Abstract
Huntington’s disease (HD) is a fatal, genetic, neurological disorder resulting from a trinucleotide repeat expansion in the gene that encodes for the protein huntingtin. These excessive repeats confer a toxic gain of function on huntingtin, which leads to the degeneration of striatal and cortical neurons and a devastating motor, cognitive, and psychological disorder. Trophic factor administration has emerged as a compelling potential therapy for a variety of neurodegenerative disorders, including HD. We previously demonstrated that viral delivery of glial cell line-derived neurotrophic factor (GDNF) provides structural and functional neuroprotection in a rat neurotoxin model of HD. In this report we demonstrate that viral delivery of GDNF into the striatum of presymptomatic mice ameliorates behavioral deficits on the accelerating rotorod and hind limb clasping tests in transgenic HD mice. Behavioral neuroprotection was associated with anatomical preservation of the number and size of striatal neurons from cell death and cell atrophy. Additionally, GDNF-treated mice had a lower percentage of neurons containing mutant huntingtin-stained inclusion bodies, a hallmark of HD pathology. These data further support the concept that viral vector delivery of GDNF may be a viable treatment for patients suffering from HD.
Keywords: gene therapy, neurodegeneration, neuroprotection, polyglutamine, adenoassociated virus
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder resulting from an expanded trinucleotide (CAG: cytosine, adenine, and guanine) repeat at the IT15 locus on chromosome 4 (1) within the huntingtin gene. The abnormal DNA is translated into mutant huntingtin with an expanded glutamine stretch at the N terminus of the protein. The excessive number of glutamine repeats is responsible for the misfolding of huntingtin and the subsequent formation of neuronal inclusions, degeneration of striatal and cortical neurons, and a triad of symptoms including severe motor, cognitive, and psychological disturbances that are ultimately fatal.
To date, HD remains incurable. Several therapies have yielded positive results in animal models, including those that alleviate potential glutamate-induced excitotoxicity such as riluzole and remacemide (2–4); those that increase the production of energy in the form of ATP in the cell, including creatine and coenzyme Q10 (5–9); those that inhibit caspase activation and apoptosis, such as minocycline (10, 11); and those that aim at replacing degenerating cells by means of fetal tissue transplantation (12–18). However, when tested clinically, none has made a major impact in the symptomatic treatment of HD, nor have any demonstrated the ability to alter the natural history of the disease by preventing cell death.
Genetic testing can identify mutated gene carriers destined to suffer from HD. Unlike other neurodegenerative disorders, identification of the genetic marker provides the unique opportunity to intercede therapeutically before the onset of symptoms that result from neuronal degeneration. Toward this end, trophic factors in general, and glial cell line-derived neurotrophic factor (GDNF) in particular, have shown promise in animal models of several different neurodegenerative disorders, including HD (19–22). In addition to its potent trophic effects on dopaminergic midbrain neurons (23, 24), GDNF has also been shown to protect striatal, medium-sized spiny GABA projection neurons, the neuronal population most vulnerable in HD (22). Moreover, the expression of GDNF’s receptors (GFRα-1 and Ret) is up-regulated in striatal neurons and astrocytes after injury (25), supporting its role as a potential trophic factor for HD. We and others have characterized GDNF’s protective effects in neurotoxic rat models of HD (22, 26–30). However, before being tested in patients, GDNF’s ability to provide neuroprotection in the HD transgenic mouse should be demonstrated, because this model more closely mimics the genetic nature of human HD.
In the present study we tested the hypothesis that delivery of GDNF into the striatum by a recombinant adenoassociated viral vector (rAAV) can preserve motor function and prevent striatal cell loss in the N171-82Q transgenic mouse model (3). N171-82Q mice contain a human cDNA encoding for the N-terminal fragment of huntingtin with 82 glutamine repeats. This is a shorter number of glutamine repeats compared with those of other transgenic and knockin mouse models (31, 32) and may be more clinically relevant to the number of repeats found in HD patients. Moreover, the shorter repeat length leads to a protracted course of disease compared with what has been observed in the other transgenic mouse models, allowing a potentially larger therapeutic window in which to administer trophic factors such as GDNF. N171-82Q mice exhibit evidence of degenerating neurons, astrogliosis, and the formation of inclusions in the striatum along with motor deficits including loss of coordination, gait abnormalities, hypokinesia, hind limb clasping behavior, and muscle weakness (3, 33). Here we report that bilateral AAV-GDNF delivery provides neuroprotection in the N171-82Q mouse model of HD by enhancing rotorod performance, diminishing hind limb clasping, reducing the density of mutant huntingtin-containing inclusions, and preventing the death and atrophy of striatal neurons.
