Abstract
We studied item and source memory with fMRI in healthy volunteers and carried out a parallel study in memory-impaired patients. In experiment 1, volunteers studied a list of words in the scanner and later took an item memory test and a source memory test. Brain activity in the hippocampal region, perirhinal cortex, and parahippocampal cortex was associated with words that would later be remembered (item memory). The activity in these regions that predicted subsequent success at item memory predicted subsequent source memory to a similar degree. In experiment 2, memory-impaired patients with damage thought to be limited to the hippocampal region were given an item memory test and a source memory test, as in experiment 1. The patients were similarly impaired on the item memory test and the source memory test. Together, the findings suggest that medial temporal lobe structures broadly support recognition memory function and that item memory and source memory similarly depend on these structures.
Keywords: amnesia, hippocampus, recognition memory
One of the most widely studied examples of declarative memory is recognition memory, the ability to judge an item as having been encountered previously. Recognition memory depends on the integrity of the medial temporal lobe (MTL), which includes the hippocampal region (subicular complex, CA fields, and dentate gyrus) and the entorhinal, perirhinal, and parahippocampal cortices (1). Much of what is known about the anatomy and organization of human recognition memory has come from the systematic study of patients with circumscribed damage to MTL structures. More recently, recognition memory has been studied in the healthy brain by using functional magnetic resonance imaging (fMRI).
A useful technique in many of these fMRI studies is the subsequent memory paradigm (2). In this paradigm, brain activity is measured with fMRI while volunteers study a list of items (e.g., words or pictures). Later, participants take a recognition memory test outside of the scanner. Brain activity associated with items that will later be remembered can then be compared to brain activity associated with items that will later be forgotten. Typically, structures within the MTL are identified by such a contrast (3).
A fundamental but controversial issue concerns the possible division of labor for recognition memory function within the MTL. Some studies using the subsequent memory paradigm reported that memory for the context in which an item is learned (source memory) is predicted especially by activity during study in the hippocampal region (and possibly parahippocampal cortex) and that memory for the item itself (item memory) is predicted especially by activity during study in the adjacent cortex (4–6). Others have found that item memory is predicted by widespread activity in the MTL during study, including in the hippocampus (7–9). Furthermore, it has been suggested that recognition memory judgments lie along a continuum of memory strength, and that in some respects categories like item memory and source memory can be viewed as representing weaker or stronger memories along the strength continuum (10, 11).
fMRI studies of healthy volunteers and studies of memory-impaired patients provide complementary methods for exploring the anatomy of recognition memory. Yet, these two methods have been used infrequently in the same study of memory and cognition. We have carried out an fMRI study of item and source memory in healthy volunteers and a parallel study of item and source memory in memory-impaired patients.
Results
Experiment 1.
Behavior.
Participants scored 82.5 ± 1.5% correct for the item memory judgment (old/new decision: 76.6 ± 2.6% hit rate and 11.5 ± 1.7% false-alarm rate, d′ = 2.03 ± 0.12) and made 82.0 ± 1.5% correct source judgments for the items that they correctly judged as old. The kind of imagery carried out at encoding had no effect on recognition performance (indoor imagery, 76.9 ± 3.0% correct; outdoor imagery, 76.2 ± 2.6% correct).
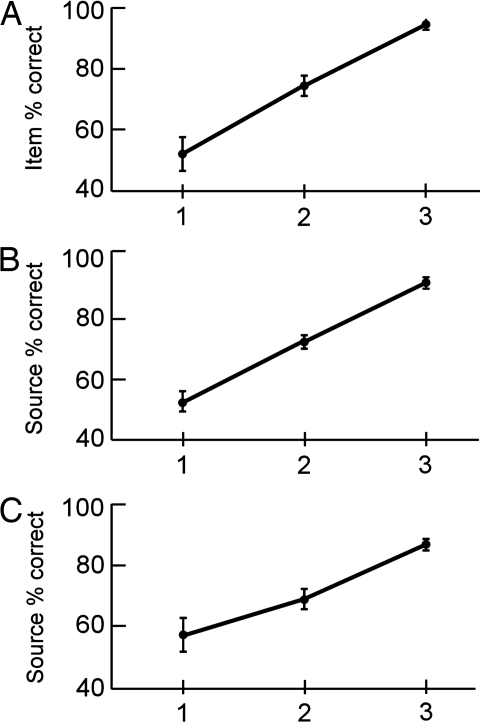
The confidence ratings given during the recognition memory test correlated with successful performance (Fig. 1). First, increasing confidence in the item memory decision correlated with item memory success (r = 0.89, P < 0.001). Specifically, item confidence ratings of 1, 2, and 3 were associated with item memory scores of 54.8%, 69.7%, and 96.9% correct, respectively (Fig. 1A). Second, increasing confidence in the source memory judgment correlated with source memory success (r = 0.90, P < 0.001). Source confidence ratings of 1, 2, and 3 were associated with source memory scores of 56.0%, 74.2%, and 91.2% correct, respectively (Fig. 1B). Finally, increasing confidence in the item memory judgments correlated with increasing source memory success (r = 0.82, P < 0.001). Item confidence ratings of 1, 2, and 3 were associated with source memory scores of 56.3%, 69.9%, and 86.8% correct, respectively (Fig. 1C).
