Abstract
Talpid moles are small insectivores that live in dark underground tunnels. They depend heavily on touch to navigate and find food. Most species have an array of complex epidermal sensory structures called Eimer’s organs that cover the tip of the nose. In this study, the anatomy of Eimer’s organ was examined in the coast mole and star-nosed mole by using the fluorescent styryl pyridinium dye AM1-43 and immunocytochemical staining for neurofilament 200 and substance P. In addition, DiI was used to label neural components of Eimer’s organ. AM1-43 labeled all of the Eimer’s organ receptors after systemic injection, suggesting a role in mechanotransduction. Immunostaining with neurofilament 200 and substance P labeled distinct subtypes of sensory fibers. Substance P labeled a group of free nerve endings along the outer edge of Eimer’s organ, indicating a nociceptive role for these fibers. In contrast, neurofilament 200 labeled a more central set of nerve endings, suggesting that these fibers function as low-threshold mechanoreceptors. By labeling subsets of trigeminal afferents distant from the receptor array with DiI, we revealed innervation patterns indicating that one afferent supplies the outer, substance P-positive set of free nerve endings, whereas several afferents differentially innervate the central free nerve endings. Our results suggest that the free nerve endings innervating Eimer’s organ are largely mechanosensitive and may play an important role in the rapid sensory discrimination observed in these species.
Keywords: mechanosensation, nociception, sensory neurons, star-nosed mole
Moles are fossorial insectivores in the family Talpidae that exhibit a number of adaptations to their underground lifestyle. The most obvious specializations are powerful shoulders and large clawed forelimbs used for digging tunnels (Fig. 1C). However, the less apparent sensory specializations for touch are perhaps the most important innovations underlying the survival and success of these species. Unlike most small mammals, which depend heavily on their large vibrissae for mechanosensation, moles primarily make use of the tips of their noses to investigate objects through touch.
Fig. 1.
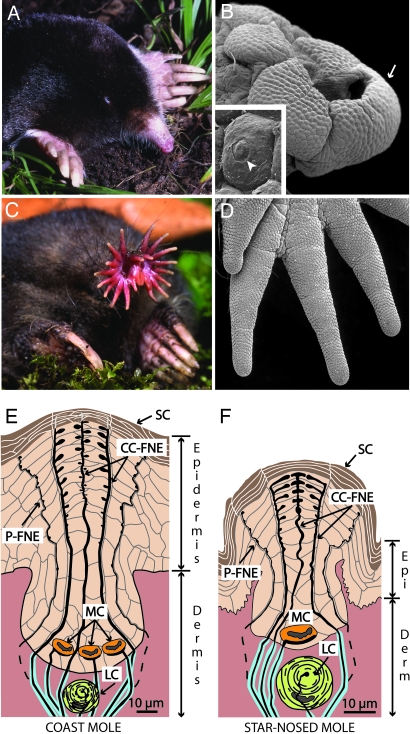
The coast mole (Scapanus orarius) and the star-nosed mole (Condylura cristata) showing adaptations to a fossorial lifestyle. (A) The coast mole has large claws and tiny eyes and lacks an external pinna. (B) A scanning electron micrograph of the coast mole’s snout covered with Eimer’s organs. An arrow marks organs worn by soil abrasion (see Discussion). (Inset) An Eimer’s organ showing the circular disk at the top of the central cell column (arrowhead). (C) The star-nosed mole showing the rhinarium composed of 22 appendages. (D) A scanning electron micrograph of appendages covered with Eimer’s organs from the right lower quadrant of the star. (E) Schematic representation of Eimer’s organ in the coast mole. The epidermis contains a central epithelial cell column associated with intraepidermal free nerve endings (CC-FNE) that course to the stratum corneum (SC). A ring of smaller peripheral free nerve endings surrounds the central column free nerve endings (P-FNE). Merkel cell–neurite complexes (MC) and lamellated corpuscles (LC) reside at the base of each organ. (F) Schematic representation of the smaller Eimer’s organ in the star-nosed mole with only one Merkel cell and a smaller central column.
At first glance, the noses of most moles appear nondescript, although the flourish of nasal appendages on the star-nosed mole is an obvious exception (Fig. 1). Closer examination of a mole’s nose under the scanning electron microscope reveals a honeycomb pattern of tiny epidermal swellings covering the skin surface in nearly every species (1). These small swellings are called Eimer’s organs (2) and are complex, well organized, and densely innervated structures used to make rapid sensory discriminations of small objects (3, 4). Each Eimer’s organ contains an array of sensory receptors associated with a column of epidermal cells running through the center of each swelling. A circular disk that makes up the tip of the cell column is visible on the outer skin surface (Fig. 1B, arrowhead).