Results
GDNF and eGFP Expression After rAAV Delivery.
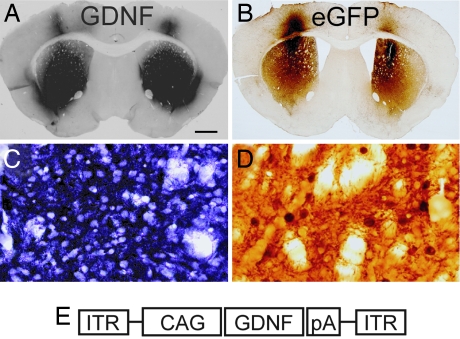
Eleven weeks after injection, numerous GDNF or eGFP immunoreactive (ir) cells were seen bilaterally in the striatum in all mice receiving AAV-GDNF or AAV-eGFP, respectively (Fig. 1A and B). Qualitative observations revealed that GDNF immunoreactivity covered approximately >90% of the striatum and eGFP-ir in >75% of the striatum; this discrepancy may be because GDNF is a secreted protein and eGFP is not. Both cell bodies and fibers coursing through the striatal gray matter stained positive for the appropriate transgene (Fig. 1 C and D). GDNF and eGFP immunoreactivity was also observed in the overlying cortex as a result of injection spread. Retrograde transport of both GDNF and eGFP was seen in the substantia nigra pars compacta, and anterograde transport was seen in the globus pallidus and substantia nigra pars reticulata (data not shown).
Fig. 1.
AAV2 delivers widespread expression of GDNF and eGFP throughout the striatum. Robust GDNF (A and C) and eGFP (B and D) immunoreactivity was seen in the striatum of N171-82Q transgenic mice 11 weeks after injection (week 16 of life). High-power magnification (C and D) shows positive staining in the cell bodies as well as the neurites. GDNF immunoreactivity was never detected in striatal tissue from AAV-eGFP-injected transgenic mice or WT littermates (data not shown). (E) Schematic of the AAV-GDNF viral vector genome. (Scale bar: A and B, 400 μm; C and D, 16.5 μm.)
Striatal Delivery of AAV-GDNF Improves Behavioral Function in HD Mice.
The accelerating rotorod and hind limb clasping tests evaluated the development of behavioral deficits over the 11 weeks of the study. AAV-eGFP-injected mice performed significantly worse over the course of the study compared with WT littermates (P < 0.05, Fig. 2A). This effect emerged as statistically significant at week 10 of life. Importantly, AAV-GDNF-treated mice performed significantly better than the AAV-eGFP-injected mice from week 10 to week 16 (P < 0.05 for all weeks analyzed). AAV-GDNF-treated mice performed similarily to WT mice until the last 3 weeks of testing (P < 0.05).
Fig. 2.
AAV-GDNF administration attenuates behavioral deficits in N171-82Q transgenic mice. (A) AAV-eGFP-injected N171-82Q mice (n = 6) performed significantly worse over the course of the study compared with WT (n = 7) on the rotorod (∗∗, P < 0.05). AAV-GDNF-injected mice (n = 7) performed significantly better than AAV-eGFP-injected mice (∗∗, P < 0.05) and were only significantly different from WT at the last three time points measured (#). (B) Clasping behavior emerged later in life in AAV-GDNF-treated mice compared with AAV-eGFP controls. (C) Fewer GDNF-treated mice clasped throughout the study compared with eGFP-treated mice (∗, P < 0.05).
Mice were evaluated twice a week on the hind limb clasping test. Clasping emerged at week 12 for AAV-eGFP-injected mice, with greater numbers of these mice exhibiting this behavior as the experiment progressed. AAV-GDNF treatment delayed the emergence of clasping, and fewer AAV-GDNF-treated mice clasped at each time point compared with AAV-eGFP-treated mice (Fig. 2B). The total number of clasping events per mouse over the duration of the experiment was summed, and significant differences between transgenic groups were observed. (P < 0.05) (Fig. 2C). WT mice never exhibited clasping behavior.
AAV-GDNF Treatment Prevents Neuronal Atrophy and Death in the Striatum.
