Abstract
In Alzheimer’s disease, both acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) colocalize with brain fibrils of amyloid-β (Aβ) peptides, and synaptic AChE-S facilitates fibril formation by association with insoluble Aβ fibrils. Here, we report that human BChE and BSP41, a synthetic peptide derived from the BChE C terminus, inversely associate with the soluble Aβ conformers and delay the onset and decrease the rate of Aβ fibril formation in vitro, at a 1:100 BChE/Aβ molar ratio and in a dose-dependent manner. The corresponding AChE synthetic peptide (ASP)40 peptide, derived from the homologous C terminus of synaptic human (h)AChE-S, failed to significantly affect Aβ fibril formation, attributing the role of enhancing this process to an AChE domain other than the C terminus. Circular dichroism and molecular modeling confirmed that both ASP40 and BChE synthetic peptide (BSP)41 are amphipathic α-helices. However, ASP40 shows symmetric amphipathicity, whereas BSP41 presented an aromatic tryptophan residue in the polar side of the C terminus. That this aromatic residue is causally involved in the attenuating effect of BChE was further supported by mutagenesis experiments in which (W8R) BSP41 showed suppressed capacity to attenuate fibril formation. In Alzheimer’s disease, BChE may have thus acquired an inverse role to that of AChE by adopting imperfect amphipathic characteristics of its C terminus.
Keywords: cholinesterase, Alzheimer’s disease, aromatic, C-terminal peptide, site-directed mutagenesis
Amyloid plaques are one of the pathological hallmarks of Alzheimer’s disease (AD). The major constituent of these amyloid deposits has been identified as an aggregated 39–43-aa-long β-sheet polypeptide, amyloid β (Aβ), primarily suspected as a main cause of neurodegeneration (1). Subsequent biophysical studies of fibrilogenesis, using well characterized, homogeneous starting peptide preparations, demonstrated new protofibrillar intermediates that appeared transiently during Aβ fibril formation. These Aβ species showed a β-sheet structure and significantly inhibited neuronal viability. Thus, substances that can inhibit protofibrils formation could be of great therapeutic value for AD (2–4).
Butyrylcholinesterase (BChE), the major acetylcholine-hydrolyzing enzyme in the circulation, is structurally and functionally related to acetylcholinesterase (AChE), the primary cholinesterase in the central nervous system (5, 6). In the mammalian brain, BChE is found in complementary and overlapping sites with AChE, in nuclei related to cognitive and behavioral functions, and in the human thalamus (7). In the brain of patients with AD, BChE colocalizes with AChE in the insoluble fibrils, known as senile plaques, within the cerebral cortex.
The proportional plaque area displaying BChE as compared with Aβ was significantly higher in the brains of demented than nondemented patients (8). Together with BChE’s localization in neurofibrillary tangles, the second pathological hallmark of AD, this finding suggested active involvement for BChE in the disease process but left its role unclear.
AChE is another component of senile plaques (8). Synaptic AChE-S, the primary AChE 3′ splice variant (also known as AChE-T) (9), promotes Aβ aggregation in vitro (10–12) and enhances amyloid toxicity in cultured neuronal cells (13). In hybrid transgenic mice, AChE-S promotes plaque accumulation (14, 15), supporting the notion of its causal involvement with the fibril-formation process.
Several studies attempted to delineate the specific AChE-S structural domain(s) accentuating the nucleation and progression of the fibril-formation process. A hydrophobic sequence positioned close to the peripheral anionic binding site (PAS) in the AChE core domain, was found to directly interact with Aβ (16) and was proposed to be actively involved in the acceleration of amyloid fibril formation (11). BChE also harbors a PAS region that shares some structural and physicochemical properties with that of AChE. However, the BChE PAS lacks three of four aromatic residues of the AChE PAS and displays an inverse biochemical property, namely, substrate activation rather than the substrate-inhibition feature characterizing the AChE PAS (17).
To study the functional involvement of BChE as compared with AChE in amyloid fibril formation, we studied the effects of purified AChE-S and BChE as well as synthetic peptides derived from their C termini on in vitro fibril formation from Aβ. We report here an attenuating role for BChE on fibril formation that involves an interaction of the C terminus of BChE with the soluble species of β-amyloids.
