Abstract
There are three known high-affinity targets for cocaine: the dopamine transporter (DAT), the serotonin transporter (SERT), and the norepinephrine transporter (NET). Decades of studies support the dopamine (DA) hypothesis that the blockade of DAT and the subsequent increase in extracellular DA primarily mediate cocaine reward and reinforcement. Contrary to expectations, DAT knockout (DAT-KO) mice and SERT or NET knockout mice still self-administer cocaine and/or display conditioned place preference (CPP) to cocaine, which led to the reevaluation of the DA hypothesis and the proposal of redundant reward pathways. To study the role of DAT in cocaine reward, we have generated a knockin mouse line carrying a functional DAT that is insensitive to cocaine. In these mice, cocaine suppressed locomotor activity, did not elevate extracellular DA in the nucleus accumbens, and did not produce reward as measured by CPP. This result suggests that blockade of DAT is necessary for cocaine reward in mice with a functional DAT. This mouse model is unique in that it is specifically designed to differentiate the role of DAT from the roles of NET and SERT in cocaine-induced biochemical and behavioral effects.
Keywords: addiction, amphetamine, conditioned place preference, knockin
Cocaine inhibits the dopamine transporter (DAT), serotonin transporter (SERT), and norepinephrine transporter (NET) with similar potencies and elevates extracellular concentrations of these monoamine neurotransmitters, thereby producing complex neurochemical and behavioral effects (1, 2). However, there is a wealth of evidence indicating that the dopaminergic system, especially DAT, is most important in mediating cocaine’s addictive properties (3–6). For instance, the potencies of cocaine analogs for producing self-administration, a measure of drug reward, correlate to their affinities for binding DAT, but not SERT or NET (1, 6). Among the drugs that block all three transporters, those with a high affinity for DAT are self-administered or produce conditioned place preference (CPP), another measure of drug reward, whereas SERT or NET selective inhibitors do not produce reward in WT animals (7–9).
The generation of DAT knockout (DAT-KO) mice (10) allowed a direct test of whether DAT inhibition is required for cocaine reward. Contrary to the expectations, these mice still self-administer cocaine (11) and exhibit cocaine-induced CPP (12, 13). These results suggest that DAT inhibition is not solely required for cocaine reward, at least in DAT-KO mice, leading to the reevaluation of the dopamine (DA) hypothesis and the proposal that redundant systems might mediate cocaine reward (14–16). However, complete deletion of DAT causes tremendous adaptive changes in DA homeostasis, including alterations in DA synthesis, storage, extracellular levels, and receptor expression and functions (10, 17). These adaptive changes may significantly alter normal reward pathways. For instance, fluoxetine and nisoxetine, selective inhibitors for SERT and NET, respectively, produce CPP in DAT-KO mice but not in WT mice (9). More importantly, cocaine still increases extracellular DA in the nucleus accumbens (NAc) of DAT-KO mice, probably through NET and/or SERT inhibition, suggesting that the elevated extracellular DA might still underlie the mechanism of cocaine reward in mice lacking DAT (18, 19).
Instead of removing the major cocaine target by knocking out DAT, an alternative way to directly test the DA hypothesis of cocaine reward is to generate a mouse line that bears a mutant DAT that can still transport DA but has substantially reduced sensitivity to cocaine. Therefore, doses of cocaine that normally inhibit activity of WT DAT would not significantly inhibit this mutant DAT. This mouse line would provide a unique tool for differentiating the role of DAT inhibition from the roles of NET and SERT inhibition in cocaine reward.
Given the different chemical structures of DA and cocaine, it is likely that mutations at certain residues in DAT may have a greater impact on cocaine binding than on DA transport. Therefore, it should be possible to generate a functional DAT mutant that is much less sensitive to cocaine inhibition. As previously reported, we determined by species scanning mutagenesis that residue F105 in transmembrane domain 2 (TM2) of mouse DAT is important for high-affinity cocaine binding (20). Through three rounds of random mutagenesis at F105 and adjacent residues, we identified a mouse DAT mutant with the triple mutations L104V/F105C/A109V that retains >50% transport activity but is ≈70-fold more insensitive to cocaine inhibition than WT DAT in cultured cells (21). It has been shown that TM2 is inaccessible, based on biochemical data (22, 23) and the recently solved structure of LeuT (a bacterial homolog of DAT) (24), which suggests that the reduced cocaine affinity is likely an indirect effect of the triple mutations. Because heterozygous (DAT+/−) and homozygous (DAT−/−) DAT-KO mice with DAT activity reduced to 50% and 0% still exhibit cocaine reward, the reduction of DAT activity in itself should not preclude cocaine reward. Therefore, the triple mutant DAT was used to generate the DAT knockin mouse line.
Results
Generation of the Knockin Mouse Line.
