., Goldberg D. E., Ullman B. J. Biol. Chem. 2000;275:10683–10691. doi: 10.1074/jbc.275.14.10683. [DOI] [PubMed] [Google Scholar]
- 9.Rager N., Ben Mamoun C., Carter N. S., Goldberg D. E., Ullman B. J. Biol. Chem. 2001;276:41095–41099. doi: 10.1074/jbc.M107037200. [DOI] [PubMed] [Google Scholar]
- 10.Parker M. D., Hyde R. J., Yao S. Y., McRobert L., Cass C. E., Young J. D., McConkey G. A., Baldwin S. A. Biochem. J. 2000;349:67–75. doi: 10.1042/0264-6021:3490067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duraisingh M. T., Triglia T., Cowman A. F. Int. J. Parasitol. 2002;32:81–89. doi: 10.1016/s0020-7519(01)00345-9. [DOI] [PubMed] [Google Scholar]
- 12.Ben Mamoun C., Gluzman I. Y., Goyard S., Beverley S. M., Goldberg D. E. Proc. Natl. Acad. Sci. USA. 1999;96:8716–8720. doi: 10.1073/pnas.96.15.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santiago T. C., Zufferey R., Mehra R. S., Coleman R. A., Ben Mamoun C. J. Biol. Chem. 2004;279:9222–9232. doi: 10.1074/jbc.M310502200. [DOI] [PubMed] [Google Scholar]
- 14.Traut T. W. Mol. Cell. Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 15.Carter N. S., Landfear S. M., Ullman B. Trends Parasitol. 2001;17:142–145. doi: 10.1016/s1471-4922(00)01806-7. [DOI] [PubMed] [Google Scholar]
- 16.Daddona P. E., Kelley W. N. J. Biol. Chem. 1977;252:110–115. [PubMed] [Google Scholar]
- 17.Lewis A. S., Lowy B. A. J. Biol. Chem. 1979;254:9927–9932. [PubMed] [Google Scholar]
- 18.Yamada K. A., Sherman I. W. Mol. Biochem. Parasitol. 1981;2:349–358. doi: 10.1016/0166-6851(81)90086-4. [DOI] [PubMed] [Google Scholar]
- 19.Kirk K. Physiol. Rev. 2001;81:495–537. doi: 10.1152/physrev.2001.81.2.495. [DOI] [PubMed] [Google Scholar]
- 20.Martin R. E., Henry R. I., Abbey J. L., Clements J. D., Kirk K. Genome Biol. 2005;6:R26. doi: 10.1186/gb-2005-6-3-r26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trager W., Jensen J. B. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 22.Lambros C., Vanderberg J. P. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]