Fig. 1.
Fifteen participants rated their confidence in both their item- and source-memory decisions. (A) Increasing confidence in the item decision (1, 2, or 3) correlated with increasing item memory success. (B) Increasing confidence in the source decision (1, 2, or 3) correlated with increasing source memory success. (C) Increasing confidence in the item decision (1, 2, or 3) also correlated with increasing source memory success. Brackets show SEM.
fMRI results.
Four contrasts were of interest: (i) remembered vs. forgotten; (ii) item and source vs. forgotten; (iii) item only vs. forgotten; and (iv) item and source vs. item only. Figs. 2–5 show the results of these contrasts for the MTL. Activity reflects the area under the hemodynamic response function for the 12 s after stimulus presentation. Figs. 2–5 Bottom show the center-of-mass of voxel clusters. Table 2, which is published as supporting information on the PNAS web site, lists the regions in the MTL and in other brain areas in which significant activity was identified.
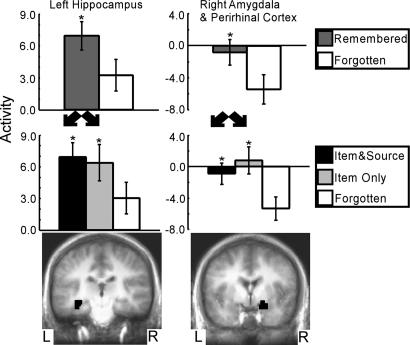
Fig. 2.
The remembered vs. forgotten contrast found two regions in the medial temporal lobe that predicted subsequent item memory success, irrespective of source memory success (Top and Bottom). Within both regions, activity for words that would later be remembered along with correct source memory judgments (item and source) was similar to activity for words that would later be remembered but with incorrect source memory judgments (item only) (Middle). Asterisks indicate a difference relative to the forgotten condition (P < 0.05). Brackets show SEM.
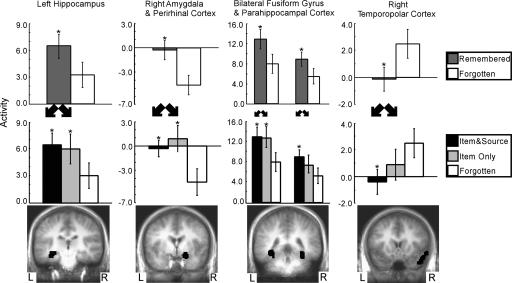
Fig. 3.
The item and source vs. forgotten contrast found five regions in the medial temporal lobe that predicted subsequent item memory and source memory success (Middle and Bottom). Within all of these regions, activity predicted subsequent item memory success (Top). Also, activity for words that would later be remembered along with correct source memory judgments (item and source) was similar to activity for words that would later be remembered but with incorrect source memory judgments (item only) (Middle). Asterisks indicate a difference relative to the forgotten condition (P < 0.05). Brackets show SEM.
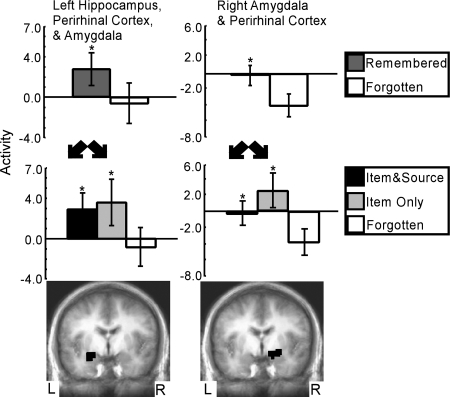
Fig. 4.
The item-only vs. forgotten contrast found two regions in the medial temporal lobe that predicted subsequent item memory success along with incorrect source memory judgments (Middle and Bottom). Within both regions, activity predicted subsequent item memory success (Top). Also, activity for words that would later be remembered along with correct source judgments (item and source) was similar to activity for words that would later be remembered but with incorrect source judgments (item only). Asterisks indicate a difference relative to the forgotten condition (P < 0.05). Brackets show SEM.