The triple mutations were introduced into the mouse DATgene by homologous recombination in mouse ES cells (129/Sv) as depicted in Fig. 1A. A knockin mouse line was generated by injecting the positive ES cells into blastocysts of C57BL/6J mice. The targeted DAT gene had a LoxP flanked selection marker [a neomycin resistance gene with its promoter and poly(A) signal] inserted in intron 3. To minimize possible interferences with DAT expression, the selection marker was removed by crossing with mice that overexpressed Cre recombinase. PCR (Fig. 1 B–D) and Southern blotting (Fig. 1E) indicated the correct homologous recombination. The modified region of the DAT gene was PCR amplified and sequenced, confirming the presence of the intended modifications. Gross and microscopic examinations of 40 tissues, including the brain, the spinal cord, and all other major organs, revealed no significant anatomic differences between homozygous mutant mice with cocaine-insensitive DAT (DAT-CI mice) and their WT littermates. Unlike DAT-KO mice, DAT-CI males and females were fertile and produced normal-sized litters.
Fig. 1.
Generation of DAT mutant knockin mice. (A) Targeting strategy. Thin lines, mouse genomic DNA; thick lines, sequences included in the targeting construct; K, KpnI sites; open box, mDAT exon 3; open box with a line, exon 3 with the triple mutation L104V/F105C/A109V and a KpnI site; open arrow, Neo cassette; triangles, LoxP sites; shaded arrow, thymidine kinase gene; shaded box, probe for Southern blot analysis; arrows, PCR primers. (B) PCR using primer f1 external of the short arm and mutant-specific primer r1. (C) PCR using specific primer f2 and primer r2 external of the long arm. (D) PCR using primers f3 and r3, which amplify a 564-bp fragment from the WT allele and a 667-bp fragment from the mutant allele. (E) Southern blot analysis of mouse genomic DNA digested with KpnI; the additional KpnI in the mutant allele reduces the probed fragment from 4.2 to 3.4 kb. HET, heterozygous; DAT-CI, homozygous mutant mice with cocaine-insensitive DAT; M, DNA marker.
Brain DAT Expression Levels and DA Contents.
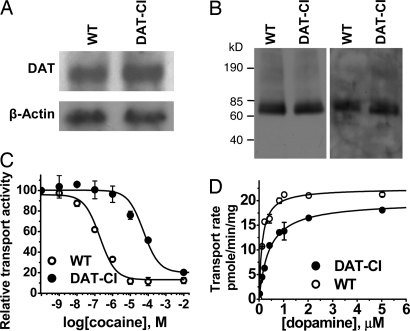
Northern and Western blot analyses did not reveal significant differences in brain DAT mRNA and protein levels between WT and DAT-CI mice (Fig. 2A and B), in contrast to DAT-KO mice that do not express DAT. The surface DAT expressions, assessed by biotinylation of stiatal synaptosomes, were also similar in DAT-CI and WT mice (Fig. 2B). However, HPLC analyses of the whole-brain DA contents indicated a statistically significant reduction (P < 0.05, t test; n = 4) of ≈40% in DAT-CI mice compared with that in WT mice (0.35 ± 0.03 vs. 0.58 ± 0.10 ng/mg of wet tissue). The reduction of DA content in our DAT-CI mice is similar to the reported 30% DA reduction in DAT+/− mice but is unlike the 95% reduction in DAT−/− mice (25).
Fig. 2.
Expression and pharmacological properties of mutant DAT in the knockin mice. (A and B) Northern (A) and Western (B) blot analysis of total (Left) or cell surface (Right) proteins did not reveal any significant difference (P > 0.05, t test; n = 3–5) in DAT expression levels between WT and DAT-CI mice. DAT activity was measured in striatal synaptosomes in the presence of increasing concentrations of cocaine or unlabeled DA. (C) The mutant DAT was 89-fold more insensitive to cocaine inhibition than the WT DAT. (D) The maximum DA uptake activities (Vmax) did not differ significantly between DATs from WT and DAT-CI mice, but apparent affinity (Km) of the mutant DAT was significantly higher (t test; n = 7) than that of the WT DAT.
Synaptosomal DA Uptake Kinetics and Cocaine Inhibition.
Synaptosomal DA uptake assays were performed to evaluate cocaine sensitivity and DAT activity in mice. The modified DAT from DAT-CI mice was 89-fold more insensitive to cocaine inhibition (IC50 = 35 ± 4 μM, n = 7) than DAT from WT mice (IC50 = 0.39 ± 0.08 μM, n = 6; Fig. 2C), further confirming the intended modification of the DAT gene. Although the Vmax values of DA transport for WT and mutant DAT mice (19 ± 3 and 21 ± 3 pmol/min per mg of protein, respectively) were not significantly different, the Km value for DAT from DAT-CI mice (455 ± 64 nM) was significantly higher (P < 0.05, t test; n = 4) than that from WT mice (200 ± 22 nM; Fig. 2D). This is different from the observation in cell cultures (21). The higher Km of mutant DAT might result in a reduced uptake rate at low DA concentrations and a higher basal extracellular DA concentration.
DA Release and Reuptake and Cocaine Effect by Fast Cyclic Voltammetry (FCV) in Brain Slices.