The central cell column is associated with a number of intraepidermal free nerve endings that originate from myelinated afferents in the underlying dermis. Fig. 1 shows schematics of the complex innervation of Eimer’s organ in the coast mole and the star-nosed mole. The free nerve endings traverse the cell column in a circular arrangement, with one to two free nerve endings in the center of the column and a ring of satellite free nerve endings at the margins of the column. A few previous reports (2, 5, 6) described a second ring of smaller free nerve endings around the periphery of each Eimer’s organ of some species. One or more Merkel cell–neurite complexes are located at the bottom of each Eimer’s organ in the basal layer of the epidermis (stratum germinativum). Finally, there are one to two lamellated corpuscles in the dermis just below the epidermal cell column.
Although electrophysiological investigations indicate that Eimer’s organs are sensitive to mechanosensory stimuli (7–9), how the different components of Eimer’s organs function remains largely unexplored. The goal of this study was to characterize the individual neural components of Eimer’s organ to better understand how this structure transduces tactile information. We used the styryl pyridinium dye AM1-43 to examine the distribution of mechanoreceptive elements while simultaneously appraising the utility of AM1-43 as an anatomical marker for primary sensory afferents. In addition, we explored the primary afferent terminals through immunocytochemical analysis and revealed innervation patterns of the receptors with the carbocyanine dye DiI.
Results
Labeling Patterns of AM1-43 Within Eimer’s Organ.
Styryl pyridinium dyes have been shown to label mechanosensory cells (10–18). In this study we used AM1-43, the fixable analog of the styryl pyridinium dye FM1-43 (19), to identify and examine the presumptive mechanosensory receptors in the rhinarium of the mole. We were particularly interested in the intraepidermal free nerve endings supplied by myelinated fibers (20), because they may play a part in the transduction of high-resolution tactile signals. Here we show robust labeling of each sensory receptor component of Eimer’s organ by AM1-43.
Coast Mole Intraepidermal Free Nerve Endings.
In the coast mole the structure of the intraepidermal free nerve endings associated with the central cell column was clearly visible 25–33 h after systemic injection of AM1-43 (Figs. 2 and 3). The column of epidermal cells with its accompanying nerve fibers formed the central core of each Eimer’s organ. The nerve terminals associated with the column extended from basal layers of the epidermis to the outer skin surface in a circular arrangement, with one to three fibers in the center and a series of 8–24 satellite fibers around the perimeter of the column (Fig. 3A).
Fig. 2.
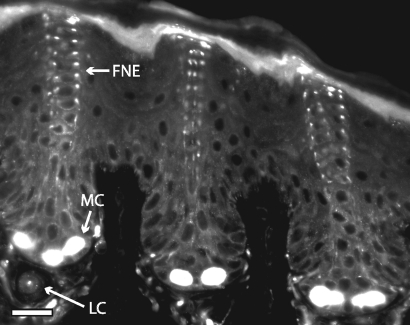
Confocal image of Eimer’s organ in an AM1-43-treated coast mole. This view shows the position and arrangement of the different sensory receptor units. The central column free nerve endings (FNE) rise up through the epidermis and terminate at the stratum corneum. The Merkel cell–neurite complexes (MC) reside within the stratum germinativum. A lamellated corpuscle (LC) (paciniform corpuscle) is situated in the dermis. (Scale bar: 20 μm.)
Fig. 3.
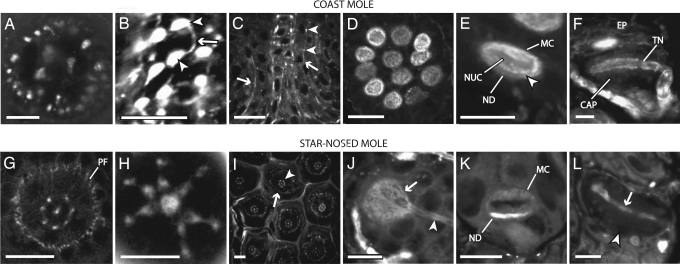
Confocal images showing AM1-43 labeling in Eimer’s organ of the coast mole (A–F) and star-nosed mole (G–L). (A) A horizontal section near the middle of the cell column showing a ring of 22 satellite nerve endings surrounding the central nerve fibers in the coast mole. (B) Brightly labeled varicosities (arrowheads) connected by thin neurites (arrow) give the free nerve ending terminals a bead-on-a-string appearance. (C) Vertical section in the coast mole showing short lengths of peripheral fibers on both sides of the central cell column fibers (arrows). The terminals are smaller than their central column counterparts but have a similar bead-on-a-string morphology (arrowheads). (D) Clustering of Merkel cell–neurite complexes at the base of the coast mole organ. (E) Magnified view of a Merkel cell–neurite complex (MC) with its characteristic lobulated nucleus (NUC) and terminal neurite disk (ND). The division is visible between the terminal neurite disk and the Merkel cell (arrowhead). (F) A vertical section through a lamellated corpuscle. The terminal neurite (TN) is aligned within the capsule (CAP) parallel to the skin surface. The whole complex sits directly below the epidermis (EP). (G) Horizontal section from a star-nosed mole ≈15 μm below the apex of the Eimer’s organ showing seven satellite fibers surrounding a single central fiber. The second ring of smaller diameter peripheral fibers is evident (PF) surrounding the center and satellite fibers. (H) Horizontal view of the most superficial free nerve ending terminals of an Eimer’s organ. The swellings of the satellite free nerve endings extend inward in close apposition to the central swelling. (I) Horizontal section showing star-nosed mole Eimer’s organs in a hexagonal array. The central free nerve endings (arrowhead) are surrounded by the peripheral ring of smaller, less organized nerve terminals (arrow). (J) Horizontal view showing the AM1-43-labeled neurite disk (arrow) and afferent neuron (arrowhead) of a single Merkel cell–neurite complex. (K) Vertical view of a Merkel cell–neurite complex. Note the higher level of staining of the neurite disk (ND) compared with the Merkel cell (MC). (L) Horizontal section showing a lamellated corpuscle. The terminal neurite (arrow) runs through the center of the capsule (arrowhead). (Scale bars: 10 μm in A, B, E, F, H, and J–L; 20 μm in C, D, G, and I.)