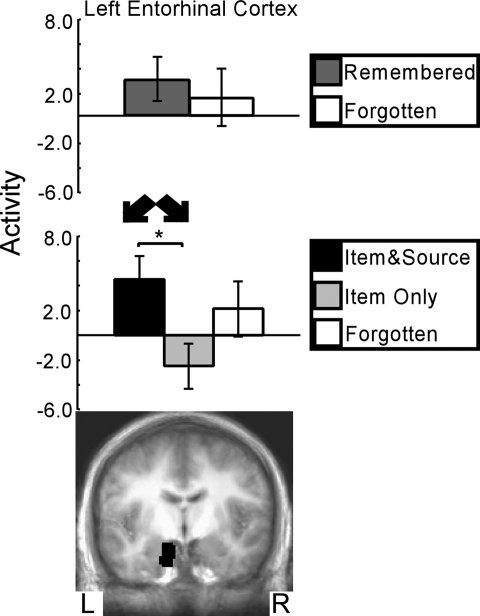
Fig. 5.
The item and source vs. item-only contrast found one region in the medial temporal lobe that predicted item memory success along with correct source memory judgments relative to item memory success but with incorrect source memory judgments (Middle and Bottom). Within this region, activity for words that would later be remembered along with correct source judgments (item and source) was greater than activity for words that would later be remembered but with incorrect source judgments (item only). Activity did not predict subsequent item memory success (Top). Asterisks indicate a difference between the activity for item and source words relative to item-only words (P < 0.05). Brackets show SEM.
The contrast of remembered vs. forgotten (Fig. 2) identified two regions within the MTL: left hippocampus and a region that included both right amygdala and perirhinal cortex. For both these regions, activity correlated with remembered words was greater than activity correlated with forgotten words (P < 0.05, Fig. 2 Top). Also, for both regions, activity correlated with item and source words was similar to activity correlated with item-only words (P > 0.40, Fig. 2 Middle). Thus, activity in these regions predicted whether words would be remembered or forgotten but did not predict whether the source judgment would be correct or incorrect.
The contrast of item and source vs. forgotten (Fig. 3) identified five regions within the MTL: left hippocampus, a region that included both right amygdala and perirhinal cortex, a region that included both right parahippocampal cortex and fusiform gyrus, a region that included both left parahippocampal cortex and fusiform gyrus, and right temporopolar cortex. For four of the five regions, activity correlated with remembered words was greater than activity correlated with forgotten words (P < 0.05, Fig. 3 Top); in the fifth region (right temporopolar cortex), the activity correlated with remembered words was less than the activity correlated with forgotten words (P < 0.05, Fig. 3 Top). For all five regions, activity correlated with item and source words was similar to activity correlated with item-only words (P > 0.45, Fig. 3 Middle). Thus, activity in all five regions predicted whether words would be remembered or forgotten but did not predict whether the source judgment would be correct or incorrect.
Initially, the contrast of item only vs. forgotten did not identify any regions within the MTL. When the contrast included item-only words that received confidence ratings for the old/new decision of 1 on the 1-to-3 scale (3 words ± 0.8 words per subject), the contrast of item only vs. forgotten (Fig. 4) identified two regions within the MTL: one region that included left hippocampus, perirhinal cortex, and amygdala; and a second region that included both right amygdala and perirhinal cortex. For both regions, activity correlated with remembered words was greater than activity correlated with forgotten words (P < 0.05, Fig. 4 Top). For the first region, activity correlated with item and source words was similar to activity correlated with item-only words (P > 0.65, Fig. 4 Middle Left). For the second region, activity correlated with item-only words was numerically greater than activity correlated with item and source words (P < 0.07, Fig. 4 Middle Right).
The contrast of item and source vs. Item only (Fig. 5) identified one region within the MTL: left entorhinal cortex. For this region, activity correlated with remembered words was similar to activity correlated with forgotten words (P > 0.50, Fig. 5 Top), but activity correlated with item and source words was greater than activity correlated with item-only words (P < 0.05, Fig. 5 Middle). Thus, activity in this region did not predict whether words would be remembered or forgotten but did predict whether the source judgment would be correct or incorrect.
Experiment 2.