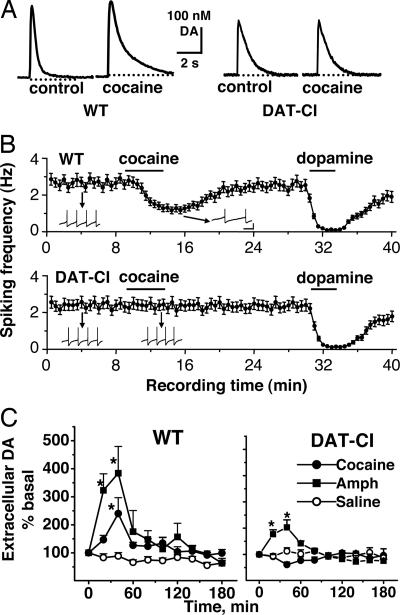
We also examined the effect of cocaine on DA release and reuptake in brain slices of WT and DAT-CI mice with FCV. Brain slices containing NAc were perfused in the bath solution with or without cocaine. DA release was triggered with local electrical stimulations, and DA concentrations were measured with FCV. The amplitude of the DA peak reflects the amount of DA release, and the rate of DA signal decay measures primarily the uptake activity. As shown in Fig. 3A, the amplitude of DA release was 25% lower (P < 0.05, t test) and DA decay was 3-fold slower (P < 0.05, t test) in DAT-CI mice than in WT mice. These changes are comparable to those in DAT+/− mice with 35% lower DA release and 2-fold slower DA decay but are quite different from DAT−/− mice with 75% lower DA release and 300-fold slower DA decay (25). The reduced DA release and slower DA decay in DAT-CI mice agree with their lower DA content and higher Km, respectively. More importantly, as shown in Fig. 3B, 4 μM cocaine prolonged the DA decay time by 2.6-fold in WT mice (P < 0.05, t test) but had no significant effect in DAT-CI mice (P > 0.05, t test), confirming the substantial reduction of DAT sensitivity to cocaine blockade.
Fig. 3.
Differential effects of cocaine on DA release, uptake, and DA neuron firing between genotypes measured in vitro and in vivo. (A) FCV was used to measure DA release and reuptake in accumbal slices in the absence or presence of 4 μM cocaine. Cocaine prolonged the DA decay time from brain slices of WT mice but not of DAT-CI mice. (B) The frequencies of spontaneous action potentials in DA neurons were recorded with whole-cell patch clamp and presented as mean ± SEM. The effects of bath application of 5 μM DA and 5 μM cocaine are shown. Cocaine significantly decreased the frequency of DA neuron firing in WT mice but not in DAT-CI mice. Insets show actual spike traces. Spikes were truncated for display. (Scale bars: 500 ms and 20 mV.) (C) Extracellular DA in the NAc was assessed by microdialysis in free-moving mice. The average of the two samples before the treatment (i.p.) of cocaine (20 mg/kg), AMPH (2.5 mg/kg), or saline was used as the baseline (100%, 0 min). Cocaine significantly increased extracellular DA level in the NAc of WT mice (F1,113 = 28.4; P < 0.0001) but not in DAT-CI mice (F1,113 = 2.89; P > 0.05). AMPH elevated extracellular DA level in both WT (F1,81 = 52.0; P < 0.0001) and DAT-CI (F1,81 = 6.5; P < 0.05) mice. Two-way ANOVAs were performed. Data represent mean ± SEM (n = 5–8).
DA Neuron Firing and Cocaine Effect by Whole-Cell Patch Clamp Recording.
It is well established that DAT blockade by cocaine causes DA accumulation and activation of the presynaptic inhibitory D2 autoreceptor, resulting in reduced spontaneous firing in DA neurons (26). In DAT-KO mice, the DA system is compromised, as indicated by a total loss of D2 autoreceptor function (17). We examined D2 receptor function and cocaine effects in our knockin mice by monitoring spontaneous firing in DA neurons with whole-cell patch clamp recording. Fig. 3B shows that DA neurons from WT and DAT-CI mice fired spontaneous action potentials at similar frequencies (2.7 ± 0.3 vs. 2.3 ± 0.3 Hz, n = 6). The firing was almost completely inhibited by bath application of 5 μM DA in both genotypes (n = 6), indicating that the D2 autoreceptor in WT and DAT-CI mice was equivalently functional. However, a dose of 5 μM cocaine inhibited spontaneous firing by 53.2 ± 4.8% in WT mice (n = 6) and had no significant effect on DA neuron firing in DAT-CI mice (n = 6), which is in agreement with the reduced sensitivity of the mutant DAT to cocaine inhibition.
Extracellular DA Concentrations and Cocaine Effect by in Vivo Microdialysis.