Upon gross examination, brains from both groups of transgenic mice (AAV-GDNF- and AAV-eGFP-injected) appeared smaller in size compared with those of WT controls. Both AAV-GDNF-injected (12.3 ± 0.4) and AAV-eGFP-injected (11.6 ± 0.9) mice had smaller striatal volumes compared with WT cohorts (15.0 ± 0.3) (P < 0.001) (Fig. 3A).
Fig. 3.
AAV-GDNF treatment prevents striatal cell death and cell atrophy. (A) Both AAV-GDNF-injected mice (18% decrease) and AAV-GFP-injected mice (23% decrease) had significantly smaller striata compared with WT littermates (P < 0.01). (B) Stereological counts of striatal NeuN-ir neurons demonstrated that AAV-GDNF-treated mice had significantly more striatal neurons compared with AAV-eGFP-treated mice (P < 0.05). (C) GDNF treatment also prevented neuronal atrophy. AAV-GDNF-injected mice had 15% larger cell bodies compared with AAV-eGFP-injected mice. Striatal volume, neuronal number, and neuronal size analyses were performed at week 16 of life.
Although AAV-GDNF treatment did not prevent the reduction in striatal volume engendered by the HD mutation, it significantly preserved the number and volume of striatal neurons. Unbiased stereological counts estimated 2.9 × 106± 1.2 × 105 NeuN-positive cells in the striatum of WT mice. Significantly fewer (24%) striatal NeuN-ir neurons were estimated in AAV-eGFP-treated HD mice (2.2 × 106± 1.7 × 105) (P < 0.05). In contrast, AAV-GDNF-treated mice had significantly more (19%) NeuN-positive striatal neurons (2.7 × 106± 1.2 × 105) (P < 0.05) compared with AAV-eGFP-treated mice, and estimated counts were statistically similar to WT controls(P = 0.27).
In addition to having more striatal NeuN-positive neurons, mice injected with AAV-GDNF also had larger NeuN-positive neurons. The nucleator method was used to quantify the average volume of neuronal cell bodies in the striatum. The mean volume (cubic micrometers) of NeuN-ir striatal neurons from AAV-eGFP-injected mice (502 ± 36.6) was significantly less (20%) than those of WT littermate mice (630 ± 17.3) (P < 0.01). NeuN-positive cell bodies in the AAV-GDNF-treated mice (591 ± 26.0) were significantly larger (15%) than those measured from AAV-eGFP-treated mice (P < 0.05) and statistically similar to WT controls (P > 0.05).
In addition to stereological estimation of the number and size of striatal neurons, we evaluated GDNF’s potential effects on the nigrostriatal dopamine system by measuring the optical density of tyrosine hydroxylase (TH)-positive fibers in the striatum. Average values for striatal optical density expressed as mean ± SEM were as follows: AAV-eGFP, 140 ± 6.6; AAV-GDNF, 160 ± 7.3; WT, 150 ± 4.2. There were no statistical differences in TH optical density values among the three groups (P = 0.26).
AAV-GDNF Alters Mutant Huntingtin Pathology in HD Mice.
Mutant huntingtin-positive (mHtt+) inclusion bodies are a prominent pathological feature in HD patients that are replicated in most transgenic HD models. mHtt+ inclusions were never present in WT mice (Fig. 4A) but were present in N171-82Q transgenic mice, regardless of treatment group (Fig. 4B) and characterized by dark, dense inclusion bodies (E). Stereological counts of EM48-ir striatal inclusions demonstrated no significant differences between mice injected with AAV-GDNF (235,710 ± 20,159) and mice injected with AAV-eGFP (264,621 ± 49,612) (Fig. 4C) (P > 0.05). However, because there were significantly more NeuN-ir neurons in the striata of AAV-GDNF-injected mice, we also evaluated the ratio of neurons in the striatum that contained inclusion bodies. Mice treated with AAV-GDNF had a significantly lower percentage (8.6 ± 0.03%) of striatal neurons that contained EM48-ir inclusions compared with AAV-GFP-treated mice (12.2 ± 1.4%) (Fig. 4D) (P < 0.01).
Fig. 4.
