Abstract
The basic premise of gene therapy is that genes can be used to produce in situ therapeutic proteins. The controlled delivery of DNA complexes from biomaterials offers the potential to enhance gene transfer by maintaining an elevated concentration of DNA within the cellular microenvironment. Immobilization of the DNA to the substrate to which cells adhere maintains the DNA in the cell microenvironment for subsequent cellular internalization. Here, layer-by-layer (LBL) films made from poly(l-glutamic acid) (PLGA) and poly(l-lysine) (PLL) containing DNA were built in the presence of charged cyclodextrins. The biological activities of these polyelectrolyte films were tested by means of induced production of a specific protein in the nucleus or in the cytoplasm by cells in contact with the films. This type of coating offers the possibility for either simultaneous or sequential interfacial delivery of different DNA molecules aimed at cell transfection. These results open the route to numerous potential applications in patch vaccination, for example.
Keywords: gene delivery, layer-by-layer films, transfection
The basic premise of somatic gene therapy is that genes can be used to cause in vivo production of therapeutic proteins. Controlled and efficient gene delivery has implications in many fields ranging from basic science to clinical medicine. Current strategies to enhance gene delivery involve the complexation of DNA with cationic polymers or lipids delivery. Cationic polymers or lipids can self-assemble with DNA to form particles that are capable of being endocytosed by cells (1). These complexes reduce effective size and cellular degradation of DNA (2) and are often delivered as a bolus, added to culture wells in vitro. Bolus delivery of these complexes can be hindered by mass transport limitations or deactivation processes, such as degradation or aggregation. For example, in vitro studies have estimated that bolus addition of complexes to the culture media results in internalization of only 20% of the DNA added (3). These limitations motivated the development of alternative delivery strategies.
The controlled delivery of DNA complexes from biomaterials offers the potential to enhance gene transfer by maintaining an elevated concentration of DNA within the cellular microenvironment (4). DNA delivery systems from biomaterials are designed to maintain elevated concentrations locally by supplying DNA to balance the loss by degradation. The continued presence of the DNA during cell division seems to facilitate entry into the nucleus (5). In recent years, considerable effort has been devoted to the design and the controlled fabrication of structured materials with functional properties (6). The layer-by-layer (LBL) buildup of polyelectrolyte films from oppositely charged polyelectrolytes (7) offers opportunities for the preparation of functionalized biomaterial coatings. This technique allows the preparation of supramolecular nano-architectures (8–13) exhibiting specific properties in terms of control of cell activation (8–11) and may also play a role in the development of local drug delivery systems (12). Peptides and proteins, chemically bound, adsorbed, or embedded in LBL films, have been shown to retain their biological activities (14–16). Cyclodextrins (CDs) constitute a group of cyclic oligosaccharides that have been shown to improve the bioavailability of many drugs by forming inclusion complexes with them (17). CDs and certain derivatives also play an important role in drug formulation due to their effect on solubility, dissolution rate, chemical stability, absorption of drugs, and conformational stabilization of proteins and lipids through encapsulation of their hydrophobic moieties (13, 14). This so-called “molecular chaperone” effect of CDs has been used to stabilize the conformation of lipid A adsorbed on LBL films (13).
CDs present many advantages as potential core molecules for the development of new vectors. The natural, and some modified, CDs have well researched pharmaceutical and toxicological profiles as drug delivery agents (18). Properties of CDs as excipients in conventional drug delivery include the ability to protect drugs from physical, chemical, and enzymatic degradation and to enhance cell membrane permeability (19). Neutral CDs have been shown to interact with nucleic acids and nucleotides and to enhance their transfection efficiency in vivo (20). Cationic CD derivatives present an even greater ability to bind nucleotides (21). In addition, CDs have been incorporated into polycationic polymer and dendrimer vectors (22, 23). Films containing DNA are of great interest for applications in sensing (24), diagnostic (25), electronics (26), and gene delivery (27, 28). Recently, Zhang et al. (27) reported multilayered polyelectrolyte assemblies that sustain the release of functional plasmid DNA from the surfaces of model substrates under physiological conditions.