We performed microdialysis to measure extracellular DA concentrations in free-moving mice. The basal dialysate from the NAc of DAT-CI and WT mice contained 37.0 ± 6.8 and 22.5 ± 4.1 fmol/20 μl of DA, respectively (P < 0.05, t test). DAT-CI mice had 64% higher basal extracellular DA levels than WT mice. The higher extracellular DA concentration paralleled the higher Km of mutant DAT mice. In comparison, DAT+/− and DAT−/− mice have 70% and 500% higher basal extracellular DA concentrations, respectively, compared with WT mice (25).
A dose of 20 mg/kg cocaine significantly increased extracellular DA in the NAc of WT mice as indicated in Fig. 3C but not in DAT-CI mice. The absence of cocaine-induced extracellular DA elevation in DAT-CI mice is in contrast to cocaine-induced extracellular DA increases in DAT-KO mice (18, 19). Importantly, 2.5 mg/kg amphetamine (AMPH) elevated accumbal DA in both WT mice and DAT-CI mice, as shown in Fig. 3C, but the amplitude of the extracellular DA increase was smaller in DAT-CI mice than that in WT mice.
Cocaine Effect on Locomotor Stimulation.
DAT-CI mice displayed significantly higher baseline locomotor activity than WT mice, which might be explained by the elevated basal extracellular DA as indicated in microdialysis. In addition, novelty seeking may have contributed to the increased locomotion in our DAT-CI mice, similar to that observed in DAT-KO mice. As expected, doses of cocaine ranging from 10 to 40 mg/kg increased locomotion in WT mice (Fig. 4A) but did not increase locomotion in DAT-CI mice. Interestingly, cocaine appeared to produce locomotor suppression in DAT-CI mice in a dose-dependent manner. However, 10 mg/kg AMPH and 10 mg/kg morphine were still able to stimulate locomotor activity in DAT-CI mice (Fig. 4 B and C), in contrast to the lack of locomotor stimulation by AMPH and morphine in DAT-KO mice. As shown in Fig. 4B, 5 mg/kg AMPH increased locomotion in WT mice but not in DAT-CI mice. The weakened locomotor stimulation by AMPH in DAT-CI mice paralleled the smaller extracellular DA increase induced by AMPH in comparison with WT mice.
Fig. 4.
Locomotor activity in WT and DAT-CI mice. The locomotor activities are presented as horizontal distances traveled (in centimeters) in 15 or 120 min (mean ± SEM) after drug injection in mice (n = 6–12). (A) Cocaine stimulated locomotion (15 min) in WT mice but suppressed it in DAT-CI mice (for genotype difference, F1,33 = 32.16 and P < 0.0001; for genotype × drug interaction, F2,33 = 21.84 and P < 0.0001). (B) AMPH elevated locomotor activity (15 min) in both WT and DAT-CI mice (for drug effect, F2,45 = 29.20 and P < 0.0001). (C) Morphine also stimulated locomotion (120 min) in both genotypes (for drug effect, F1,29 = 28.54 and P < 0.0001). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 between drug and saline in the same genotype.
Cocaine Effect on CPP.
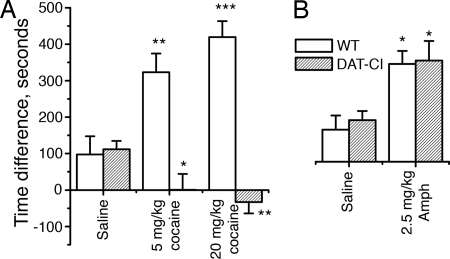
Finally, we investigated whether cocaine could produce reward in DAT-CI mice. In the CPP test, mice were given a total of four injections of cocaine on alternating days. As shown in Fig. 5A, DAT-CI mice clearly failed to develop CPP when given 5 and 20 mg/kg cocaine (P < 0.05 compared with saline treatment in DAT-CI mice), whereas both doses induced robust CPP in WT mice (P < 0.05 compared with saline treatment in WT mice). The absence of cocaine reward in our DAT-CI mice is in contrast to the persistence of cocaine reward in DAT-KO mice. Furthermore, the 2.5 mg/kg dose of AMPH produced similar levels of CPP in both WT and DAT-CI mice (Fig. 5B), which might be explained by the AMPH-induced extracellular DA increase in both genotypes of mice as indicated in the microdialysis study.
Fig. 5.
Drug-induced CPP in WT and DAT-CI mice. CPP is represented by the time difference (in seconds) between pre- and postconditioning (mean ± SEM) that mice spend in the drug-paired chamber (n = 8–15). (A) Five and 20 mg/kg cocaine produced significant CPP in WT mice but not in DAT-CI mice compared with saline mice (for genotype difference, F1,66 = 49.96 and P < 0.0001; for genotype × drug interaction, F2,66 = 16.7 and P < 0.0001). (B) AMPH (2.5 mg/kg) produced CPP in both WT and DAT-CI mice (for drug effect, F1,47 = 18.86 and P < 0.0001). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 between drug and saline in the same genotype; n = 8–15.
Discussion
In the present study, we generated a DAT knockin mouse line carrying a mutant DAT (L104V/F105C/A109V) that is functional but insensitive to cocaine inhibition. This DAT knockin mouse line, as an alternative model of DAT-KO mice, allows us to test whether DAT is required for cocaine reward in mice that preserve substantial DAT activity.