AAV-GDNF reduces the percentage of neurons with mHtt+ inclusions. WT mice never exhibited mHtt+ inclusions in the striatum (A). In contrast, transgenic mice, regardless of group (the example above is an AAV-eGFP-injected mouse), showed evidence of mHtt+ inclusions in the striatum (B). (E) Evidence of cells with robust inclusions. (C) Whereas no differences in the total number of striatal inclusions were observed between AAV-GDNF-injected mice and AAV-eGFP-injected mice (P > 0.05), a significant decrease in the percentage of neurons with inclusions was observed (D) (P < 0.01). (Scale bar: A and B, 500 μm; E, 10 μm.) Inclusion quantification was performed at week 16 of life.
Discussion
To our knowledge, this study is the first to demonstrate that viral delivery of GDNF to the striatum of presymptomatic mice can prevent the structural and functional degeneration seen in a transgenic model of HD. We observed robust gene expression of GDNF and eGFP for up to 11 weeks after viral delivery to the striatum. AAV-GDNF improved rotorod performance, delayed and attenuated clasping behavior, and preserved the number and size of striatal neurons in this model. Both clinical (34–36) and preclinical (3, 31, 33) studies suggest that HD symptoms can occur before the frank loss of striatal neurons. The prevention of neuronal atrophy by AAV-GDNF suggests that an early aspect of the degenerative process that mediates symptom onset might be prevented by this treatment. The lack of difference in whole striatal volume of AAV-GDNF-treated mice compared with AAV-eGFP-treated mice suggests potential atrophy of neuronal processes within the striatum, either those that arise from striatal neurons themselves or those that course through the striatum via the internal capsule. Interestingly, the protection of striatal neurons was associated with a lower percentage of neurons containing inclusion bodies consisting of pathogenic, mutated huntingtin protein. It is unknown whether GDNF partially prevented the formation of inclusion bodies or assisted in their breakdown and subsequent processing by the ubiquitin proteosome pathway. Alternatively, GDNF may have been capable only of protecting cells that had not yet formed inclusions. Empirical studies should address this question, the answer to which will be important in the timing of AAV-GDNF administration, especially if the latter is the case and GDNF is unable to elicit trophic effects on cells that have already formed inclusion bodies. These data extend previous demonstrations by our laboratory and others (22, 26–30) that AAV-GDNF is protective in neurotoxin-based HD models and now establish its beneficial effects in a transgenic mouse model.
The data presented in this study stand in contrast to the study by Popovic et al. (37), who delivered GDNF via a lentiviral vector into the striata of R6/2 transgenic HD mice. Although excellent gene expression was demonstrated in the striatum, they did not observe functional improvement or differences in the number of mHtt+ striatal inclusions compared with control mice. This difference may be attributable to the transgenic mouse model used. R6/2 mice contain a human cDNA that encodes mutant huntingtin protein with 145 CAG repeats, the larger number of repeats resulting in a more severe model in terms of the time course of behavioral and pathological sequelae compared with N171-82Q mice. In their study, R6/2 mice were treated with lentiviral GDNF at week 5, when the anatomical and behavioral symptoms had potentially already commenced. Recent reports demonstrate that inclusions have already formed in the striatum of R6/2 mice by postnatal day 1, brain weight is significantly reduced by 4 weeks of age (38), and by week 5 R6/2 mice show significant reductions in running, climbing, and open-field behavior compared with WT littermates (39). In the present study, AAV-GDNF was administered to the N171-82Q mice at week 5, and GDNF was already optimally expressed before the behavioral syndrome began, around week 10. Taken together, these two studies suggest that gene delivery of GDNF may be beneficial when applied to asymptomatic HD gene carriers but may be less effective for patients already displaying behavioral symptoms. If further studies validate this hypothesis, testing surgical treatment strategies in asymptomatic patients will be a future challenge given the number of patients required and the time needed to ascertain a clinical readout.
GDNF’s ability to rescue dopaminergic neuron loss in patients with Parkinson’s disease has been evaluated in three published clinical trials to date (40–42). Although the efficacy of GDNF to improve motor scores on the Unified Parkinson’s Disease Rating Scale has been variable, reported side effects in these studies were mostly attributed to problems with the delivery methods used, including putamenal delivery via a pump (41, 42) and intracerebroventricular delivery (40). Side effects that were most likely due to GDNF itself were minimal and included nausea, vomiting, headache, and L’Hermitte’s sign (40–42).