Along their course through the central column, each neurite exhibited a series of brightly labeled swellings that increased in size close to the outer skin surface. The swellings were connected in bead-on-a-string fashion by a thin neurite (Fig. 3B). Interestingly, AM1-43 labeling was also observed in terminal swellings within the stratum corneum.
A second ring of smaller free nerve endings was variably apparent peripheral to the central cell column (Fig. 3C). These smaller fibers also had a bead-on-a-string morphology. The path of the peripheral fibers was convoluted and appeared to follow the borders between the epithelial cells at a distance of one to two cell diameters from the central column.
Star-Nosed Mole Intraepidermal Free Nerve Endings.
In the star-nosed mole the structure of the free nerve endings was revealed by AM1-43 5 min after systemic injection accompanied by mechanosensory stimulation (Fig. 3). Star-nosed moles have Eimer’s organs that are smaller than those of other mole species. As a result, several features of the star-nosed mole Eimer’s organ are unique. For example, the central cell column in star-nosed moles is only one cell in diameter, forming a single stack of keratinocytes (20). This configuration leaves less room for free nerve endings associated with the column, and, as might be expected, there are fewer satellite fibers in star-nosed mole Eimer’s organs. Typically, one central fiber was surrounded by 5–10 satellite fibers.
At the top of the cell column, the terminal swellings of the neurites in the star-nosed mole form a concise geometric pattern with a single swelling in the center surrounded by a ring of closely opposed terminal swellings from the satellite fibers (Fig. 3H). This remarkable configuration of nerve terminals is the predictable result of reducing the size of the Eimer’s organ in the coast mole (the likely ancestral condition for moles) to that observed in the star-nosed mole (see scales in Fig. 1 E and F).
Finally, AM1-43 consistently revealed the second ring of less organized and thinner free nerve endings surrounding the central cell column (Fig. 3 G and I). These smaller peripheral free nerve endings were located at a distance of one epithelial cell diameter from the central cell column.
Merkel Cell–Neurite Complexes and Lamellated Corpuscles.
The Merkel cell–neurite complexes in the coast mole were brightly labeled after systemic injection of AM1-43. The coast moles had an average of 11 Merkel cell–neurite complexes at the base of the Eimer’s organ in the stratum germinativum. These receptor end organs were tightly packed in a relatively flat circular arrangement (Fig. 3D), with the Merkel cells themselves characteristically situated above their respective neurite disks. Both the Merkel cell and the neurite were well labeled by AM1-43 (Fig. 3E). The lobular nucleus of the Merkel cell was visible, as was the division between the Merkel cell and the terminal neurite.
One hour after systemic injection of star-nosed moles AM1-43 also labeled Merkel cells. The terminal neurite disk appeared to be more robustly labeled with AM1-43 than was the Merkel cell after this shorter time course of labeling (Fig. 3K). Fig. 3J shows the labeled neurite disk just below the Merkel cell terminating in an expanded filamentous ending. Similar results were seen in the neurofilament 200 (NF 200) immunolabeling in coast moles (see Fig. 4B).
Fig. 4.
Confocal images showing the main neural components of Eimer’s organ in the coast mole immunolabeled for NF 200. (A) Horizontal section showing the free nerve endings near the middle in the stratum spinosum, where the center and satellite arrangement of the nerve fibers is clearly visible. (B) Horizontal section showing NF 200 labeling in the base of a Merkel cell–neurite complex. The label appears to be concentrated in a ring around the perimeter of the disk. (C) The terminal neurite of the lamellated corpuscle is brightly labeled for NF 200 (arrow), whereas the capsule of the corpuscle is devoid of label (arrowhead). (Scale bars: 10 μm.)