Results
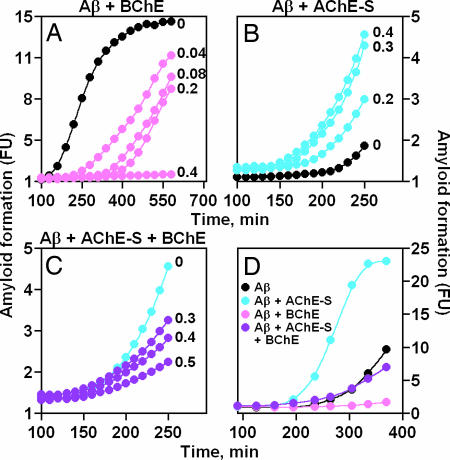
In vitro, Aβ fibrils formed spontaneously, provided the Aβ (1–40 amino acid residues) peptide was present >10 μM. Fibril formation from 33 μM Aβ peptide was quantified by measuring changes in thioflavin T (ThT) fluorescence in reaction mixtures, including increasing concentrations of purified human BChE (Fig. 1A), recombinant AChE-S (Fig. 1B), or both (Fig. 1 C and D). Characteristic experiments demonstrated that the presence of AChE-S or BChE conferred distinct kinetics of Aβ sheet formation.
Fig. 1.
BChE suppresses, whereas AChE-S accelerates, amyloid formation. Kinetics of change in ThT fluorescence with time are shown. Aβ (1–40), at 33 μM, was shaken at 30°C with 1 μM ThT and BChE (A) or AChE-S (B) at the noted micromolar concentrations or with both enzymes at 0.4 μM (C and D). One of 12–16 reproducible experiments is shown. Note time scale differences.
BChE Attenuates Amyloid Fibril Formation.
Purified BChE, at a 1:100 molar ratio to Aβ, prolonged the lag and reduced the apparent rate of amyloid fibril formation. The effect was dose-dependent to the extent that addition of 0.4 μM BChE to 33 μM Aβ totally prevented fibril formation for >600 min (Fig. 1A and Table 1). In contrast, addition of similar doses of AChE-S to Aβ inversely shortened by half the lag time before fibril formation (from 240 to 150 min), again in a dose-dependent manner, and increased the apparent maximal rate of fibril formation, from 0.057 fluorescence units/min for Aβ alone to 0.116 for Aβ in the presence of 0.36 μM AChE-S (Fig. 1B and Table 1). Increasing doses of human (h)BChE added to a mix of 33 μM Aβ with 0.36 μM AChE-S caused dose-dependent interference with the fibril-formation process (Fig. 1C). Under these conditions, the rate of fibril formation receded to the slow rate observed for Aβ alone, demonstrating a capacity of BChE to attenuate the fibril formation-accelerating effect of AChE-S (Fig. 1 C and D).
Table 1.
Cholinesterases and their Aβ fibrillation effects
| Cholinesterase | Concentration, mg/liter | Molecular mass, Da | ε280 | Fold change |
|
|---|---|---|---|---|---|
| Rate | Lag | ||||
| No addition | – | – | – | – | – |
| AChE-S | 25.8 | 64,575.1 | 124,570 | 2.1* | 0.6* |
| ASP40 | 2.0 | 5,074.5 | 19,750 | 0.8 | 1.1 |
| BChE | 27.4 | 58,418.1 | 134,310 | 0.3* | 1.4* |
| BSP41 | 2.0 | 5,029.5 | 25,440 | 0.4* | >1.9* |
| (W8R)BSP41 | 2.0 | 5,000.0 | 19,750 | 0.53 | 1.25 |
| (R8W)ASP40 | 2.0 | 5,104.0 | 25,440 | 0.62 | 1.0 |
Peptides following the C-terminal sequences of human AChE-S and BChE (SwissProt accession nos. P06276 and P22303) were synthesized by using a Pioneer or a 443A peptide synthesizer (Applied Biosystems), followed by purification (>90%) by reverse-phase HPLC and analysis by MALDI-TOF mass spectrometry. Synthesized peptides and Aβ fibril formation were characterized as detailed in Materials and Methods. Statistically significant differences (P < 0.05, Student’s t test) from control (No addition) for triplicate measurements and up to 20 repetitive experiments are marked by asterisks.
BChE Is Present in a Soluble Fraction of Aβ.