The aim of the present work is to present a type of material coating based on polyelectrolyte multilayers containing sequentially adsorbed DNA and cationic CD that show great transfection ability and allow for multiple and sequential activity. Our first objective is to demonstrate that such a functionalization is possible. We also show that DNA embedded in the multilayers without CD does not lead to transfection. Cationic CD thus plays the role of transfection enhancer. Finally, we show that multiple and sequential biological activity can be induced and that the order of the sequence is solely determined by the level at which the DNA is embedded in the multilayer architectures. For this purpose, we selected a model system made from LBL poly(l-glutamic acid) (PLGA) and poly(l-lysine) (PLL) films into which cationic CD [pyridylamino-β-cyclodextrin (pCD)] and DNA have been embedded. We examined whether the DNA activity could be retained as reagent for stimulating specific protein production.
The biological activities of these polyelectrolyte films were tested by means of induced production of a specific protein in the nucleus or in the cytoplasm in three different types of cells, which were brought into contact with the polyelectrolyte films. We show here that these polyelectrolyte multilayers can act as an efficient gene delivery tool to transfect cells. These types of coatings combine the simplicity of the construction, because it requires only adsorption processes and the simultaneous interfacial delivery of different effector molecules.
Results and Discussion
The polyelectrolyte multilayer gene transfer system we tested was based on plasmid DNA and sequentially deposited CDs. The buildup of (PLL-PLGA)5-pCD-DNA-pCD-PLGA-PLL multilayered films was monitored by quartz crystal microbalance (QCM). The evolution of the normalized frequency shift (−Δf/ν) after each successive injection is plotted in Fig. 1. The increase in −Δf/ν with the number of deposited layers suggests the regular film deposition. Successive injections of pCD on PLGA and DNA and then again pCD also led to an increase in this normalized frequency shift −Δf/ν (see Fig. 1 Inset). DNA adsorbed on pCD induces a shift in −Δf/ν of 56 Hz, corresponding to a surface concentration of ≈1 μg/cm2.
Fig. 1.
Normalized frequency shifts (−Δf/ν) measured with QCM after successive injections of polyelectrolytes (PLL or PLGA), pCD, and DNA. The measurements are performed at 15 MHz resonance frequency. The deposition of the pCD/DNA/pCD sequence as a function of time is detailed in the Inset. R, rinsing.
In vitro tests were conducted on three types of cells (CHO, macrophages, and COS) by using SPT7pTL, a vector expressing the human SPT7 nuclear transcription factor, and pEGFP expressing GFP as cytoplasmic protein.
Before each study, we verified the viability of cells to have information about the toxicity of the concentration used. In this study, we have analyzed the viability by using an MTT test. Our results indicate clearly that, for the same concentration of DNA adsorbed on the top of the multilayered film, in the presence or in the absence of CD, no loss of the viability of cells was detected after 72 h of culture.
To check the gene transfer power of our plasmid DNA-CDs coatings, we compared cells transfected with SPT7pTL by the calcium phosphate method or by the transfection reagent FuGENE 6 with cells grown on (PLL-PLGA)5-pCD-SPT7pTL-pCD-(PLGA-PLL)5 multilayered films (embedded DNA in bold). The expression of SPT7 in the cells was detected by using anti-SPT7 monoclonal antibody and a Cy3-fluorochrome-conjugated secondary antibody and investigated by fluorescence microscopy (Fig. 2). Nuclei were visualized by Hoechst 33258 staining.
Fig. 2.
Comparison of COS-1 cells transfected with SPT7pTL by the calcium phosphate method (A and B) [10 ± 5% (SD), n = 4] or by the transfection reagent FuGENE 6 (C and D) [25 ± 7% (SD), n = 4] with COS-1 cells grown (PLL-PLGA)5-pCD-SPT7pTL-pCD-(PLGA-PLL)5 multilayered films (E and F) [100 ± 10% (SD), n = 4]. (A, C, and E) Hoechst DNA staining. (B, D, and F) Immunodetection of SPT7 expression using mouse monoclonal anti-SPT7 and Cy3-conjugated goat anti-mouse antibodies. Similar results were obtained on the two other cellular types.