AM1-43 also labeled the terminal neurite and primary afferent neuron of the lamellated corpuscles in both species (Fig. 3 F and L). The lamellated corpuscles were located directly beneath the epidermis, with the long axis of the receptor aligned parallel to the skin surface. The terminal neurites were brightly labeled, but the capsule was devoid of label. The most obvious difference between the two species was the occurrence of one to two corpuscles in coast moles and only one corpuscle in star-nosed moles.
NF 200 Immunocytochemistry.
The stereotypical neural structure of Eimer’s organ allows convenient comparisons to be made between AM1-43 labeling and immunofluorescence that may reflect the functional characteristics of the receptor terminals. Neurons that are immunoreactive for the heavy chain (200-kDa) NF 200 have been shown to be primarily large-diameter, myelinated, peripheral mechanoreceptors (21, 22). Here we show that the majority of AM1-43-positive components in the Eimer’s organ complex also label for NF 200 (Fig. 4). NF 200 staining was observed throughout the free nerve endings in the central column, but the fibers appeared to become thinner and decreased in luminescence as they approached the stratum corneum, where no label was evident.
When viewed from above, the circular configuration of the free nerve endings associated with the central column was clearly evident (Fig. 4A). The nerve terminal disk of the Merkel cell–neurite complexes was immunoreactive for NF 200, with the majority of the neurofilaments concentrated in a ring around the periphery (Fig. 4B). This pattern was similar to the expanded region of the neurite seen in AM1-43 (Fig. 3J) but with a lower intensity of label in the center of the disk. The terminal neurites of the lamellated corpuscles also labeled strongly with NF 200 (Fig. 4C).
NF 200 and Substance P Double Labeling.
The two rings of free nerve endings described above (satellite fibers associated with the cell column and the smaller, peripheral fibers that form an outer ring) were of particular interest. Based on location and morphology, it seemed possible that they serve different functions. The outer peripheral ring is seldom identified or discussed in accounts of Eimer’s organ. In reports from the 19th century there are descriptions of these “peripheral” free nerve endings (2, 5), but in later examinations there is only cursory mention of them (6), and in two other reports the peripheral ring is evident in micrographs but is not discussed (23, 24).
To further explore these different neural components, we used double labeling immunofluorescence for both NF 200 and substance P. Our results provide compelling evidence that these systems play different roles in sensory transduction. Specifically, the fibers associated with the central cell column were labeled exclusively with NF 200 (Figs. 4A and 5A). However, the peripheral ring of finer neurites was labeled for substance P (Fig. 5B), a marker for nociceptive primary afferent neurons (25, 26). A role in nociception is consistent with the location of these peripheral fibers, which encircle the entire organ such that damaging stimuli might be readily detected (see Discussion).
Fig. 5.
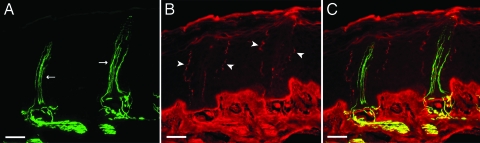
Fluorescent micrographs of Eimer’s organ in the coast mole double-labeled for NF 200 (green) and substance P (red). (A) The central and satellite free nerve endings label specifically with NF 200 (arrows). (B) The peripheral free nerve endings are immunoreactive exclusively for substance P (arrowheads). (C) The overlay shows the lack of colocalization between the two different markers. The convoluted course that the peripheral fibers take to the surface can be seen in contrast to the more direct route taken by the central and satellite fibers. (Scale bars: 20 μm.)
DiI Tracing.
DiI can be used to trace neuronal pathways in fixed tissues when the cell membrane has been directly exposed to dye crystals. We randomly labeled only a few fibers within a peripheral nerve to explore the patterns of innervation for different components of Eimer’s organ in star-nosed moles (Fig. 6). Fig. 6A illustrates the nerve terminals at the apex of the star-nosed moles’ central cell column when all of the afferents are labeled. By sparsely labeling fewer fibers, we found evidence that different fibers supplying the cell column originate from different afferents. For example, we selectively labeled the satellite fibers of the rings (Fig. 6B), the central fiber (Fig. 6C), and more often a small portion of the satellite ring (Fig. 6D). Importantly, we always observed labeling of multiple terminal swellings, suggesting that one afferent branches to supply a number of satellite fibers, consistent with previous observations in star-nosed moles (27).
Fig. 6.