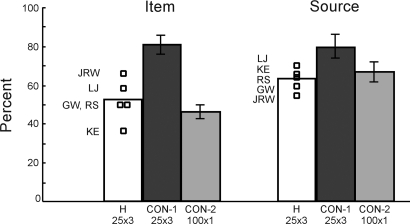
Fig. 6 shows performance for item memory (hit rate minus false alarm rate) and performance for source memory (% correct source judgments for items correctly judged as old). The patients were impaired at item memory relative to controls (CON-1) who took the same memory test (52.0 ± 5.0% for the patients vs. 80.7 ± 5.2% for CON-1, P < 0.05, Fig. 6 Left). Patients scored 76.0 ± 2.6% correct on the item memory judgment (old/new decision: 72.8 ± 5.39% hit rate and 20.8 ± 6.7% false alarm rate, d′ = 1.62 ± 0.23), and the CON-1 group scored 90.3 ± 2.6% correct on the item memory judgment (old/new decision: 87.3 ± 2.5% hit rate and 6.7 ± 3.7% false alarm rate, d′ = 2.86 ± 0.30). On the source memory test, patients made 62.7% ± 2.5% correct source judgments for the items that they correctly judged as old, and controls made 79.9 ± 5.9% correct source judgments for the items that they correctly judged as old (P < 0.05, Fig. 6 Right). Both source judgment scores were above chance levels (50%, P < 0.05).
Fig. 6.
Five memory-impaired patients with damage limited to the hippocampus (H) and six controls (CON-1) learned 25 words by imagining an indoor or outdoor scene associated with each word. Each participant saw the 25 words three times each (25 × 3). Patients were impaired relative to controls on both item judgments and source judgments. Six additional controls (CON-2) saw 100 words once each (100 × 1) and performed similarly to the patients on both item judgments and source judgments. The item score is the hit rate minus the false alarm rate (chance = 0%). The source score is the proportion of hits that were followed by a correct indoor/outdoor judgment (chance = 50%).
Fig. 6 also shows that patients performed similarly to controls (CON-2) who studied 100 words a single time, instead of 25 words three times. The patients performed similarly at item memory relative to the CON-2 group (45.5 ± 3.7% for CON-2, P > 0.30, Fig. 6 Left). The CON-2 group scored 72.8 ± 1.8% correct on the item memory judgment (old/new decision: 67.7 ± 6.0% hit rate and 22.2 ± 6.8% false alarm rate, d′ = 1.41 ± 0.15). The patients also performed similarly at source memory relative to the CON-2 group (Con-2, 66.5 ± 5.0% correct source judgments for the items that they correctly judged as old, P > 0.50, Fig. 6 Right). Thus, when the controls and patients matched on item memory performance, they also matched on source memory performance.
Discussion
In experiment 1, brain activity in 15 healthy volunteers was assessed during the encoding of 175 words. Activity predicting whether words would be subsequently remembered was found throughout the MTL, including the hippocampus, perirhinal cortex, and parahippocampal cortex. Activity in these regions that predicted subsequent success at item memory predicted source memory to the same degree. In experiment 2, memory-impaired patients with damage thought to be limited to the hippocampal region studied words and then took a recognition memory test as in experiment 1. The patients were similarly impaired at item memory and source memory.
Henson (3) surveyed 23 studies that used the subsequent memory paradigm that we used in experiment 1. Most of the studies found activity within the MTL that predicted subsequent success at remembering studied items, although the active areas varied across studies. Our findings agree with this literature in that we also found activity predicting subsequent item recognition in three different MTL regions: hippocampus, perirhinal cortex, and parahippocampal cortex. Notably, activity in the hippocampus at encoding has often been found to predict subsequent performance on relatively difficult memory tests, such as tests of source memory or tests of associative information (e.g., refs. 4, 5, 12, and 14). Here, as in other studies that focused on item memory (7–9, 15, 16), we found that hippocampal activity predicted subsequent success on a simple test of item memory.
The present findings for perirhinal cortex also accord with findings from earlier fMRI studies of recognition memory and source memory (4, 5). These two studies, like our study, found that activity in perirhinal cortex at encoding predicted whether a word would be subsequently remembered, and furthermore that activity in perirhinal cortex did not predict whether correct item memory judgments would be accompanied by correct source memory judgments. However, our finding that MTL activity did not differentially predict correct source memory judgments (with the exception of left entorhinal cortex) contrasts with the findings from these same two studies. These studies found that activity at encoding differed in both the hippocampus and parahippocampal cortex, depending on whether source memory was subsequently available (4, 5).
The source question in these two studies involved a relatively salient aspect of the encoding condition, such as imagining a scene associated with the word vs. read the word backwards (4). In comparison, the source question in the present study involved a potentially more difficult judgment (indoor image vs. outdoor image). Accordingly, one can ask whether participants in the present study might in fact have had available a significant amount of source memory, even for item-only words, and lacked only the specific information (indoor image vs. outdoor image) that was the basis for the source memory question. If so, it might not be surprising that activity in MTL structures did not differ in the case of remembered words that were also assigned the correct source (item and source) and remembered words that were assigned the incorrect source (item only).