The exact mechanism of how mutant huntingtin leads to the devastating course of events that culminates in cell death in HD remains elusive. It has been suggested that mutant huntingtin is cleaved and translocates into the nucleus, where it activates apoptosis (43). Additionally, it has been demonstrated that the lack of normal huntingtin in HD patients and transgenic mice leads to a decrease in the transcription of trophic factors, such as BDNF (44). Upon binding to its GFRα1/Ret receptor complex, GDNF activates the inositol triphosphate and the mitogen-activated protein kinase intracellular cascades. Stimulation of these pathways results in cell and neurite outgrowth and inactivation of caspases 3 and 9 (via Akt), effectively inhibiting apoptosis. Although GDNF’s exact role in preventing cell death in the N171-82Q transgenic model of HD remains to be established, we speculate that increasing trophic support and inhibiting apoptosis via these two pathways likely play integral roles.
The present study demonstrates that the viral delivery of GDNF protects striatal neurons from mutant huntingtin-induced cell death. Additionally, AAV-GDNF treatment significantly attenuates impairments with balance and coordination and delays the hind limb clasping phenomenon. These results support the concept that the striatal delivery of AAV-GDNF may be a viable therapy for patients with HD.
Materials and Methods
Animals.
N171-82Q transgenic mice [strain B6C3F1/J-TgN(HD82Gln)81Dbo] and WT littermates (B6C3F1/J background strain) were used in this experiment. Breeding pairs were obtained from The Jackson Laboratory, and all mating took place at Rush University. Male N171-82Q transgenic mice were always bred with female WT littermates. Mice were housed in groups of either two or three per cage on a 12-h light/dark cycle, with chow and water provided ad libitum. For all analyses, the same three groups of mice were used: group 1, vehicle-injected WT mice (n = 7); group 2: AAV-GDNF-injected N171-82Q mice (n = 7); group 3, AAV-eGFP-injected N171-82Q mice (n = 6). All experiments were carried out in accordance with federal guidelines of proper animal care and with the approval of the Rush University Medical Center Animal Care Committee. PCR was performed to genotype all mice by using primers previously described (3). CAG repeat number was confirmed by a separate PCR assay with primers flanking the repeat sequence, followed by polyacrylamide gel electrophoresis of products and comparison of bands with known size standards from human HD patients.
Construction of AAVs.
AAV-GDNF and AAV-eGFP viral vectors (serotype 2) were used in this study. The vector genome consisted of the GDNF or eGFP expression cassette flanked by the inverted terminal repeats from AAV2. The expression cassette consisted of the hybrid CAG promoter (including a human cytomegalovirus enhancer, a chicken β-actin promoter and splice donor, and a rabbit β-globin splice acceptor) driving expression of the GDNF or eGFP cDNAs and the polyadenlylation sequence from human growth hormone gene. Viral vectors were produced in human embryonic kidney 293 (HEK293) cells by using the calcium phosphate triple plasmid transfection method. Three days after transfection cells were harvested and lysed. AAV was purified from the cell lysates by heparin and ion-exchange column chromatography. Purified particles were concentrated by centrifugal filtration, and vector titer was determined by quantitative PCR. All vectors were created by Ceregene.
rAAV Injections.
At 5 weeks of life, mice were anesthetized with a ketamine (10 mg/kg) and xylazine (100 mg/kg) mix (0.1 ml per 10 g per mouse, administered i.p.), and their heads were shaved, sterilized with betadine, and placed in a mouse stereotaxic frame (Kopf Instruments, Tujunga, CA). A midline incision was made, and bilateral burr holes were created over the striatum with a dental drill. One injection (2 μl) of AAV-GDNF, AAV-eGFP, or vehicle was made on each side of the striatum (0.86 mm rostral to bregma, 1.8 mm lateral to midline, and 3.5 mm ventral to the skull surface). Each mouse received a total of 4 × 109 vector genomes, distributed bilaterally. All injections were performed through a 10-μl Hamilton syringe connected to an infusion pump at a rate of 0.2 μl/min. Needles (33-gauge, blunt-tipped) were left in situ for an additional 5 min to allow the injectate to diffuse from the needle tip. The scalp was closed with 5-0 polyvicryl suture.
Behavioral Analyses.
All behavioral analyses were performed in a blinded fashion.
Accelerating Rotorod Test.
All mice were assessed (twice per week) on the accelerating rotorod test beginning at week 4 of life (1 week before injection of viral vectors) and every week thereafter until they were killed at postnatal week 16. The rod accelerated from 0 to 30 rotations per minute over 300 s, and the latency to fall was recorded. Three trials were performed in each testing day, with a 1-h interval between trials. At each time point and for each animal, the three trials latency-to-fall were recorded, and the average was used for statistical analysis.