The calcium phosphate method (Fig. 2 A and B) and FuGENE 6 reagent (Fig. 2 C and D) allowed expression of SPT7 in a limited number of cells (25% for FuGENE 6 and 10% for the calcium phosphate method). In cells grown on (PLL-PGA)5-pCD-SPT7pTL-pCD-(PGA-PLL)5 multilayers, 100% transfection efficiency was obtained for the three cellular types (Fig. 2 E and F). The same results were obtained when the SPT7pTL vector was adsorbed on the top of the mutilayered film (PLL-PLGA)5-pCD-DNA-pCD.
We investigated the effect of the same architectures by using another vector (PEGFP), instead of SPT7pTL, expressing GFP as cytoplasmic protein. This study was performed only on COS cells. These results evidence the specific transfection by this vector adsorbed on top of or embedded into the multilayered films (data not shown).
We checked that DNA availability for cells is not due to passive release but rather to uptake of DNA from the surface by cells. This was done by verifying that the supernatants from the cells grown on the multilayers containing embedded SPT7pTL or PEGFP with pCDs are not able to transfect cells, suggesting that the presence of the cells on the functionalized multilayers is needed for the interaction with the active compounds. It is thus strongly expected that the mechanism of action is through local enzymatic degradation induced by the cells in contact with the film. Finally, we also checked that DNA embedded in the same architectures but without CD does not transfect cells. This finding clearly indicates the abilities of charged CDs to preserve DNA activity.
We also analyzed the passive release of DNA by the amount of fluorescein-labeled DNA measured by fluorometry. Our results indicate clearly no significant DNA release from PLL/PLGA architectures versus time over a 6-day period of incubation with the culture medium, indicating that DNA availability for cells was not mainly through passive release but was due to the uptake of complexes from the surface by cells. This fact would support the idea that multilayered film might provide cells for controlled DNA availability.
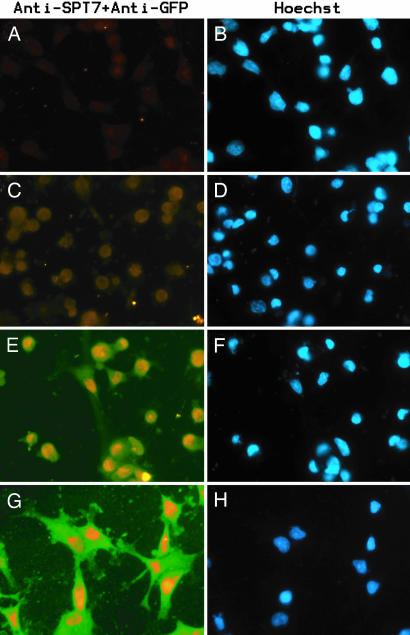
To demonstrate the sequential activity when two types of DNA molecules are embedded at different film levels, COS cells were grown for 2, 4, and 8 h on the surface of (PLL-PLGA)5-pCD-pEGFP-pCD-(PLGA-PLL)5-PLGA-pCD-SPT7pTL-pCD-(PLGA-PLL)5 multilayered films containing both SPT7pTL (nuclear) and pEGFP (cytoplasmic).
The expression of SPT7 and EGFP in COS cells were detected by double labeling, and the nuclei were visualized by Hoechst 33258 staining (Fig. 3). SPT7 was detected in the nuclei of all COS cells and started to accumulate as early as 2 h (Fig. 3 C and D) and increased over the next few hours [Fig. 3 E and F (4 h) and Fig. 3 G and H (8 h)]. GFP was detected in the cytoplasm and started to accumulate after 4 h (Fig. 3 E and F) and increased after 8 h (Fig. 3 G and H). No staining was observed without plasmids (Fig. 3 A and B) or by using secondary antibodies only.
Fig. 3.