One way to view studies of recognition memory (with respect to activity in the MTL) is to suppose that item information and source information vary in memory strength and that items for which source information is available are remembered with greater memory strength than items for which less source information is available (refs. 10 and 11; also see ref. 17). Indeed, our Fig. 1 shows that words for which participants have stronger memory are also words for which they are likely to make correct source judgments. This finding has been reported for other item memory tasks and source memory tasks as well (18, 19) and is consistent with the idea that item memory and source memory lie on a continuum of memory strength. Furthermore, in our study, the confidence ratings associated with forgotten words, item-only words, and item and source words were 2.04 ± 0.09, 2.47 ± 0.07, and 2.76 ± 0.04, respectively. Notably, the difference between the confidence ratings associated with item-only words and forgotten words was marginally greater than the difference between item and source words and item-only words (0.43 ± 0.06 vs. 0.30 ± 0.04, P < 0.08). Perhaps this observation can account for why hippocampal activity at encoding predicted subsequent success at recognizing items but did not differentially predict success at making source judgments.
None of the recent studies of item memory and source memory (4–6) reported average confidence ratings for remembered words, forgotten words, item and source words, or item-only words. If the difference in memory strength between remembered words (or item-only words) and forgotten words in those studies was small, such a result could explain why hippocampal activity did not predict subsequent item memory. Similarly, if the difference in memory strength in those studies between item and source words and item-only words was great, such a result could explain why hippocampal activity in these studies did predict subsequent source memory success. Studies that manipulate the strength of item memory and source memory could provide a test of these possibilities.
The results from experiment 2 are consistent with the findings from experiment 1. Patients with damage to the hippocampal region were impaired on both the item memory test and the source memory test. Furthermore, when patients and controls were equated for item memory performance by having controls study more words fewer times each, the two groups performed similarly to controls on both the item memory test and the source memory test. This finding suggests that the hippocampus supports item memory and source memory judgments to a similar degree. Accordingly, experiment 2 provides no basis for supposing that the hippocampus is especially important for source memory. Note, however, that the present findings are not an argument against the utility of the distinction between item and source memory itself (or the related concepts of recollection vs. familiarity and remembering vs. knowing) (11, 20). For example, source memory may depend especially on the strategic, effortful search that is the province of the frontal lobes (21).
In an important study (22), elderly participants were divided into groups (high or low MTL function and high or low frontal lobe function) based on their performance on standard neuropsychological tests. High MTL function was related to good performance on an independent test of item memory, whereas high frontal lobe function was related to good performance on an independent test of source memory. This finding suggests that memory for items depends on the MTL, whereas memory for sources depends importantly on the frontal lobe. Subsequent work by the same group (23) tried to rule out the possibility that individuals with low frontal lobe function are impaired at source memory tests simply because source memory judgments are more difficult than item memory judgments. Item memory was assessed by using a more difficult test than previously, yielding a score of ≈70% correct instead of ≈85% correct. (The source memory score was ≈60% correct.) Despite the greater difficulty of the item memory test, participants with low frontal lobe function still performed well on the item memory test but poorly on the source memory test. Other evidence also relates source memory to frontal lobe function (24, 25).
The present results are consistent with these earlier findings. In experiment 1, the hippocampus was identified by the remembered vs. forgotten contrast (item memory) but not by the item and source vs. item-only contrast (source memory). However, the item and source vs. item-only contrast identified three brain regions in the right frontal lobe (Table 2). Activity in these three frontal regions was greater for item-only words than for item and source words. Participants may have expended more effort when it was difficult to create a mental image than when a mental image came easily to mind. (In the former case, the image would not be expected to be remembered as well as in the latter case.) Further work with fMRI investigating the role of the frontal lobes in source memory is warranted.
In summary, with the exception of a region of left entorhinal cortex, activity within the MTL predicted which items would be subsequently remembered but did not differentially predict which items would later be accompanied by accurate source judgments (experiment 1). In addition, patients with hippocampal damage were similarly impaired on item memory and source memory tasks (experiment 2). Note that the present findings are not an argument for the view that the structures of the MTL carry out one undifferentiated function. However, the findings do caution against the simple idea that processes like item memory and source memory can be neatly dichotomized and assigned to separate MTL structures.
Materials and Methods
Experiment 1.
Participants.
The participants were eight males and seven females (mean age = 25.3 ± 1.0 years) recruited from the University of California at San Diego community.
Stimuli.
Stimuli were adjectives with a mean frequency of 55 (range 10–500). Four lists of 175 words were constructed, two study lists and two foil lists. Eight participants saw one study list (in one of two possible orders), and the seven other participants saw the other study list (also in one of two possible orders). The yes–no recognition test consisted of the 175 study words and 175 foils presented in a mixed order such that no more than three studied words or three foils appeared consecutively.
fMRI test procedure.