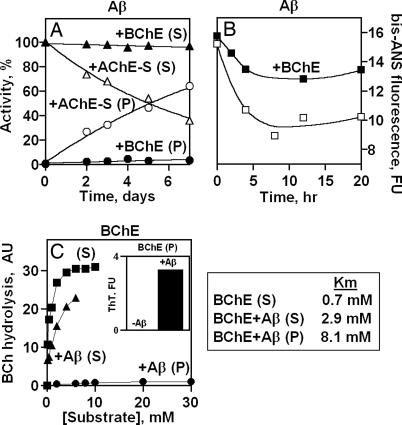
To reveal which of the different intermediates of the Aβ fibril-formation process are affected by BChE, we incubated 40 μM Aβ (1–40) peptide with 0.4 μM BChE or AChE and, at the indicated time points, separated the soluble and insoluble fractions by centrifugation and assessed the presence of cholinesterases in each of the fractions by measuring their enzymatic activities. AChE-S, predictably, disappeared with time from the soluble fraction and appeared in the pellet, in agreement with the findings of Alvarez et al. (18) (Fig. 2A). In contrast, BChE was found to be present exclusively in the soluble fraction of Aβ over the entire time range of fibril formation (Fig. 2A). The same distribution of BChE activity was obtained when the fibril formation involved shaking and followed the time scale of hours (data not shown).
Fig. 2.
BChE associates with the soluble fraction of Aβ peptide. (A) BChE is localized in the soluble fraction of Aβ. Shown are the enzymatic activities associated with the soluble or pelleted fractions of Aβ formed by incubating Aβ (1–40) peptide (40 μM) with 0.4 μM BChE or AChE at 25°C without shaking, removal of samples at the indicated time points, centrifugation for 30 min at 20,800 × g, and three washes of the pelleted material with 10 volumes of PBS. (B) BChE intervenes with the conversion of soluble to insoluble Aβ-fibrils. Shown is bis-ANS fluorescence measured as described in Materials and Methods for Aβ (1–40), shaken, at 200 μM and 25°C in the presence or absence of 10 μM BChE. Aliquots were removed at the indicated time points and diluted 10 times with 27.5 μM bis-ANS. (C Left) Interaction of BChE with Aβ affects the affinity of the enzyme to its substrate. BChE (2 μM without or with 30 μM Aβ was incubated for 22 h with shaking, and the mixture was fractionated as in A. Enzymatic activity was determined at 4°C over the range of increasing butyrylthiocholine concentrations (0–30 mM). (Inset) ThT fluorescence of Aβ pellets formed in the presence of BChE. (C Right) Corresponding Km values for BChE in the different fractions.
Kinetics of Appearance of the Different Conformers of Aβ.
To reveal the step of the fibril-formation process that is affected by BChE, we followed with time the changes in the fluorescence of bis-ANS (4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonate), which binds to the soluble, low-molecular-mass isoforms of Aβ. BChE increased the fraction of the bis-ANS-sensitive conformers that accumulated during 20 h of incubation (Fig. 2B) and demonstrated significantly lower associations with the ThT-labeled fraction (data not shown), indicating that BChE, by binding to the soluble, probably low oligomeric, form of Aβ slows down its conversion to the larger, β-sheets-rich Aβ species. Because bis-ANS cannot distinguish between monomers and low oligomers of Aβ peptides, further research will be needed to reveal the exact composition of the soluble intermediate sensitive to BChE.
Enzymatic Properties of the Soluble BChE–Aβ Mixture.
To demonstrate the presence of the BChE–Aβ complex in the soluble fraction, the affinity of BChE to its substrate was determined after 22-h preincubation with or without Aβ. To preserve the steady state of BChE association to Aβ, the preincubation mixture was cooled to 4°C, supernatant and pellet were separated, and hydrolytic activities were determined at 4°C. Substrate affinity of soluble BChE preincubated with Aβ (EC50 of 1.7 mM) was eight times lower than that of BChE incubated without Aβ (EC50 of 0.2 mM) (Fig. 2C). Enzyme activity in the soluble fraction reflects the activity of both free and Aβ-bound enzyme species, suggesting that the measured affinity of BChE to its substrate is, likely, an underestimation. Importantly, the very minor fraction of BChE that was associated with the Aβ pellet (resuspended in the original volume of PBS buffer and exhibiting high binding of ThT (Fig. 2C Inset) bound its substrate with >30-fold-lower affinity (EC50 of 6.5 mM) than the soluble unbound enzyme (Fig. 2C Right). Similar decreases in substrate affinity were reported for AChE present in AChE–Aβ complexes of the insoluble fibrillar fraction (18). The currently observed decrease in substrate affinity thus likely reflects binding of BChE to one or several soluble intermediates of the Aβ fibrillation process, reinforcing the notion that BChE is present in soluble BChE–Aβ complexes.