Expression of SPT7 and EGFP in COS cells grown on the surface of (PLL-PLGA)5-pCD-(PLGA-PLL)5-PLGA-pCD-(PLGA-PLL)5 multilayered films (A and B) or (PLL-PLGA)5-pCD-pEGFP-pCD-(PLGA-PLL)5-PLGA-pCD-hSPT7pTL-pCD-(PLGA-PLL)5 multilayered films for 2 h (C and D), 4 h (E and F), and 8 h (G and H). The expression of SPT7 (red) and GFP (green) was detected by using mouse monoclonal anti-SPT7 and rabbit polyclonal anti-GFP as primary antibodies and Cy3-conjugated goat anti-mouse and Alexa Fluor 488 goat anti-rabbit as secondary antibodies (A, C, E, and G). Nuclei were visualized by Hoechst 33258 staining (B, D, F, and H).
We also investigated the same architecture by embedding first the nuclear vector and second the cytoplasmic one. The results demonstrate that cells can be transfected first with a cytoplasmic response and later on with the nuclear response (data not shown). These results indicate clearly that we are able to propose a biomaterial coated with multilayer films that interact with cells by inducing sequential and specific interactions (nuclear or cytoplasmic) depending upon the embedding level of the active vectors. Our results also show that CDs can act as promising molecular templates, with large potential for development as a generic series of gene delivery vectors. Here, we have described the fabrication of substrates containing β-cyclodextrin–DNA complexes embedded in a multilayered polyelectrolyte film in which specific expression of nuclear or cytoplasmic proteins is selectively and sequentially produced. We have shown that polyelectrolyte multilayers on which CD–DNA complexes are adsorbed can act as an efficient gene delivery tool to transfect cells. This type of coating combines the simplicity of the construction by adsorption processes and the simultaneous interfacial delivery of the molecules of different effectors. These results should have a significant impact on the development of localized gene therapies and should open the route to numerous potential applications.
Methods
Chemicals.
PLL (molecular mass = 30.3 kDa) and PLGA (molecular mass = 54 kDa) were purchased from Sigma. Bisbenzimide H 33258 (Hoechst) used for microscopy was purchased from Invitrogen, Molecular Probes, and Sigma. The pCD (13) was from R. Darcy (University College Dublin).
Polyelectrolyte Multilayer Preparation.
For all in vitro experiments, polyelectrolyte multilayer films were prepared on glass coverslips (CML, Nemours, France) pretreated with 10−2 M SDS and 0.12 M HCl for 15 min at 100°C, and then extensively rinsed with deionized water. Glass coverslips were deposited in 24-well plates (Nunc). Films made of n(PLL-PLGA) bilayers were built by alternated immersion of the pretreated coverslips during 10 min in polyelectrolyte solutions (300 μl) at a concentration of 1 mg·ml−1 for PLL and PGA in the presence of 0.15 M NaCl at pH 7.4. pCDs or DNA solution (300 μl) were added on polyanion-ending architectures for 1 h, and the buildup was pursued by the further addition of layers. After each deposition, the coverslips were rinsed three times during 10 min with 0.15 M NaCl. All the films were sterilized for 10 min by UV light (254 nm). Before use, the architectures were put in contact with 1 ml of cell culture medium without serum during 24 h.
QCM.
The film buildups were monitored in situ by QCM by using the axial flow chamber QAFC 302 (QCM-D, D300; Q-Sense, Götenborg, Sweden). This QCM technique consists in measuring the resonance frequency shift (Δf) of a quartz crystal induced by polyelectrolyte or protein adsorption on the crystal, when compared with the crystal in contact with buffer. Changes in the resonance frequencies were measured at the third overtone (ν = 3) corresponding to the 15-MHz resonance frequency. A shift in Δf can be associated, in a first approximation, to a variation of the mass adsorbed on the crystal through the Sauerbrey relation (29): m = −CΔf/ν, where C is a constant characteristic of the crystal used (C = 17.7 ng·cm−2·Hz−1). The measurement methodology has been addressed in detail elsewhere (30) and is applied in the present work.
Transfection Protocol.