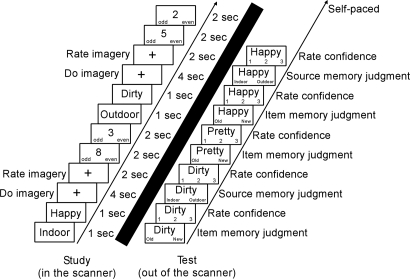
For the task (Fig. 7), participants saw a cue (indoor/outdoor) followed by a study word (e.g., happy) and formed a mental image of an indoor or outdoor scene that was associated with the study word. Across participants, each of the 175 study words was equally likely to be presented with indoor or outdoor imagery instructions. Participants were asked to remember the study words for a subsequent memory test but were not asked to remember the image they formed. A fixation cross then appeared for four seconds, during which participants formed their image. After 4 s, the cross turned red, and participants rated their success at forming an image (0–3). Words assigned a 0 rating were removed from subsequent analysis (9.2 ± 1.7 words per subject). Between word presentations, participants were given zero, two, four, or six trials of a baseline task in which they saw a single digit (1–9) and made odd/even judgments. This baseline task is known to result in relatively little medial temporal lobe activity (26). A short break (≈1 min) occurred after each group of 25 words.
Fig. 7.
Inside an fMRI scanner, participants learned 175 words by imagining an indoor or outdoor scene associated with that word. At study, participants were shown a cue (indoor/outdoor) for 1 s, the study word for 1 s, and then had 4 s to form an indoor or outdoor image as they viewed a fixation cross. Trials were separated by zero, two, four, or six 2-s trials of the baseline task (odd/even number judgments). At test (5–10 min later), participants made old/new decisions for the 175 studied words and 175 novel foils followed by confidence ratings (1–3). For words endorsed as “old,” participants also made source judgments (indoor or outdoor) and confidence ratings (1–3).
After scanning (5- to 10-min delay), participants took a recognition memory test. A total of 350 words (175 studied words and 175 novel foils) were presented one at a time. For each word, participants made an old/new judgment (item memory) and gave a confidence rating for their judgment (1 = “not sure,” 2 = “somewhat sure,” and 3 = “very sure”). If a word was endorsed as “old,” participants were also asked whether the word was learned in association with an indoor or an outdoor image (source memory), and they gave a confidence rating for that judgment as well (on the same 1–3 scale). The test was self-paced.
Study words were classified into one of four groups according to subsequent performance on the recognition test: (i) remembered words (studied words later endorsed as studied, irrespective of the source memory judgment); (ii) forgotten words (studied words later endorsed as new); (iii) item and source words (studied words later endorsed as old that were also assigned correct source memory judgments); and (iv) item-only words (studied words later endorsed as old but assigned incorrect source memory judgments).
fMRI imaging parameters.
Imaging was carried out in a 3T GE scanner at the Center for Functional MRI (University of California at San Diego). Functional images were acquired by using a gradient-echo, echo-planar, T2*-weighted pulse sequence (repetition time, 2 s; two shots per repetition; echo time, 30; flip angle, 70°; bandwidth, 250 MHz). Twenty sections covering the whole brain were acquired perpendicular to the long axis of the hippocampus (4 × 4 × 7 mm voxels). High-resolution (1 × 1 × 1 mm3) T1-weighted, magnetization-prepared rapid gradient echo (MP-RAGE) anatomical images were also collected for each participant after the first 100 study words had been presented.
fMRI data analysis.
For each group of study words (remembered, forgotten, item and source, and item-only words), a hemodynamic response (relative to the baseline condition) was first estimated for the 22 s after the presentation of the indoor/outdoor cue using signal deconvolution and the afni suite of programs (27). Data analysis was then based on the area under the hemodynamic response function from 0 to 12 s after the presentation of the indoor/outdoor cue (at ≈12 s, the hemodynamic response function returned to baseline). Standard landmarks were defined manually on the anatomical scans (28). The anatomical scans and the fMRI data were then transformed into Talairach space by afni using nearest-neighbor interpolation. These data were used for whole brain analysis. To improve alignment of the medial temporal lobe, the ROI-AL alignment method (9, 29, 30) was also used. These data were used for the analysis of medial temporal lobe activity.