BChE Synthetic Peptide (BSP)41, BChE’s C-Terminal Peptide, Attenuates Fibril Formation.
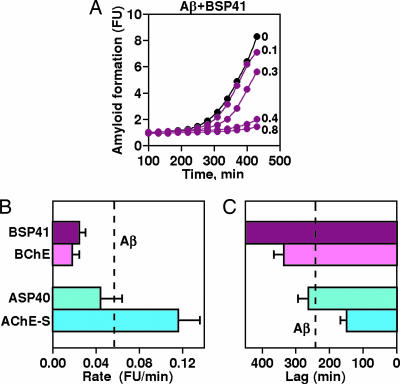
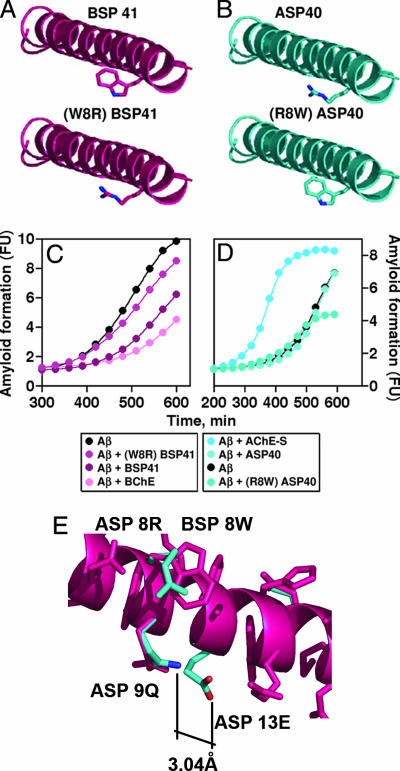
In search of the region(s) within the BChE molecule that cause the observed interference with Aβ fibril formation, synthetic peptides of the homologous C-terminal domains of BChE and AChE-S (Table 2) were examined for their effects on amyloid fibril formation. The 41-aa-long BSP41 peptide suppressed the fibril-formation process in a dose-dependent manner and in similar molar ratios to those of BChE (Fig. 3A). Like BChE, BSP41 decreased the rate of fibril formation and prolonged the lag period (Fig. 3 B and C). In absolute quantity terms, complete attenuation of Aβ fibril formation for >400 min was achieved with 2 μg/ml BSP41 as compared with 30 μg/ml BChE (Table 1). In contrast, the 40-aa-long AChE synthetic peptide (ASP)40 peptide derived from the corresponding domain in AChE-S showed no capacity to activate or inhibit fibril formation (Fig. 3 B and C).
Table 2.
Native or mutated C-terminal peptides of cholinesterases
| Cholinesterase | Sequence |
|---|---|
| ASP40 | DTLDEAERQWKAEFHRWSSYMVHWKNQFDHY-SKQDRCSDL |
| (R8W)ASP40 | DTLDEAEWQWKAEFHRWSSYMVHWKNQFDHY-SKQDRCSDL |
| BSP41 | GNIDEAEWEWKAGFHRWNNYMMDWKNQFNDYTSKKESCVGL |
| (W8R)BSP41 | GNIDEAEREWKAGFHRWNNYMMDWKNQFNDYTSKKESCVGL |
The mutated trytophan and arginine residues are shown in bold.
Fig. 3.
Effects of synthetic BSP and ASP on amyloid formation. (A) Aβ fibril formation was followed under addition of the noted micromolar concentrations of BSP41. (B) Average rates ± SE of changes in ThT fluorescence are shown for the cumulative data of each protein and peptide tested. (C) Average lags ± SE preceding the onset of fibril formation in the presence of Aβ and the noted proteins or peptides.
Structure–Function Relationships.