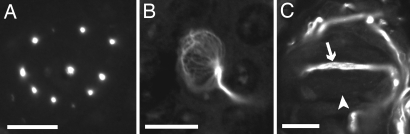
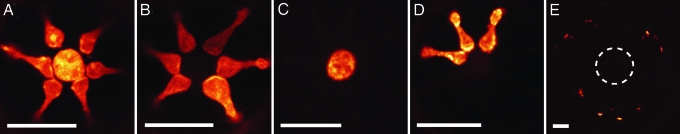
Confocal images in the horizontal plane of DiI-treated free nerve ending terminals within individual star-nosed mole Eimer’s organs. (A) The central fiber and satellite fibers all labeled with a broad application of DiI crystals. (B) Labeling of the satellite fibers only. (C) Only the central fiber in this Eimer’s organ is labeled. (D) DiI label in only three satellite fibers. (E) A single Eimer’s organ showing the outer ring of peripheral fibers labeled with DiI. There is no label visible in the central and satellite fibers that are denoted by the dashed circle. (Scale bars: 5 μm.)
Finally, the peripheral ring of substance P-positive fibers was occasionally labeled (Fig. 6E). As would be expected from their unique immunohistochemistry, these were typically labeled independent of other neuronal elements. In each case where the peripheral ring was labeled, a single afferent in the underlying dermis supplied all of the free nerve endings for an organ. We were unable to determine whether it was myelinated; however, it was clear that afferents supplying the central cell column fibers were myelinated.
Discussion
In this study we were able to label the different sensory components of Eimer’s organ using the fluorescent styryl pyridinium dye AM1-43, immunolabeling for substance P and NF 200, and using the lipophilic neuronal tracer DiI. The results provide new insights into the structure and possible functions of different components of this sensory organ. They also provide further evidence that AM1-43 is a useful anatomical marker for examining the neural components of mammalian skin.
Anatomy and Function of Eimer’s Organ.
Eimer’s organ was first described by Eimer in the European mole (2) and has since been found in a number of additional species. This structure is restricted to moles (family Talpidae, order Insectivora), where it has been identified in most of the 17 genera (1). As illustrated in Fig. 1, Eimer’s organ is a small but complexly organized receptor organ that includes free nerve endings, Merkel cell–neurite complexes, and lamellated corpuscles. The function of this organ has been a matter of speculation since the 1800s, and most investigators conclude that it plays a critical role in mechanosensation. This conclusion seems obvious from mole exploratory behavior, during which the tip of the nose is repeatedly touched to the ground. However, direct evidence for mechanosensory responses from Eimer’s organs was only recently obtained during recordings from the neocortex (7–9). Neuronal recordings indicate that these organs respond to very slight depressions of the skin surface, and responses include both rapidly and slowly adapting classes (8). These findings are consistent with the proposed role of Eimer’s organ based on anatomical and behavioral studies that indicate that moles can make rapid discriminations of objects based on brief contact with the skin of the snout (4).
Despite these findings, a number of questions remain regarding the function of Eimer’s organ. For example, although Merkel cell–neurite complexes and lamellated corpuscles in mammalian skin have been well characterized as slowly and rapidly adapting mechanoreceptors, respectively, the function of the conspicuous free endings associated with Eimer’s organ has not been clearly established. These are of special interest, because the central cell column is the defining feature of Eimer’s organ, and the associated free nerve endings have a geometric arrangement and structure unparalleled by receptor arrays of most other mammals. Thus, one of the goals of the present study was to learn more about the general anatomy, immunohistochemistry, and innervation of Eimer’s organ and to provide additional clues to the possible functions of the different components.
AM1-43 and Immunocytochemistry.
One of the most interesting and obvious results of our systemic injection with AM1-43 in moles is the ability to visualize sensory receptors in adult mammalian skin. Although this has been shown in previous investigations of juvenile rodents, we show that AM1-43 is clearly useful for additional mammalian species at various stages of development. The hypothesized mechanism for dye entry, through the associated transduction channels, raises the possibility that AM1-43 could be used for in vivo studies of receptor responses in adult mammalian skin. In the present study we were unable to make enough systematic observations to determine whether stimulation of mechanoreceptors resulted in differential labeling patterns for Eimer’s organs.
Each of the components of Eimer’s organ was labeled after systemic dye injection in moles. Our current results with AM1-43 are consistent with the mechanosensory hypothesis for Eimer’s organ generally and for the free nerve endings within the column specifically. However, this is not a definitive test for mechanosensation, because this dye is known to label nonmechanosensitive cells, such as taste buds (15).
By combining the data from AM1-43 with the results of substance P and NF 200 immunolabeling we are able to obtain addition functional clues. Perhaps the most compelling results come from the double-labeling experiments with substance P and NF 200 (Fig. 5). The outermost peripheral ring of free nerve endings was uniquely positive for substance P, whereas the central fibers of the column were positive for NF 200. This finding strongly suggests that the outer ring is composed of high-threshold, peptidergic nociceptors. The outer ring of fibers has been inconsistently reported in previous studies and is seldom included in descriptions of Eimer’s organ. This omission may be because of the smaller size of the fibers (resulting in less consistent appearance in classical silver stains). However, the position of these fibers (surrounding the central cell column and associated sensory elements) is ideal for detecting the earliest stages of damage to the organ.