Voxel-wise t tests (two-tailed) were then carried out across all 15 participants for both the whole brain and medial temporal lobe analyses, based on the area under the hemodynamic response function for (i) the remembered vs. forgotten contrast; (ii) the item and source vs. forgotten contrast; (iii) the item-only vs. forgotten contrast; and (iv) the item and source vs. item-only contrast. Monte Carlo simulations were used to correct for multiple comparisons and to determine how large a cluster of voxels was needed to be statistically meaningful (P < 0.05). Within all of the clusters that emerged from the four contrasts described above, we then identified activity (relative to baseline) that was correlated with remembered, forgotten, item and source, and item-only words.
For detailed fMRI methods, see Supporting Text, which is published as supporting information on the PNAS web site.
Experiment 2.
Participants.
The memory-impaired patients were four males and one female (Table 1) with lesions thought to be limited to the hippocampus (dentate gyrus, CA fields, and subiculum). GW and RS became amnesic after a drug overdose and associated respiratory failure in 2001 and 1998, respectively. KE became amnesic in 2004 after an episode of ischemia associated with kidney failure and toxic shock syndrome. JRW became amnesic in 1990 after an episode of cardiac arrest. LJ became amnesic in 1988 during a 6-month period with no known precipitating event.
Table 1.
Characteristics of amnesic patients
| Patient | Age, years | Education, years | WAIS-III IQ | WMS-R |
||||
|---|---|---|---|---|---|---|---|---|
| Attention | Verbal | Visual | General | Delay | ||||
| K.E. | 63 | 13.5 | 108 | 114 | 64 | 84 | 72 | 55 |
| L.J. | 67 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| R.S. | 45 | 12 | 99 | 99 | 85 | 81 | 82 | <50 |
| G.W. | 45 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| J.R.W. | 38 | 12 | 90 | 87 | 65 | 95 | 70 | <50 |
The Wechsler Adult Intelligence Scale-III (WAIS-III) and the Wechsler Memory Scale-Revised (WMS-R) yield mean scores of 100 in the normal population with a standard deviation of 15. The WMS-R does not provide numerical scores for individuals who score <50. IQ scores for JRW and RS are from the Wechsler Adult Intelligence Scale-Revised.
Estimates of medial temporal lobe damage were based on quantitative analysis of magnetic resonance images (MRI), compared to data for 19 controls (KE, RS, GW, and JRW) or 11 controls (the female, LJ) (31). The volume of the full anterior–posterior length of the hippocampus and the parahippocampal gyrus were measured by using criteria based on histological analysis of healthy brains (32–34). For each patient, the volumes of the hippocampus and parahippocampal gyrus were divided by the intracranial volume (ICV normalized) to correct for brain size. KE, LJ, RS, GW, and JRW have an average bilateral reduction in hippocampal volume of 49%, 46%, 33%, 48%, and 44%, respectively (all values >3.0 SD below the control mean). In comparison, the volume of the parahippocampal gyrus (temporopolar cortex, perirhinal, entorhinal, and parahippocampal cortices) is reduced by 17%, −8%, 1%, 12%, and 6%, respectively (all values within 2 SD of the control mean). On the basis of two patients (LM and WH) with similar bilateral volume loss in the hippocampus for whom detailed postmortem neurohistological information was obtained (35), this degree of volume loss likely reflects nearly complete loss of hippocampal neurons (also see ref. 31).
Additional measurements, based on four controls for each patient, were carried out for the insular cortex, fusiform gyrus, frontal lobes, lateral temporal lobes, parietal lobes, and occipital lobes. The only volume reduction in these regions >1.3 SD of the control mean was the parietal lobe for RS (36).
Two groups of six control subjects (CON-1, one female, mean age = 56.7 ± 10.3 years; CON-2, two females, mean age = 62.0 ± 15.4 years) also participated.
Stimuli.
The word lists used in experiment 1 were used to generate materials for experiment 2.
Procedure.
Five patients and six controls (Con-1) studied 25 words three times. The procedure was the same as in experiment 1 (e.g., imagery of indoor/outdoor scenes during learning, intermixed odd/even digit trials). One to 2 min after the third presentation of the study list, the recognition memory test was given (25 study words and 25 foils). As in experiment 1, when a word was endorsed as old (item memory), participants were asked whether the word was learned in association with an indoor or outdoor image (source memory). The patients (but not the controls) were also tested again 1–8 weeks later with a second set of words.
To match the item memory performance of controls and patients, a second group of six controls (Con-2) studied 100 words. The procedure was otherwise the same as described above. In this way, it was possible to ask whether patients and controls with similar item memory performance would also exhibit similar source memory performance.
Supplementary Material
Acknowledgments
We thank Jen Frascino, Leah Swalley, and Mark Starr for assistance and Giedrius Buracas and Geoffrey Boynton for advice. This work was supported by the Department of Veterans Affairs, National Institute of Mental Health (NIMH) Grant MH24600, the Metropolitan Life Foundation, Public Health Services (PHS) Training Grant AG000216 (to C.N.S.), an National Defense Science and Engineering Graduate Fellowship (to Y.S.), and NIMH Training Grant 5-T32-MH20002 (to J.J.G.).