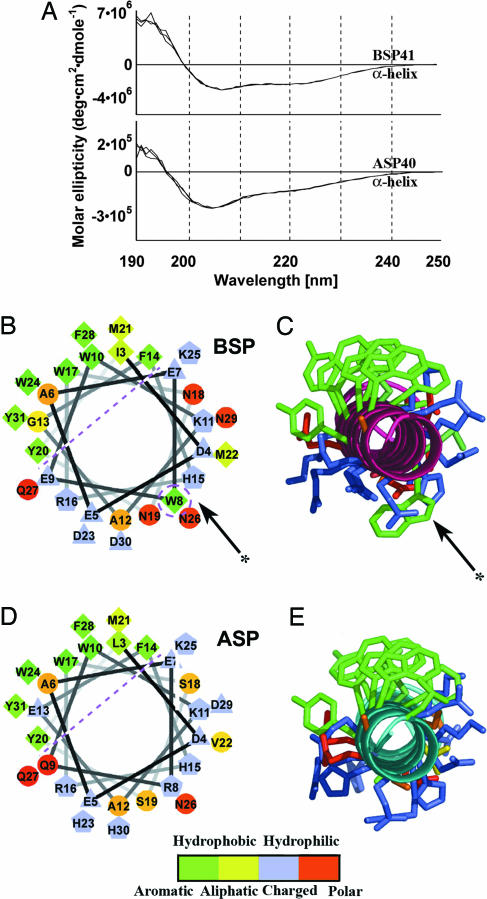
To further pursue the structural basis for the functional differences between ASP and BSP, we measured molar elipticity circular dichroism (CD) of BSP41 and ASP40. A clear positive band at 192 nm and two negative bands at 209 and 220 nm were obtained for both peptides, characteristic of α-helical structures (Fig. 4A). In structural modeling, both ASP40 and BSP41 peptides emerged as symmetric amphipathic helices with similar distributions of polar and nonpolar residues, compatible with findings of others (Fig. 4 B–E) (19). However, BSP41 displayed imperfect amphipathicity, locally disturbed by the protruding aromatic W8 residue in the polar side of this helix, indicating that the aromatic W8 residue in the polar side of BSP41 could potentially be responsible for the functional difference between ASP40 and BSP41. To challenge this hypothesis, we prepared a W8R mutant of BSP41 and an R8W mutant of ASP40 (Table 2 and Fig. 5 A and B). (W8R)BSP41 showed a significantly reduced ability to attenuate Aβ fibril formation, corroborating the importance of this aromatic tryptophan. Reciprocally, (R8W)ASP40 showed a decreased yield and a marginally reduced rate of fibril formation as compared with the native ASP40 (Fig. 5C and D), suggesting that other feature(s) of ASP40 determine its incapacity to attenuate Aβ fibril formation. Structural modeling further highlighted an H-bond of 3.04 Å formed between Q9 and E13 of ASP40 but not between the corresponding E9 and G13 residues of BSP41 (Fig. 5E). This difference, next to W8, forms a polarized microenvironment, shielding the tryptophan effect in the polar side. That both (W8R)BSP41 and (R8W)ASP40 failed to affect Aβ fibril formation thus confirmed that W8 is an essential, but not sufficient, cause of attenuating Aβ fibril formation.
Fig. 4.
Structural characteristics of BSP and ASP peptides. (A) CD α-helix spectra of 1 × 10−4 M BSP41 (Upper) and ASP40 (Lower) in aqueous solutions. (B) Helical wheel projection of residues 3–30 in human BSP. Residues appear as circles (hydrophilic), diamonds (hydrophobic), triangles (potentially negatively charged), or pentagons (potentially positively charged); for further division, see color scale. A division line divides the BSP amphipathic helix into polar and nonpolar sides. Tryptophan 8 (dashed circle, arrow), disturbs amphipathicity. (C) Combined molecular modeling with colored aromaticity (green, see scale) highlights the asymmetric protrusion by tryptophan 8. Arrows mark tryptophan 8. (D) ASP shows minor differences from BSP in the hydrophobicity and charge of its residues (see scale). (E) ASP’s amphipathicity is intact, completed by arginine 8.
Fig. 5.
W8 is essential, but not sufficient, to attenuate Aβ fibril formation by BSP41. (A and B) Molecular models of BSP41 and substituted (W8R)BSP41 and ASP40 and (R8W)ASP40, respectively. (C) BSP41 attenuates Aβ fibril formation more effectively than (W8R)BSP41. (D) Neither ASP40 nor (R8W)ASP40 attenuates fibril formation. One of six reproducible experiments is shown. (E) Superimposed BSP (purple) and ASP (blue). Residues Q9 and E13 of ASP form a H-bond (3.04 Å) that cannot be formed between E9 and G13 of BSP, raising the microenvironment polarity to ASP.