It seems worth pointing out in this regard the critical role that nociception probably plays in the maintenance of these receptor arrays. Skin has two important functions: protecting underlying tissues and acting as a sensory surface. These two roles are at odds with one another in Eimer’s organ. The skin associated with Eimer’s organ is quite thin and vascular (e.g., note the thin stratum corneum covering the organs in Figs. 1 and 2), yet these sensory arrays are constantly touched to the soil. As a result, the delicate Eimer’s organs of wild-caught specimens invariably show signs of abrasion at the distal end of the snout, where the most frequent contact occurs (e.g., arrow in Fig. 1B). It seems that there is a delicate balance maintained between the two functions of skin in these species, and nociception probably plays a critical role in this balance. As a testament to the effects of the environment of these sensory arrays, the eastern American mole (Scalopus aquaticus), which lives in the driest, most abrasive soil, has an uncharacteristically thick stratum corneum on its rhinarium with degenerate Eimer’s organs (1).
In contrast to the peripheral ring of free nerve endings, the fibers associated with the central cell column were not positive for substance P. However, as was the case for the mechanoreceptor neurites associated with Merkel cells and lamellated corpuscles, the central cell column fibers were positive for NF 200. These results suggest that the central cell column fibers are not nociceptive, consistent with the hypothesis that they play a role in low-threshold mechanoreception (i.e., non-noxious touch) (21, 22).
DiI and Eimer’s Organ Innervation.
To investigate how the components of Eimer’s organ are differentially innervated we randomly labeled small sets of afferents by applying a few crystals of DiI to the exposed nerve in fixed tissue. The results showed that different parts of the central cell column were innervated by different afferents. For example, the central fiber in the middle of the column was often labeled independently. In addition, groups of satellite terminals supplied by single fibers were often separately labeled.
This innervation pattern is similar to what has been reported for the push-rod skin receptors of monotremes (28). Both the push-rod and Eimer’s organ include a similar arrangement of intraepidermal free nerve endings (vesicle chain receptors in push-rods) showing the bead-on-a-string appearance (28–30). In both organs the fiber terminals form swellings below an epithelial dome at the surface of the skin, and in both organs the terminal nerve swellings are anchored by a basket-like complex of tonofibrils that may play a role in sensory transduction (20, 28). Each organ also has Merkel cell–neurite complexes in the base of the epidermal compartment and lamellated corpuscles in the dermis below. The peripheral ring of fibers outside of the cell column has not been identified in the monotremes, but this has been variably reported in moles and may be similarly hard to discern in monotremes. Thus, both monotremes and moles appear to have convergently evolved a similar sensory complex.
It has been suggested that the radial arrangement of free nerve endings within push-rods provides the organs with directional sensitivity to mechanical displacement (28, 29). This function has also been suggested for Eimer’s organ (1). We hypothesize that an array of Eimer’s organs provides moles with textural information about minute surface features on objects of interest through the differential stimulation of the terminal nerve swellings within the central cell column (see ref. 1 for more details). The present results are consistent with this hypothesis; however, the specific responses of the free nerve endings within the central column remain to be investigated.
Materials and Methods
Experimental Animals.
Five coast and seven star-nosed moles were examined. Coast moles were provided by Kevin L. Campbell (University of Manitoba, Winnipeg, Canada). Star-nosed moles were collected in Potter County, Pennsylvania, under permit COL00087. All procedures followed guidelines for the care and use of laboratory animals from the National Institutes of Health and were approved by the Vanderbilt University Institutional Animal Care and Use Committee.
AM1-43 Labeling.
AM1-43 experiments in coast moles were performed in conjunction with terminal electrophysiology experiments. Each mole was injected i.p. with a 3 mg/kg dose of AM1-43 (Biotium, Hayward, CA) mixed in sterile saline. The animals were allowed to forage and tunnel overnight and then were anesthetized with an i.p. dose of 1.0 g/kg urethane supplemented with 20 mg/kg ketamine hydrochloride and 0.5 mg/kg xylazine, respectively, as needed. After recordings animals were euthanized with a 250 mg/kg i.p. injection of sodium pentobarbital and perfused with 1× PBS (pH 7.3) followed by 4% paraformaldehyde (PFA) in PBS. The dye remained in the system of these animals for an average of 30 h (ranging from 25 to 33.5 h) before the animals were euthanized. Star-nosed moles were injected with 3 mg/kg AM1-43 in sterile saline (one-half i.p. and one-half s.c.). One star-nosed mole was injected, returned to its cage for 1 hour, and then euthanized and perfused as described above. The remaining five star-nosed moles were first anesthetized with a 1.0 g/kg i.p. injection of urethane followed by administration of 20 mg/kg ketamine as needed. The left surface of the nose was then brushed back and forth with a piezo-electric driven probe for 5 min before the mole was euthanized and perfused as outlined above.