Hind Limb Clasping Test.
All mice were assessed (twice per week) via the clasping test beginning at week 4 of life and every week thereafter until they were killed. The clasping test evaluated the mice’s hind limb response during tail suspension 10 cm above their home cage. Mice received a score of 0 for a normal hind limb extension and a score of 1 when hind limbs were clasped.
Immunohistochemical Analysis.
By using the biotin-labeled antibody procedure described previously (30), 40-μm-thick, free-floating, coronal sections were submitted to a series of protein detection analyses using antibodies against NeuN (1:1,000; Chemicon), GDNF (1:250; R & D Systems), eGFP (1:2,000; Clontech), TH (1:10,000; Chemicon), and mutant huntingtin (mEM48 clone, 1:50; Chemicon). Briefly, primary antibody incubations (overnight) were followed by secondary antibody incubations (1 h) with the appropriate biotinylated IgG secondary antibodies (1:200; Vector Laboratories). Nickel intensification was used in the GDNF staining procedure. Controls consisted of substitution of an irrelevant IgG in lieu of the primary antibody.
Stereological Analysis.
Neuronal counts of NeuN-ir or mutant huntingtin-ir neurons were performed by using an unbiased, design-based stereology procedure as described previously (30). Neuronal and inclusion counts for each subject were made throughout the striatum by using five equally spaced serial sections spaced 480 μm apart. Using stereoinvestigator software (Microbrightfield), the optical fractionator method estimated the number, and the nucleator procedure quantified the cell volume of NeuN-ir cells. The optical fractionator method was also used to estimate the number of mHtt+ inclusions. Striatal volume was quantified on the same five equally spaced sections of NeuN-stained tissue by using the Cavalieri method.
TH Optical Density Analysis.
The striatum was outlined at ×2 magnification and the optical density of TH-positive fibers was assessed by using the scion image analysis program. Background levels were captured from the corpus callosum in each section and subtracted from the total optical density measurement. The optical density number reported is the average optical density from each of the five measured sections. All analyses were performed in a blinded fashion.
Statistical Analysis.
A two-way repeated-measures ANOVA with Student–Neuman–Keul’s post hoc analyses was used to compare group performance on the rotorod test. Hind limb clasping behavior was summed for each animal across time, and Kruskal–Wallis nonparametric analyses, along with Mann–Whitney pairwise comparisons, assessed significant differences between individual groups. The number and size of NeuN-ir cells in the striatum, the number of inclusions, total striatal volume, and TH optical density were compared by using a one-way ANOVA with Scheffé’s post hoc tests to assess for significant differences between individual groups.
Acknowledgments
This research was funded by the Charles and M. V. Shapiro Foundation and National Institutes of Health Grant T32AG00269.
Abbreviations
- AAV
adenoassociated viral vector
- HD
Huntington’s disease
- GDNF
glial cell line-derived neurotrophic factor
- TH
tyrosine hydroxylase
- ir
immunoreactive
- mHtt+
mutant huntingtin-positive.