Abbreviations
- MTL
medial temporal lobe
- fMRI
functional MRI.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Squire L. R., Stark C. E., Clark R. E. Annu. Rev. Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 2.Paller K. A., Wagner A. D. Trends Cognit. Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- 3.Henson R. Q. J. Exp. Psychol. B. 2005;58:340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- 4.Davachi L., Mitchell J. P., Wagner A. D. Proc. Natl. Acad. Sci. USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranganath C., Yonelinas A. P., Cohen M. C., Dy C. J., Tom S. M., D’Esposito M. Neuropsychologia. 2003;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Kensinger E. A., Schacter D. H. J. Neurosci. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchhoff B. A., Wagner A. D., Maril A., Stern C. E. J. Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otten L. J., Henson R. N., Rugg M. D. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- 9.Stark C. E., Okado Y. J. Neurosci. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wixted J. T., Stretch V. Psychon. Bull. Rev. 2004;11:616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- 11.Wixted J. T. Psychol. Rev. 2006 in press. [Google Scholar]
- 12.Sperling R., Chua E., Cocchiarella A., Rand-Giovannetti E., Poldrack R., Schacter D. L., Albert M. NeuroImage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson O., III, Schacter D. L. NeuroImage. 2004;21:456–462. doi: 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 14.Kirwan C. B., Stark C. E. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reber P. J., Siwiec R. M., Gitelman D. R., Parrish T. B., Mesulam M. M., Paller K. A. J. Neurosci. 2002;22:9541–9548. doi: 10.1523/JNEUROSCI.22-21-09541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morcom A. M., Good C. D., Frackowiak R. S., Rugg M. D. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- 17.Cansino S., Maquet P., Dolan R. J., Rugg M. D. Cereb. Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- 18.Slotnick S. D., Klein S. A., Dodson C. S., Shimamura A. P. J. Exp. Psychol. Learn. Mem. Cognit. 2000;26:1499–1517. doi: 10.1037//0278-7393.26.6.1499. [DOI] [PubMed] [Google Scholar]
- 19.Slotnick S. D., Dodson C. S. Mem. Cognit. 2005;33:151–170. doi: 10.3758/bf03195305. [DOI] [PubMed] [Google Scholar]
- 20.Wais P., Wixted J. T., Hopkins R. O., Squire L. R. Neuron. 2006;49:459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckner R. L., Wheeler M. E. Nat. Rev. Neurosci. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- 22.Glisky E. L., Polster M. R., Routhieaux B. C. Neuropsychology. 1995;9:229–235. [Google Scholar]
- 23.Glisky E. L., Rubin S. R., Patrick S. R. D. J. Exp. Psych. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- 24.Janowsky J. S., Shimamura A. P., Squire L. R. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- 25.Johnson M. K., O’Connor M., Cantor J. Brain Cognit. 1997;34:189–206. doi: 10.1006/brcg.1997.0873. [DOI] [PubMed] [Google Scholar]
- 26.Stark C. E., Squire L. R. Proc. Natl. Acad. Sci. USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox R. W. Comp. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 28.Talairach J., Tournoux P. A Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgard: Thieme; 1998. [Google Scholar]
- 29.Law J. R., Flanery M. A., Wirth S., Yanike M., Smith A. C., Frank L. M., Suzuki W. A., Brown E. N., Stark C. E. J. Neurosci. 2005;25:5720–5729. doi: 10.1523/JNEUROSCI.4935-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller M. I., Beg M. F., Ceritoglu C., Stark C. E. Proc. Natl. Acad. Sci. USA. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold J. J., Squire L. R. Hippocampus. 2005;15:79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amaral D. G, Insausti R. In: The Human Nervous System. Paxinos G., editor. San Diego: Academic; 1990. pp. 711–755. [Google Scholar]
- 33.Insausti R., Insausti A. M., Sobreviela M. T., Salinas A., Martinez-Penuela J. M. Microsc. Res. Tech. 1998;43:8–15. doi: 10.1002/(SICI)1097-0029(19981001)43:1<8::AID-JEMT2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Insausti R., Juottonen K., Soininen H., Insausti A. M., Partanen K., Vainio P., Laakso M. P., Pitkänen A. Am. J. Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- 35.Rempel-Clower N. L., Zola S. M., Squire L. R., Amaral D. G. J. Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bayley P. J., Gold J. J., Hopkins R. O., Squire L. R. Neuron. 2005;46:799–810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.