COS-1 cells were harvested by trypsinization from a confluent 100-mm tissue culture dish and resuspended at a density of 5 × 104 cells/cm2 in 24-well plates in DMEM containing 10% FCS. Transfections were performed by using eukaryotic vectors SPT7pTL and pEGFP (0.1 μg·cm−2) expressing human transcription factor SPT7 and GFP, respectively. Transfection with the calcium phosphate precipitation method was done for 24 h. Transfection with FuGENE 6 (Roche Diagnostics) was performed over 24 h according to the manufacturer’s instructions. Transfection with pCD-SPT7pTL-pCD, embedded into multilayered film, was done for 24 h and with pCD-SPT7pTL-pEGFP-pCD for 2, 4, and 8 h.
Immunofluorescence.
COS-1 cells were fixed with 2% paraformaldehyde in PBS for 4 min at room temperature and incubated twice for 10 min with PBS containing 0.1% Triton X-100 (PBS-Tx). After a PBS wash, the cells were incubated overnight at room temperature in a humidified chamber with primary antibody diluted at 1/1,000 in PBS. Mouse monoclonal anti-SPT7 or rabbit polyclonal anti-GFP was used as the primary antibody. After overnight incubation at room temperature, the cells were washed with PBS-Tx and incubated with a fluorochrome-conjugated secondary antibody diluted at 1/500 in PBS-Tx for 1 h at room temperature. Cy3-conjugated goat anti-mouse (Jackson ImmunoResearch) and Alexa Fluor 488 goat anti-rabbit (Molecular Probes) were used as secondary antibodies. The cells were washed with PBS-Tx, rinsed with PBS, and counterstained with Hoechst 33258 DNA dye (5 μg·ml−1 bisbenzimide; Sigma) for 20 s. The cells were covered with mounting medium and analyzed by fluorescence microscopy.
Acknowledgments
We thank Professor Pierre Chambon (IGBMC, ICS, Collège de France, Strasbourg) for helpful discussions. N.J. is indebted to Bernard Senger for many fruitful and stimulating discussions, to Christiane Bouthier for help, and to Raphael Darcy (University College Dublin) for the generous gift of cyclodextrin (pCD). This work was supported by the Ligue Contre le Cancer (Région Alsace).
Abbreviations
- LBL
layer-by-layer
- CD
cyclodextrin
- pCD
pyridylamino-β-cyclodextrin
- PLGA
poly(l-glutamic acid)
- PLL
poly(l-lysine)
- QCM
quartz crystal microbalance.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kabanov A. V., Kabanov V. A. Bioconjugate Chem. 1995;6:7–20. doi: 10.1021/bc00031a002. [DOI] [PubMed] [Google Scholar]
- 2.Ledley F. D. Pharm. Res. 1996;13:1595–1614. doi: 10.1023/a:1016420102549. [DOI] [PubMed] [Google Scholar]
- 3.Tseng W. C., Haselton F. R., Giorgio T. D. J. Biol. Chem. 1997;272:25641–25647. doi: 10.1074/jbc.272.41.25641. [DOI] [PubMed] [Google Scholar]
- 4.Shea L. D., Smiley E., Bonadio J., Mooney D. J. Nat. Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 5.Tseng W. C., Haselton F. R., Giorgio T. D. BBA-Gene Struct. Expr. 1999;1445:53–64. doi: 10.1016/s0167-4781(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 6.Wang D. Y., Rogach A. L., Caruso F. Nano Lett. 2002;2:857–861. [Google Scholar]
- 7.Decher G. Science. 1997;277:1232–1237. [Google Scholar]