As expected, DAT in our DAT-CI mice showed significantly reduced cocaine sensitivity compared with WT mice, as demonstrated in several experiments. DAT from DAT-CI mice displayed 89-fold more cocaine insensitivity than DAT from WT mice in synaptosomal DA uptake assays. In addition, results from in vivo microdialysis, voltammetry studies in brain slices, and patch clamp of DA neurons all confirmed that DAT from DAT-CI mice were much less sensitive to cocaine than DAT from WT mice. Although the brain DAT expression level in DAT-CI mice was equivalent to that in WT mice, DAT activity was lower at lower DA concentrations because of the increased Km value of DAT from DAT-CI mice. The lowered DAT activity resulted in moderate, but significant, changes in DA homeostasis that were similar to those seen in heterozygous DAT-KO mice. Therefore, the most relevant comparison of DAT-CI mice is probably with heterozygous DAT-KO mice. However, these changes in DA homeostasis cannot account for the lack of cocaine effects in our mice.
The mesolimbic DA system, especially the NAc, is believed to play a critical role in the rewarding and addictive properties of cocaine and other drugs of abuse (18, 19, 27–29). The fact that cocaine elevates extracellular DA in the NAc of DAT-KO mice suggests that the elevated extracellular DA might be the mechanism of cocaine reward in mice lacking DAT (18, 19). It has been reported that the NET selective inhibitor reboxetine and the SERT selective inhibitor fluoxetine elevate DA in the NAc of DAT-KO mice but not in WT mice (18, 19). These results suggest that the complete removal of DAT altered the reward pathway in such a way that cocaine inhibition of SERT and/or NET led to an increase of DA in the NAc and thus cocaine reward in DAT-KO mice (18, 19). As shown in Fig. 3C, 20 mg/kg cocaine did not elevate extracellular DA levels in our DAT-CI mice, but it increased the DA concentration by ≈150% in WT mice as measured by microdialysis. We then used AMPH to test whether DA in the NAc could be elevated in DAT-CI mice by other drugs with rewarding properties. As shown in Fig. 3C, 2.5 mg/kg AMPH significantly elevated extracellular DA in the NAc of both WT and DAT-CI mice, suggesting that the lack of cocaine-elevated DA in DAT-CI mice was not due to the inability of DAT-CI mice to respond to stimulants but due to the lack of DAT inhibition by cocaine. The amount of AMPH-induced DA increase in DAT-CI mice was significantly smaller than in WT mice. The data presented in Fig. 3C are the percentage change from baseline. Because DAT-CI mice had higher baseline DA concentrations, the differences between the genotypes in the amounts of the DA increases were magnified. One possible contributing factor to the difference in the DA increase was that the DA content in DAT-CI mice was lower than in WT mice. It is also conceivable that the mutant DAT has higher spontaneous DA efflux rates, which might be a correlate for the relative inefficacy of AMPH in stimulating DA efflux and locomotion in the knockin animals. Moreover, 2.5 mg/kg AMPH elevated DA levels in the NAc and produced CPP, but 5 mg/kg AMPH failed to stimulate locomotion. The AMPH-induced reward and locomotor stimulation are mediated by different neurocircuitries, which may not have the same AMPH dose responses.
Cocaine stimulates locomotor activity in mice by blocking DAT and elevating extracellular DA levels. As expected, cocaine did not stimulate locomotor activity in DAT-CI mice at doses of 5, 20, or 40 mg/kg but stimulated locomotor activity in WT mice at all three doses. In fact, cocaine appeared to produce locomotor suppression in DAT-CI mice in a dose-dependent manner (Fig. 4A). This result suggests that cocaine may inhibit locomotion in the absence of DAT blockade through blocking SERT and/or NET and indirectly activating serotonin or norepinephrine receptors. We then tested whether DAT-CI mice had a defect in the drug-stimulated locomotor response. Contrary to the lack of locomotor stimulation by AMPH and morphine in DAT-KO mice, 10 mg/kg AMPH and 10 mg/kg morphine were able to stimulate locomotor activity in DAT-CI mice (Fig. 4 B and C). This finding suggests that the lack of cocaine response was not due to the inability of DAT-CI mice to respond to stimulants but was due to cocaine’s inability to block DAT and failure to elevate the extracellular DA level. However, DAT-CI mice displayed reduced locomotor response to AMPH, which is consistent with a reduced extracellular DA increase after the AMPH treatment as measured by in vivo microdialysis.