Discussion
By following the kinetics of amyloid formation rather then measuring the final yield of the fibril-formation process, we were able to show that BChE prolongs the lag (nucleation phase) and reduces the rate (propagation phase) of amyloid fibril formation in vitro. Others have observed that AChE, but not BChE, increases the final yield of the Aβ fibrils and interpreted that to imply no involvement of BChE (11). Our study, however, demonstrated that, contrary to this early prediction, BChE acts as a negative modifier in this process and is also capable of suppressing the facilitation of amyloid fibril formation enhanced by recombinant, highly purified AChE-S. Importantly, the C-terminal peptide of BChE, BSP41, mimicked the effect of BChE. In contrast, the corresponding peptide of AChE-S failed to affect the fibril-formation process, showing neither facilitation nor suppression of amyloid fibril formation. This finding is compatible with the conclusion of others (11) that AChE-S does not promote fibril formation via its C-terminal peptide but rather through its hydrophobic PAS domain. Our findings thus demonstrate that BChE, in contrast to AChE-S, interferes with the fibril-formation process in a manner involving C-terminal aromatic residues in a polar environment.
Aggregation processes are affected by hydrophobicity, β-propensity, π-stacking, and charge (20, 21). Recent reports postulate that, for amyloid-forming proteins, a unique side-chain arrangement that, by π-stacking forces, provides the energetic force supporting the formation and stabilization of the core β-sheet structure (22). In the majority of AChE-S and BChE molecules, the hydrophobic part of the amphipathic helix of the C terminus, is engaged in G4 homooligomers (19, 23). In the AChE-S molecule, the hydrophobic PAS domain located close to the lip of the active-site gorge is free to form complexes with growing fibrils, thus supporting the Aβ assembly process (24). In variance with AChE-S, BChE attenuates the fibril-formation process by the aromatic W8 residue, positioned in the polar side of the BSP helix. This residue can form heteroaromatic complexes with soluble monomeric or low-oligomeric Aβ conformers. The residue can thus interfere with oligomerization and/or the side-chain stabilization of the β-sheet structure and inhibit propagation of the fibril-formation process to form toxic protofibrils and insoluble fibers (Fig. 6B). That replacement of tryptophan to a polar residue abolishes the attenuation of Aβ fibril formation is fully compatible with this hypothesis. This concept is also strengthened by the reports of others (25, 26), that pointed to heteroaromatic interactions as being involved in the inhibition of fibril formation.
Fig. 6.
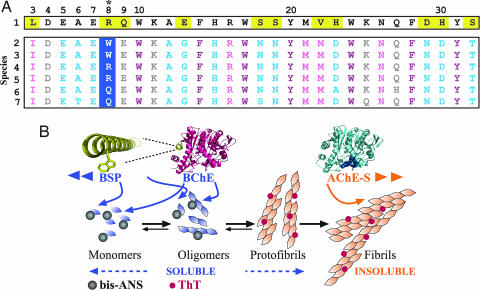
The mechanism(s) underlying BChE’s effects. (A) Evolutionary conservation of amphipathic apolarity in BSP. (Upper) The fully conserved ASP sequence (row 1) (SwissProt accession no. P22303). Differences from human BSP are marked in yellow. (Lower) blast search of the BSP sequences (rows 2–7, human, P06276; ponpy, Q5R7Z4; horse, P81908; chick, Q90ZK8; rabbit, P21927; and mouse, Q04411). Only great apes (i.e., Homo sapiens and ponpy) include tryptophan in position 8 (asterisk); thus, the currently observed effect could be deciphered only with the human enzyme. (B) Model of BChE and AChE opposing effects on Aβ fibril formation. BChE attenuates Aβ fibril formation by association with the monomeric Aβ and/or the protofibrillar-soluble Aβ species, recognized by bis-ANS. In contrast, AChE-S enhances Aβ fibril formation by binding to the highly organized insoluble amyloid fibrils and fibrils recognized by ThT.
Single amino acid substitutions affect the amyloidogenic potential of several peptides and proteins (27, 28). Our current findings highlight the importance of the structural location of such residues. Particularly, we show that two peptides sharing high homology affect fibril formation inversely because of the altered position of a single aromatic residue.
Intriguingly, the polar substitution in BSP is unique to humans and great apes (Fig. 6A), explaining why mouse studies overlooked this phenomenon and suggesting that primate BChE acquired this promiscuous function while not impairing its classical roles in ACh hydrolysis (29). Correspondingly, arginine-for-tryptophan substitutions impair the biological features in numerous proteins (30). Therefore, identifying evolutionary structural promiscuity may, perhaps, be used to predict and manipulate protein functions.