- 8.Lvov Y., Onda M., Ariga K., Kunitake T. J. Biomater. Sci. Polym. Ed. 1998;9:345–355. doi: 10.1080/09205063.1998.9753060. [DOI] [PubMed] [Google Scholar]
- 9.Serizawa T., Yamaguchi M., Akashi M. Macromolecules. 2002;35:8656–8658. [Google Scholar]
- 10.Sato K., Suzuki I., Anzai J. Langmuir. 2003;19:7406–7412. [Google Scholar]
- 11.Berth G., Voigt A., Dautzenberg H., Donath E., Mohwald H. Biomacromolecules. 2002;3:579–590. doi: 10.1021/bm0200130. [DOI] [PubMed] [Google Scholar]
- 12.Hiller J., Mendelsohn J. D., Rubner M. F. Nat. Mater. 2002;1:59–63. doi: 10.1038/nmat719. [DOI] [PubMed] [Google Scholar]
- 13.Jessel N. B., Schwinté P., Donohue R., Lavalle P., Boulmedais F., Darcy R., Szalontai B., Voegel J.-C., Ogier J. Adv. Funct. Mater. 2004;14:963–969. [Google Scholar]
- 14.Jessel N., Atalar F., Lavalle P., Mutterer J., Decher G., Schaaf P., Voegel J.-C., Ogier J. Adv. Mater. 2003;15:692–695. [Google Scholar]
- 15.Kempf M., Mandal B., Jilek S., Thiele L., Vörös J., Textor M., Merkle H. P., Walter E. J. Drug Target. 2003;11:11–18. doi: 10.1080/1061186031000072978. [DOI] [PubMed] [Google Scholar]
- 16.Mendelsohn J. D., Yang S. Y., Hiller J., Hochbaum A. I., Rubner M. F. Biomacromolecules. 2003;4:96–106. doi: 10.1021/bm0256101. [DOI] [PubMed] [Google Scholar]
- 17.Benkirane-Jessel N., Schwinté P., Falvey P., Darcy R., Haîkel Y., Schaaf P., Voegel J.-C., Ogier J. Adv. Funct. Mater. 2004;14:174–182. [Google Scholar]
- 18.Szejtli J., Osa T. Cyclodextrins, Comprehensive Supramolecular Chemistry. Vol. 3. Oxford: Pergamon; 1996. [Google Scholar]
- 19.Kilsdonk E. P., Yancey P. G., Stoudt G. W., Bangerter F. W., Johnson W. J., Phillips M. C., Rothblat G. H. J. Biol. Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 20.Freeman D. J., Niven R. W. Pharm. Res. 1996;13:202–209. doi: 10.1023/a:1016078728202. [DOI] [PubMed] [Google Scholar]
- 21.Eliseev A. V., Schneider H. J. J. Am. Chem. Soc. 1994;116:6081–6088. [Google Scholar]
- 22.Gonzalez H., Hwang S. J., Davis M. E. Bioconjugate Chem. 1999;10:1068–1074. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 23.Arima H., Kihara F., Hirayama F., Uekama K. Bioconjugate Chem. 2001;12:476–484. doi: 10.1021/bc000111n. [DOI] [PubMed] [Google Scholar]
- 24.Boon E. M., Ceres D. M., Drummond T. G, Hill M. G, Barton J. K. Nat. Biotechnol. 2000;18:1096–1100. doi: 10.1038/80301. [DOI] [PubMed] [Google Scholar]
- 25.Schouten S., Stroeve P., Longo M. L. Langmuir. 1999;15:8133–8138. [Google Scholar]
- 26.Hartwich G., Caruana D. J., de Lumley-Woodyear T., Wu Y. B., Campbell C. N., Heller A. J. Am. Chem. Soc. 1999;121:10803–10807. [Google Scholar]
- 27.Zhang J. T., Chua L. S., Lynn D. M. Langmuir. 2004;20:8015–8021. doi: 10.1021/la048888i. [DOI] [PubMed] [Google Scholar]
- 28.Johnston A. P. R., Read E. S., Caruso F. Nano Lett. 2005;5:953–956. doi: 10.1021/nl050608b. [DOI] [PubMed] [Google Scholar]
- 29.Sauerbrey G. Z. Phys. 1959;155:206–222. [Google Scholar]
- 30.Picart C., Lavalle P., Hubert P., Cuisinier F. J. G., Decher G., Schaaf P., Voegel J.-C. Langmuir. 2001;17:7414–7424. [Google Scholar]