The star was postfixed in 4% PFA in PBS at 4°C overnight and then cryoprotected in 30% sucrose in PBS. The noses of the coast moles and star-nosed moles were sectioned at 15 and 10 μm, respectively, on a Leica SM 2000R microtome. Sections were stored in 4% PFA in PBS for 48 h to reduce background staining. The sections were mounted on a subbed coverslip and covered with three drops of glycerin as a mounting medium. Cyanoacrylate adhesive was placed along each edge of the coverslip, with spaces at the four corners, and a second coverslip was laid over the samples to allow viewing from either side.
Immunocytochemistry.
Coast moles were euthanized and perfused as described above. The rhinarium was postfixed in 4% PFA in PBS for 3 h at 4°C and then cryoprotected in 30% sucrose at 4°C. For NF 200 and substance P labeling, tissue was first equilibrated and embedded in Tissue-Tek Optimal Cutting Temperature Compound (VWR Scientific, Westchester, PA) and cut on a Jung CM3000 cryostat at 10 μm (similar results were obtained by cutting on the sledge microtome and mounting in glycerin). Sections were mounted on SuperFrost Plus slides (VWR Scientific) and after staining were coverslipped with Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL). Sections were surrounded by a hydrophobic wall with an Aqua-Hold pap pen (SDL, Des Plains, IL), blocked with PBS (pH 7.4) containing 0.1% Triton X-100 and 10% normal goat serum for 1 h at room temperature. Sections were incubated overnight at 4°C with guinea pig anti-substance P antiserum diluted 1:20,000 (kindly provided by John Maggio, University of Cincinnati, Cincinnati) and mouse anti-NF 200 (Sigma-Aldrich) diluted 1:800 in PBS containing 1% normal goat serum and 0.1% Triton X-100. After four washes with 0.1% Triton X-100 in PBS sections were incubated at room temperature for 1–2 h with Alexa Fluor 594-coupled goat anti-guinea pig and Alexa Fluor 488-coupled goat anti-mouse secondary antibodies diluted 1:1,000 (Molecular Probes/Invitrogen) in PBS containing 1% normal goat serum and 0.1% Triton X-100. After four washes sections were coverslipped as described above.
DiI Labeling.
After perfusion an appendage was removed from the star and a wooden probe was used to diffusely apply DiI crystals (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Molecular Probes/Invitrogen) to the exposed nerve. The appendage was embedded in 2% agarose in PBS, immersed in 4% PFA, and stored in darkness for 3 weeks.
Imaging.
Images of the AM1-43, NF 200 single-label, and DiI-treated tissue sections were collected by using an Upright LSM510 confocal microscope (Zeiss). Measurements were made by using lsm image browser 3.2.0.115 (Zeiss). NF 200/subtance P colabeled tissue was imaged with an Axioskop (Zeiss) and captured with the metamorph imaging system (Molecular Devices). Images were processed for levels, brightness, and contrast with photoshop cs2 9.0 (Adobe Systems, San Jose, CA).
Scanning Electron Microscopy.
Tissue was washed in tap water, dehydrated in ethanol, critical point-dried, mounted on an aluminum stud, and sputter-coated with gold. The specimens were imaged on a Cambridge Stereoscan 360 scanning electron microscope and processed as above.
Acknowledgments
We thank Tim Sheehan (Coastal Mole Control, Vancouver) and Kevin Campbell for coast moles; Sam Crish, Erin Henry, Christine Dengler-Crish, and Terry Page for editorial suggestions; Bruce Appel, Haechul Park, and Drew Latimer for help with immunocytochemical experiments and imaging; and the Hermitage and Site Operations Director John Leach for assistance capturing local moles. The Vanderbilt University Medical Center Cell Imaging Shared Resource provided the Upright LSM510 Confocal Microscope and is supported by National Institutes of Health Grants CA68485, DK20593, DK58404, HD15052, DK59637, and EY08126. D.J. and D.M.B. are supported by National Institutes of Health Grants DE08973 and NS049226 and by a Burroughs Wellcome Fund Career Award in Biomedical Science (to D.M.B.). K.C.C. is supported by National Science Foundation Career Award 0238364 and National Science Foundation Grant 0518819.