In the human brain, AChE mRNA is 20-fold more abundant than BChE mRNA (31). In human blood, however, BChE, at 50 nM, is 3-fold more abundant than AChE (32). This difference might be physiologically important, because the Aβ fibril-formation process likely involves continuous communication between the brain and the circulation (33) and because BChE interacts with Aβ in the soluble phase. That others see BChE in amyloid plaques (7) may imply that BChE incorporates into Aβ fibrils at a late phase of their formation. Putative therapeutic use of the relatively short BSP peptide may involve injection, similar to erythropoietin or granulocyte–marcrophage colony-stimulating factor C (GM-CSF) (34, 35). Transfecting bone marrow cells for autologous transplantation with a BSP expression vector, similar to the gene therapy protocols used for adenosine deaminase replacement (36, 37), or intranasal administration, like that of nerve-growth factor (NGF) (38), are also plausible. In either way, the reportedly disrupted blood–brain barrier in AD (17) and that cholinergic imbalances induce blood–brain-barrier disruption (39) predict effective BSP41 penetration into the brain. The role(s) and actions of BChE in the pathogenesis of AD thus merit renewed attention.
Materials and Methods
Enzymes.
Purified human BChE (from human serum, >90% pure, as determined by gel electrophoresis) and recombinant human AChE-S were from Sigma. To define enzymatic activity, we determined hydrolysis rates of butyryl- or acetylthiocholine at 25°C or 4°C, respectively, as indicated (40). Enzyme concentrations were calculated based on the molecular mass of a protein monomer and its known amino acid composition.
The mammalian AChE-S and BChE proteins are highly homologous, and both were tightly conserved through evolution. Intriguingly, there were sequence differences in a structural context between the BChE and the AChE-S C termini, especially in aromatic residues shown by others to exert dominant effects on the fibril-formation process. This phenomenon served as the basis for our selection of the C-terminal peptides of BChE and AChE-S as putative yet distinct interactors with the Aβ peptide.
Peptides.
Peptides were synthesized, according to the C-terminal sequences of human AChE-S and BChE (SwissProt accession nos. P06276 and P22303) by using a Pioneer or 433A peptide synthesizer (Applied Biosystems). Purification (>90%) by reverse-phase HPLC was followed by MALDI-TOF mass spectrometry (41).
Kinetics of Aβ Fibril Formation Monitored by Fluorescence Measurements.
The fluorescence excitation spectrum of the benzothiazole dye ThT (Sigma) shifts from 340 to 450 nm when interacting with β-sheet amyloid structures. Fluorescence signals (excitation, 450 nm; emission, 485 nm) reflected the amount of amyloid fibrils formed (42). In contrast, bis-ANS shows increasing fluorescence when interacting in acidic buffer solutions with soluble, random coil/mixed conformations and α-helical forms of Aβ (1–40) but reacts weakly with soluble β-sheet forms or amyloid fibrils (43). Both ThT and bis-ANS fluorescence were measured by using a spectrofluorometer (Tecan, Maennedorf, Switzerland).
A synthetic Aβ (1–40) peptide (from BioSource, Camarillo, CA, or Sigma) dissolved in DMSO at 1.6 mM was sonicated (three 5-sec pulses), filtered through 0.22-μm filters, and stored in aliquots at −70°C. Aliquots of Aβ were diluted from the stock DMSO solution to a final concentration of 162 μM in 20 μl of PBS containing 0.02% sodium azide (fibril-formation buffer), with or without cholinesterases or synthetic peptides, in 96-well plates (Nunc). After 20-min preincubation at room temperature, 80 μl of 1.25 μM ThT in 50 mM glycine–NaOH buffer, pH 8.5, was added for 6–10 h, shaking at 200 rpm at 30°C. ThT fluorescence was measured at 10- to 30-min intervals. Alternatively, 162 μM Aβ was incubated at room temperature in the fibril-formation buffer, with shaking for several hours or without shaking, for up to 7 days. Aliquots of 20 μl were removed and assayed with ThT (as above) or with 30 μM bis-ANS (in 30 mM citrate buffer, pH 2.4).
Assessment of the fibril-formation process involved measuring the lag preceding the onset of fluorescence increase (the nucleation process) and the apparent maximal rate of fluorescence increase (rate of fibril formation) for 300–600 min, depending on the duration of the lag phase. Mean standard error was calculated by using the program kaleidagraph (Synergy Software, Reading, PA).