Footnotes
Conflict of interest statement: M.G., R.T.B., C.D.H., E.P.B., and J.H.K. have a financial interest in Ceregene, Inc.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Huntington’s Disease Collaborative Research Group Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Mary V., Wahl F., Stutzmann J. M. Neurosci. Lett. 1995;201:92–96. doi: 10.1016/0304-3940(95)12137-s. [DOI] [PubMed] [Google Scholar]
- 3.Schilling G., Becher M. W., Sharp A. H., Jinnah H. A., Duan K., Kotzuk J. A., Slunt H. H., Ratovitski T., Cooper J. K., Jenkins N. A., et al. Hum. Mol. Genet. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- 4.Schiefer J., Landwehrmeyer G. B., Luesse H. G., Sprunken A., Puls C., Milkereit A., Milkereit E., Kosinski C. M. Movement Disorders. 2002;17:748–757. doi: 10.1002/mds.10229. [DOI] [PubMed] [Google Scholar]
- 5.Beal M. F., Henshaw D. R., Jenkins B. G., Rosen B. R., Schulz J. B. Ann. Neurol. 1994;36:882–888. doi: 10.1002/ana.410360613. [DOI] [PubMed] [Google Scholar]
- 6.Schilling G., Coonfield M. L., Ross C. A., Borchelt D. R. Neurosci. Lett. 2001;315:149–153. doi: 10.1016/s0304-3940(01)02326-6. [DOI] [PubMed] [Google Scholar]
- 7.Matthews R. T., Yang L., Jenkins B. G., Ferrante R. J., Rosen B. R., Kaddurah-Daouk R., Beal M. F. J. Neurosci. 1998;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrante R. J., Andreassen O. A., Jenkins B. G., Dedeoglu A., Kuemmerle S., Kubilus J. K., Kaddurah-Daouk R., Hersch S. M., Beal M. F. J. Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shear D. A., Haik K. L., Dunbar G. L. NeuroReport. 2000;11:1833–1837. doi: 10.1097/00001756-200006260-00007. [DOI] [PubMed] [Google Scholar]
- 10.Chen M., Ona V. O., Li M., Ferrante R. J., Fink K. B., Zhu S., Bian J., Guo L., Farrell L. A., Hersch S. M., et al. Nat. Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 11.Wang X., Zhu S., Drozda M., Zhang W., Stavrovskaya I. G., Cattaneo E., Ferrante R. J., Kristal B. S., Friedlander R. M. Proc. Natl. Acad. Sci. USA. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isacson O., Brundin P., Gage F. H., Bjorklund A. Neuroscience. 1985;16:799–817. doi: 10.1016/0306-4522(85)90095-8. [DOI] [PubMed] [Google Scholar]
- 13.Isacson O., Dunnett S. B., Bjorklund A. Proc. Natl. Acad. Sci. USA. 1986;83:2728–2732. doi: 10.1073/pnas.83.8.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isacson O., Dawbarn D., Brundin P., Gage F. H., Emson P. C., Bjorklund A. Neuroscience. 1987;22:481–497. doi: 10.1016/0306-4522(87)90348-4. [DOI] [PubMed] [Google Scholar]
- 15.Sanberg P. R., Henault M. A., Deckel A. W. Pharmacol. Biochem. Behav. 1986;25:297–300. doi: 10.1016/0091-3057(86)90269-8. [DOI] [PubMed] [Google Scholar]
- 16.Tulipan N., Luo S. Q., Allen G. S., Whetsell W. O. Exp. Neurol. 1998;102:325–332. doi: 10.1016/0014-4886(88)90227-0. [DOI] [PubMed] [Google Scholar]
- 17.Borlongan C. V., Koutouzis T. K., Poulos S. G., Saporta S., Sanberg P. R. Cell Transplant. 1998;7:131–135. doi: 10.1177/096368979800700208. [DOI] [PubMed] [Google Scholar]
- 18.Dunnett S. B., Isacson O., Sirinathsinghji D. J. S., Clarke D. J., Björklund A. Neuroscience. 1998;24:813–820. doi: 10.1016/0306-4522(88)90069-3. [DOI] [PubMed] [Google Scholar]
- 19.Hoffer B. J., Hoffman A., Bowenkamp K., Huettl P., Hudson J., Martin D., Lin L. F. H., Gerhardt G. A. Neurosci. Lett. 1994;182:107–111. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 20.Kim B. T., Rao V. L., Sailor K. A., Bowen K. K., Dempsey R. J. J. Neurosurg. 2001;95:674–679. doi: 10.3171/jns.2001.95.4.0674. [DOI] [PubMed] [Google Scholar]
- 21.Watabe K., Ohashi T., Sakamoto T., Kawazoe Y., Takeshima T., Oyanagi K., Inoue K., Eto Y., Kim S. U. J. Neurosci. Res. 2000;60:511–519. doi: 10.1002/(SICI)1097-4547(20000515)60:4<511::AID-JNR10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Navarro E., Arenas E., Reiriz J., Calvo N., Alberch J. Neuroscience. 1996;75:345–352. doi: 10.1016/0306-4522(96)00336-3. [DOI] [PubMed] [Google Scholar]