Abbreviations
- NF 200
neurofilament 200
- PFA
paraformaldehyde.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Catania K. C. Brain Behav. Evol. 2000;56:146–174. doi: 10.1159/000047201. [DOI] [PubMed] [Google Scholar]
- 2.Eimer T. Arch. Mikr. Anat. 1871;7:181–191. [Google Scholar]
- 3.Catania K. C., Kaas J. H. J. Comp. Neurol. 1997;387:215–233. [PubMed] [Google Scholar]
- 4.Catania K. C., Remple F. E. Nature. 2005;433:519–522. doi: 10.1038/nature03250. [DOI] [PubMed] [Google Scholar]
- 5.Ranvier L. Q. J. Micr. Sci. 1880;20:456–458. [Google Scholar]
- 6.Halata Z. Z. Zellforsch. Mikrosk. Anat. 1972;125:108–120. [PubMed] [Google Scholar]
- 7.Catania K. C., Kaas J. H. J. Comp. Neurol. 1995;351:549–567. doi: 10.1002/cne.903510406. [DOI] [PubMed] [Google Scholar]
- 8.Sachdev R. N., Catania K. C. Somatosens. Motor Res. 2002;19:272–278. doi: 10.1080/0899022021000037737. [DOI] [PubMed] [Google Scholar]
- 9.Sachdev R. N., Catania K. C. J. Neurophysiol. 2002;87:2602–2611. doi: 10.1152/jn.2002.87.5.2602. [DOI] [PubMed] [Google Scholar]
- 10.Nurse C. A., Farraway L. Cell Tissue Res. 1989;255:125–128. doi: 10.1007/BF00229073. [DOI] [PubMed] [Google Scholar]
- 11.Balak K. J., Corwin J. T., Jones J. E. J. Neurosci. 1990;10:2502–2512. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collazo A., Fraser S. E., Mabee P. M. Science. 1994;264:426–430. doi: 10.1126/science.8153631. [DOI] [PubMed] [Google Scholar]
- 13.Gale J. E., Marcotti W., Kennedy H. J., Kros C. J., Richardson G. P. J. Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda J., Ishimine H., Masaki Y. Cell Tissue Res. 2003;311:325–332. doi: 10.1007/s00441-002-0688-7. [DOI] [PubMed] [Google Scholar]
- 15.Meyers J. R., MacDonald R. B., Duggan A., Lenzi D., Standaert D. G., Corwin J. T., Corey D. P. J. Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheatham M. A., Huynh K. H., Gao J., Zuo J., Dallos P. J. Physiol. 2004;560:821–830. doi: 10.1113/jphysiol.2004.069559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunzi M. G., Pisarek A., Mugnaini E. J. Neurocytol. 2004;33:359–376. doi: 10.1023/B:NEUR.0000044196.45602.92. [DOI] [PubMed] [Google Scholar]
- 18.Alagramam K. N., Stahl J. S., Jones S. M., Pawlowski K. S., Wright C. G. J. Assoc. Res. Otolaryngol. 2005;6:106–118. doi: 10.1007/s10162-004-5032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renger J. J., Egles C., Liu G. Neuron. 2001;29:469–484. doi: 10.1016/s0896-6273(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 20.Catania K. C. J. Comp. Neurol. 1996;365:343–354. doi: 10.1002/(SICI)1096-9861(19960212)365:3<343::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Lawson S. N., Waddell P. J. J. Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sann H., McCarthy P. W., Jancso G., Pierau F. K. Cell Tissue Res. 1995;282:155–161. doi: 10.1007/BF00319142. [DOI] [PubMed] [Google Scholar]
- 23.Giacometti L., Machida H. Anat. Rec. 1965;153:31–39. doi: 10.1002/ar.1091530105. [DOI] [PubMed] [Google Scholar]
- 24.Quilliam T. A. J. Zool. 1966;149:76–88. [Google Scholar]
- 25.Kuraishi Y., Hirota N., Sato Y., Hino Y., Satoh M., Takagi H. Brain Res. 1985;325:294–298. doi: 10.1016/0006-8993(85)90326-9. [DOI] [PubMed] [Google Scholar]
- 26.Lawson S. N., Crepps B. A., Perl E. R. J. Physiol. 1997;505:177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catania K. C. J. Comp. Neurol. 1995;351:536–548. doi: 10.1002/cne.903510405. [DOI] [PubMed] [Google Scholar]
- 28.Andres K. H., von Düring M. In: Sensory Receptor Mechanisms. Iggo A., editor. Singapore: World Scientific; 1984. pp. 81–89. [Google Scholar]
- 29.Andres K. H., von During M., Iggo A., Proske U. Anat. Embryol. (Berlin) 1991;184:371–393. doi: 10.1007/BF00957899. [DOI] [PubMed] [Google Scholar]
- 30.Manger P. R., Pettigrew J. D. Brain Behav. Evol. 1996;48:27–54. doi: 10.1159/000113185. [DOI] [PubMed] [Google Scholar]