As predicted by cocaine’s ability to elevate DA in WT mice and its inability to do so in DAT-CI mice, 5 and 20 mg/kg cocaine produced robust CPP in WT mice and clearly failed to produce reward in DAT-CI mice (Fig. 5A). This finding is in contrast to the preservation of cocaine-elevated DA in the NAc and cocaine reward in DAT-KO mice. However, in addition to lowered cocaine sensitivity, DAT activity in DAT-CI mice was also significantly lower than that in WT mice. The differences in DAT function might lead to defects in the reward pathway. Therefore, we tested whether DAT-CI mice could still respond to drugs with rewarding properties. As shown in Fig. 5B, AMPH was still capable of inducing CPP in DAT-CI mice, indicating that DAT-CI mice could still respond to rewarding stimuli. In addition, cocaine consistently produced reward in WT mice and in heterozygous and homozygous DAT-KO mice (with 100%, 50%, and 0% DAT activity, respectively), suggesting that reduced DAT activity would not lead to changes that disrupt cocaine reward. Therefore, the lack of cocaine-induced CPP in DAT-CI mice is likely not due to the lowered DAT activity or a defect in the reward pathway but due to the lack of cocaine inhibition of mutant DAT. Our results suggest that DAT blockade is required for cocaine reward in mice with a functional DAT.
In summary, cocaine did not stimulate locomotion, did not elevate extracellular DA, and did not produce reward in DAT knockin mice with a functional but cocaine-insensitive DAT. Our results suggest that blockade of DAT is required for cocaine reward in mice with a functional DAT, and, thus, they do not support the notion of a DAT-independent cocaine reward pathway in normal mice. Our study further confirmed the idea that cocaine-induced increase in extracellular DA in the NAc is critical in mediating cocaine reward. Our knockin mouse line is a unique animal model specifically designed to distinguish the role of DAT from the roles of NET and SERT in mediating cocaine effects, including acute and chronic cocaine-induced changes in gene expressions. Precise knowledge of the roles of relevant genes and proteins in cocaine action is important for understanding the mechanism of cocaine addiction and will facilitate the development of effective therapies for cocaine addiction.
Materials and Methods
Generation of the Knockin Mice.
The targeting strategy is illustrated in Fig. 1A. A 1.34-kb mouse genomic DNA fragment (nucleotides 13700–15040 in U12313) containing exon 3 and portions of introns 2 and 3 was PCR amplified. The triple mutation L104V/F105C/A109V and a KpnI site were incorporated within exon 3. The mutated fragment was in the short arm of the targeting construct; a 5-kb fragment (nucleotides 15082–20110 in U12313) of mDAT intron 3 was amplified and used as the long arm. The selection marker, a neomycin resistance gene with its promoter and poly(A) signal, was flanked by two LoxP sites, and the thymidine kinase gene was included for negative selection against random insertions. The linearized targeting construct was electroporated into mouse ES cells (129/SvJ) (30). G418-resistant ES cell clones displaying the correct homologous recombination were microinjected into blastocysts of C57BL/6J mice and implanted into pseudopregnant C57BL/6J foster female mice. The chimeras were bred with C57BL/6J mice. Germ line-transmitting males were then bred with a mouse strain (FVB/N-Tg[EIIa-cre]C5379Lmgd/J; The Jackson Laboratory) that carries a Cre recombinase transgene under the control of the adenovirus EIIa promoter targeting early embryo expression. The progenies were backcrossed with C57BL/6J mice twice, and mice with germ-line transmission of Neo cassette removal were selected. The heterozygous mice were used as breeding pairs to produce the mice used in this study. The final mutant allele had the triple mutation in exon 3 of the DAT gene, and a 103-bp insertion (one LoxP site and some vector sequence) in intron 3 at a site 168 bp away from its junction with exon 3. Routine genotyping was performed with PCR by using primers f3/r3 as illustrated in Fig. 1D. The Ohio State University Animal Care Committee approved all animal procedures.
Northern Blot Analysis of DAT mRNA.
The digoxigenin (DIG)-dUTP-labeled DNA probe was made from the first 950 bp of mDAT coding region by using the DIG DNA Labeling and Detection Kit II (Roche Applied Science, Indianapolis). Total RNA was purified from the mouse midbrain containing the ventral tegmental area and substantia nigra by using TRIzol reagent (Invitrogen). A DIG-dUTP-labeled probe for β-actin mRNA was included in the experiments as a control for equal loading.
Western Blot Analysis of the Total and Surface-Biotinylated DAT Protein.
Proteins were prepared from freshly dissected striata of WT and DAT-CI mice. Biotinylated surface proteins were prepared as described in ref. 31. Standard Western blot procedures were used. The primary antibody was a goat polyclonal raised against the DAT C terminus (DAT C-20; Santa Cruz Biotechnology), and the secondary antibody was a rabbit anti-goat antibody conjugated with horseradish peroxidase (Sigma).
Synaptosomal DA Uptake.
DA uptake was measured by using synaptosomes prepared from fresh mouse striata. The tissue was homogenized in ice-cold Krebs–Ringer’s buffer containing 0.32 M sucrose. The samples were centrifuged for 10 min at 1,000 × g, the pellet was discarded, and the remaining supernatant was centrifuged for an additional 15 min at 16,000 × g. The uptake of [3H]DA in the presence of increasing concentrations of unlabeled DA or cocaine was performed by using methods described in ref. 32. Km, Vmax, and IC50 values were determined by nonlinear regression.