- 23.Lin L.-F. H., Doherty D. H., Lile J. D, Bektesh S., Collins F. Science. 1993;260:1130–1134. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 24.Choi-Lundberg D. L., Lin Q., Chang Y. N., Chiang Y. L., Hay C. M., Mohajeri H., Davidson B. L., Bohn M. C. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 25.Marco S., Canudas A. M., Canals J. M., Gavalda N., Perez-Navarro E., Alberch J. Exp. Neurol. 2002;174:243–252. doi: 10.1006/exnr.2001.7859. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Navarro E., Arenas E., Marco S., Alberch J. Eur. J. Neurosci. 1999;11:241–249. doi: 10.1046/j.1460-9568.1999.00433.x. [DOI] [PubMed] [Google Scholar]
- 27.Araujo D. M., Hilt D. C. Neuroscience. 1997;81:1099–1110. doi: 10.1016/s0306-4522(97)00079-1. [DOI] [PubMed] [Google Scholar]
- 28.Araujo D. M., Hilt D. C. Neuroscience. 1998;82:117–127. doi: 10.1016/s0306-4522(97)00266-2. [DOI] [PubMed] [Google Scholar]
- 29.Gratacos E., Perez-Navarro E., Tolosa E., Arenas E., Alberch J. J. Neurochem. 2001;78:1287–1296. doi: 10.1046/j.1471-4159.2001.00538.x. [DOI] [PubMed] [Google Scholar]
- 30.McBride J. L., During M. J., Wuu J., Chen E. Y., Leurgans S. E., Kordower J. H. Exp. Neurol. 2003;181:213–223. doi: 10.1016/s0014-4886(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 31.Mangiarini L., Sathasivam K., Seller M., Cozens B., Harper A., Hetherington C., Lawton M., Trottier Y., Lehrach H., Davies S., Bates G. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 32.Menalled L. B., Sison J. D., Dragatsis I., Zeitlin S., Chesselet M. F. J. Comp. Neurol. 2003;465:11–26. doi: 10.1002/cne.10776. [DOI] [PubMed] [Google Scholar]
- 33.Yu Z. X., Li S. H., Evans J., Pillarisetti A., Li H., Li X. J. J. Neurosci. 2003;23:2193–2202. doi: 10.1523/JNEUROSCI.23-06-02193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albin R. L., Young A. B., Penney J. B., Handelin B., Balfour R., Anderson K. D., Markel D. S., Tourtellotte W. W., Reiner A. N. Engl. J. Med. 1990;322:1293–1298. doi: 10.1056/NEJM199005033221807. [DOI] [PubMed] [Google Scholar]
- 35.Albin R. L., Qin Y., Young A. B., Penney J. B., Chesselet M. F. Ann. Neurol. 1991;30:542–549. doi: 10.1002/ana.410300406. [DOI] [PubMed] [Google Scholar]
- 36.Albin R. L., Reiner A., Anderson K. D., Dure L. S., IV, Handelin B., Balfour R., Whetsell W. O., Jr, Penney J. B., Young A. B. Ann. Neurol. 1992;31:425–430. doi: 10.1002/ana.410310412. [DOI] [PubMed] [Google Scholar]
- 37.Popovic N., Maingay M., Kirik D., Brundin P. Exp. Neurol. 2005;193:65–74. doi: 10.1016/j.expneurol.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Stack E. C., Kubilus J. K., Smith K., Cormier K., Del Signore S. J., Guelin E., Ryu H., Hersch S. M., Ferrante R. J. J. Comp. Neurol. 2005;490:354–370. doi: 10.1002/cne.20680. [DOI] [PubMed] [Google Scholar]
- 39.Hickey M. A., Gallant K., Gross G. G., Levine M. S., Chesselet M. F. Neurobiol. Dis. 2005;20:1–11. doi: 10.1016/j.nbd.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 40.Nutt J. G., Burchiel K. J., Comella C. L., Jankovic J., Lang A. E., Laws E. R., Jr, Lozano A. M., Penn R. D., Simpson R. K., Jr, Stacy M., Wooten G. F. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 41.Patel N. K, Bunnage M., Plaha P., Svendsen C. N., Heywood P., Gill S. S. Ann. Neurol. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- 42.Lang A. E., Gill S., Patel N. K., Lozano A., Nutt J. G., Penn R., Brooks D. J., Hotton G., Moro E., Heywood P., et al. Ann. Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 43.Kim Y. J., Yi Y., Sapp E., Wang Y., Cuiffo B., Kegel K. B., Qin Z. H., Aronin N., DiFiglia M. Proc. Natl. Acad. Sci. USA. 2001;98:12784–12789. doi: 10.1073/pnas.221451398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuccato C., Ciammola A., Rigamonti D., Leavitt B. R., Goffredo D., Conti L., MacDonald M. E., Friedlander R. M., Silani V., Hayden M. R., et al. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]