Whole-Brain DA Content.
DA was extracted from whole brains by using an established procedure (33) and analyzed by HPLC. Samples were analyzed by using a microbore C-18 reverse-phase column (MD150, 3.2 mm), a 5014B microdialysis cell (ESA, Bedford, MA), and a mobile phase consisting of 75 mM sodium phosphate/1.7 mM octane sulfonic acid/25 μM EDTA/0.1 mM triethylamine/10% acetonitrile, pH 3.0.
Microdialysis of Extracellular DA.
Microdialysis was performed on freely moving mice by using a procedure similar to the methods described in refs. 18 and 19. Briefly, guide cannulae (CMA/7; CMA/Microdialysis, North Chelmsford, MA) were stereotaxically implanted in anesthetized mice in the NAc (anterior, 1.2 mm; lateral, 0.6 mm; vertical, 4.2 mm, relative to bregma). After a 24- to 48-h recovery, dialysis probes (CMA/7, 1-mm membrane length, 0.24-mm o.d.; CMA/Microdialysis) were lowered and perfused for 3 h at a rate of 1 μl/min with artificial cerebrospinal fluid (CSF) containing 147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, and 0.85 mM MgCl2. Samples were collected on ice every 20 min at a flow rate of 1 μl/min and stored in a solution containing 0.1 M perchloric acid and 0.2 mM EDTA at −80°C until analysis. Samples were analyzed with HPLC as described above. DA standards of 0.625, 2.5, and 10 nM dissolved in artificial CSF were interspersed throughout the samples and used as internal controls. The ESA-501 data system was used to compute peak areas.
FCV.
Brain slices (400 μm in thickness) containing NAc were prepared from 10- to 12-week-old mice, and FCVs were performed according to well established procedures (34). Local electrical stimulations (0.2-ms, 50- to 100-μA pulses) were delivered every 100 s through bipolar tungsten electrodes with a resistance of 0.5 megaohms. Nisoxetine and fluoxetine (1 μM) were used to eliminate possible DA uptake by NET and SERT.
Patch Clamp Recording.
Coronal midbrain slices (250 μm in thickness) containing substantia nigra pars compacta were prepared from 10- to 12-week-old mice and whole-cell patch clamp recording was performed according to well established procedures (35). DA neurons were first approached under visual guidance of a video microscope and were further identified by the presence of the hyperpolarization-activated current (35). Data were acquired with a Multiclamp 700B amplifier and pclamp 9 software (Axon Instruments, Union City, CA). The spontaneous action potentials and firing frequencies were recorded and analyzed.
Behavioral Tests.
Mouse movement and locations were tracked by the video and software system limelight (ActiMetrics Software, Evanston, IL). For locomotion measurement, individual mice were habituated for 45 min in a 25 × 25-cm box made of acrylic and injected with saline or drugs (cocaine and AMPH i.p.; morphine s.c.), and the horizontal distances mice traveled were recorded for 15 min immediately after the injection (120 min for morphine-injected mice). The CPP boxes were made of acrylic consisting of three chambers: a middle chamber (12.5 × 7.5 cm) and two test chambers (12.5 × 17.5 cm). The middle chamber had gray walls and no floor mat; one test chamber had a coarse floor mat and thick, straight black lines on white walls; the other test chamber had a fine floor mat and thin, wavy black lines on white walls. On day 1 (preconditioning), mice were placed in the middle chamber and allowed free access to all three chambers for 30 min. The time spent in each chamber was recorded. During the conditioning phase (days 2–9), mice were randomly assigned to one test chamber for drug pairing and to the other chamber for saline pairing. On days 2, 4, 6, and 8, mice were injected with saline and confined to their saline-paired chambers for 30 min. On days 3, 5, 7, and 9, mice were injected with drug and confined to drug-paired chambers. The saline control mice were always given saline. On day 10 (postconditioning), mice were placed in the middle chamber and allowed to explore all three chambers for 30 min, and time spent in each chamber was recorded. CPP was assessed by the time differences in the cocaine-paired chamber between the preconditioning and postconditioning days. The procedure for AMPH CPP was the same as that for cocaine except that the conditioning time was 15 min. Two-way ANOVA and post hoc Bonferroni tests were performed by using spss (SPSS, Chicago).
Acknowledgments
We thank Dr. Gary Rudnick for his support and encouragement during the early stage of this study and Drs. Laura Bohn and Megan Stephan for critical reading of the manuscript. This work was supported by National Institute on Drug Abuse Grant DA14610 (to H.H.G.), National Institute of Mental Health Grant MH067119, and the National Alliance for Research on Schizophrenia and Depression (F.-M.Z.).
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- SERT
serotonin transporter
- NET
norepinephrine transporter
- DAT-KO
DAT knockout
- NAc
nucleus accumbens
- FCV
fast cyclic voltammetry
- CPP
conditioned place preference
- AMPH
amphetamine.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ritz M. C., Lamb R. J., Goldberg S. R., Kuhar M. J. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 2.Amara S. G., Kuhar M. J. Annu. Rev. Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- 3.Wise R. A., Bozarth M. A. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 4.Di Chiara G., Imperato A. Proc. Natl. Acad. Sci. USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman J., Madras B. K., Johnson S. E., Spealman R. D. J. Pharmacol. Exp. Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- 6.Kuhar M. J., Ritz M. C., Boja J. W. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 7.Ritz M. C., Kuhar M. J. Biochem. Soc. Symp. 1993;59:51–64. [PubMed] [Google Scholar]
- 8.Rothman R. B., Glowa J. R. Mol. Neurobiol. 1995;11:1–19. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- 9.Hall F. S., Li X. F., Sora I., Xu F., Caron M., Lesch K. P., Murphy D. L., Uhl G. R. Neuroscience. 2002;115:153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- 10.Giros B., Jaber M., Jones S. R., Wightman R. M., Caron M. G. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 11.Rocha B. A., Fumagalli F., Gainetdinov R. R., Jones S. R., Ator R., Giros B., Miller G. W., Caron M. G. Nat. Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 12.Sora I., Wichems C., Takahashi N., Li X. F., Zeng Z., Revay R., Lesch K. P., Murphy D. L., Uhl G. R. Proc. Natl. Acad. Sci. USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medvedev I. O., Gainetdinov R. R., Sotnikova T. D., Bohn L. M., Caron M. G., Dykstra L. A. Psychopharmacology. 2005;180:408–413. doi: 10.1007/s00213-005-2173-y. [DOI] [PubMed] [Google Scholar]
- 14.Caine S. B. Nat. Neurosci. 1998;1:90–92. doi: 10.1038/335. [DOI] [PubMed] [Google Scholar]
- 15.Uhl G. R., Hall F. S., Sora I. Mol. Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- 16.Rocha B. A. Eur. J. Pharmacol. 2003;479:107–115. doi: 10.1016/j.ejphar.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 17.Jones S. R., Gainetdinov R. R., Hu X. T., Cooper D. C., Wightman R. M., White F. J., Caron M. G. Nat. Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- 18.Carboni E., Spielewoy C., Vacca C., Nosten-Bertrand M., Giros B., Di Chiara G. J. Neurosci. 2001;21:1–4. doi: 10.1523/JNEUROSCI.21-09-j0001.2001. RC141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mateo Y., Budygin E. A., John C. E., Jones S. R. Proc. Natl. Acad. Sci. USA. 2004;101:372–377. doi: 10.1073/pnas.0207805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X., Gu H. H. Mol. Pharmacol. 2003;63:653–658. doi: 10.1124/mol.63.3.653. [DOI] [PubMed] [Google Scholar]
- 21.Chen R., Han D. D., Gu H. H. J. Neurochem. 2005;94:352–359. doi: 10.1111/j.1471-4159.2005.03199.x. [DOI] [PubMed] [Google Scholar]
- 22.Sato Y., Zhang Y. W., Androutsellis-Theotokis A., Rudnick G. J. Biol. Chem. 2004;279:22926–22933. doi: 10.1074/jbc.M312194200. [DOI] [PubMed] [Google Scholar]
- 23.Sen N., Shi L., Beuming T., Weinstein H., Javitch J. A. Neuropharmacology. 2005;49:780–790. doi: 10.1016/j.neuropharm.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 25.Jones S. R., Gainetdinov R. R., Jaber M., Giros B., Wightman R. M., Caron M. G. Proc. Natl. Acad. Sci. USA. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacey M. G., Mercuri N. B., North R. A. Br. J. Pharmacol. 1990;99:731–735. doi: 10.1111/j.1476-5381.1990.tb12998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Chiara G. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- 28.Carboni E., Imperato A., Perezzani L., Di Chiara G. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- 29.Cass W. A., Gerhardt G. A., Mayfield R. D., Curella P., Zahniser N. R. J. Neurochem. 1992;59:259–266. doi: 10.1111/j.1471-4159.1992.tb08899.x. [DOI] [PubMed] [Google Scholar]
- 30.Hogan B. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 31.Johnson L. A., Furman C. A., Zhang M., Guptaroy B., Gnegy M. E. Neuropharmacology. 2005;49:750–758. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Ansah T. A., Ramamoorthy S., Montanez S., Daws L. C., Blakely R. D. J. Pharmacol. Exp. Ther. 2003;305:956–965. doi: 10.1124/jpet.102.047134. [DOI] [PubMed] [Google Scholar]
- 33.Fumagalli F., Gainetdinov R. R., Valenzano K. J., Caron M. G. J. Neurosci. 1998;18:4861–4869. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F. M., Liang Y., Salas R., Zhang L., De Biasi M., Dani J. A. Neuron. 2005;46:65–74. doi: 10.1016/j.neuron.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Pidoplichko V. I., DeBiasi M., Williams J. T., Dani J. A. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]