CD Measurements.
BSP and ASP peptides were dissolved in double-distilled water to a final concentration of 1×10−4 M. Direct CD spectra were recorded at room temperature by using a CD J-810 spectropolarimeter (Jasco, Easton, MD) with a 100-QS 1-mm path-length quartz cuvette (Hellma, Müllheim, Germany). Recordings were at 0.5-nm intervals in the spectral range 185–260 nm.
Peptide Modeling.
Model construction of the analyzed peptides involved the deep view spdbv 3.7 software (GlaxoSmithKline, Bredford, U.K.), followed by distance geometry minimization. Figures were created with the program pymol (DeLano Scientific, San Carlos, CA). Helical wheel projections were prepared by using wheel.pl, Ver. 0.10 (Cell Biology and Neuroscience, University of California, Riverside). The consurf server (44) was used to predict the evolutionary conservation of specific ASP and BSP residues.
Acknowledgments
Special thanks to Sir Alan Fersht (Cambridge University, Cambridge, U.K.) for advice and assistance. This work was supported by the Hurwitz Fund and Hebrew University’s Eric Roland Center for Neurodegenerative Disease (H.S.) and by a Bikura Grant from the Israel Science Foundation (to A.F.).
Abbreviations
- Aβ
β-amyloid peptide
- AChE
acetylcholinesterase
- AD
Alzheimer’s disease
- ASP
AChE-S C-terminal peptide
- bis-ANS
4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonate
- BChE
butyrylcholinesterase
- BSP
BChE synthetic peptide
- CD
circular dichroism
- PAS
peripheral anionic binding site
- ThT
thioflavin T.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Selkoe D. J. J. Biol. Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- 2.Walsh D. M., Hartley D. M., Kusumoto Y., Fezoui Y., Condron M. M., Lomakin A., Benedek G. B., Selkoe D. J., Teplow D. B. J. Biol. Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 3.Walsh D. M., Lomakin A., Benedek G. B., Condron M. M., Teplow D. B. J. Biol. Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 4.Stine W. B., Jr., Dahlgren K. N., Krafft G. A., LaDu M. J. J. Biol. Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- 5.Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 6.Mesulam M., Guillozet A., Shaw P., Quinn B. Neurobiol. Dis. 2002;9:88–93. doi: 10.1006/nbdi.2001.0462. [DOI] [PubMed] [Google Scholar]
- 7.Darvesh S., Hopkins D. A. J. Comp. Neurol. 2003;463:25–43. doi: 10.1002/cne.10751. [DOI] [PubMed] [Google Scholar]
- 8.Mesulam M. M., Geula C. Ann. Neurol. 1994;36:722–727. doi: 10.1002/ana.410360506. [DOI] [PubMed] [Google Scholar]
- 9.Morel N., Leroy J., Ayon A., Massoulie J., Bon S. J. Biol. Chem. 2001;276:37379–37389. doi: 10.1074/jbc.M103192200. [DOI] [PubMed] [Google Scholar]
- 10.Inestrosa N. C., Alvarez A., Calderon F. Mol. Psychiatry. 1996;1:359–361. [PubMed] [Google Scholar]
- 11.Inestrosa N. C., Alvarez A., Perez C. A., Moreno R. D., Vicente M., Linker C., Casanueva O. I., Soto C., Garrido J. Neuron. 1996;16:881–891. doi: 10.1016/s0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 12.Bartolini M., Bertucci C., Cavrini V., Andrisano V. Biochem. Pharmacol. 2003;65:407–416. doi: 10.1016/s0006-2952(02)01514-9. [DOI] [PubMed] [Google Scholar]
- 13.Munoz F. J., Inestrosa N. C. FEBS Lett. 1999;450:205–209. doi: 10.1016/s0014-5793(99)00468-8. [DOI] [PubMed] [Google Scholar]
- 14.Rees T., Hammond P. I., Soreq H., Younkin S., Brimijoin S. Neurobiol. Aging. 2003;24:777–787. doi: 10.1016/s0197-4580(02)00230-0. [DOI] [PubMed] [Google Scholar]
- 15.Rees T. M., Berson A., Sklan E. H., Younkin L., Younkin S., Brimijoin S., Soreq H. Curr. Alzheimer Res. 2005;2:291–300. doi: 10.2174/1567205054367847. [DOI] [PubMed] [Google Scholar]
- 16.De Ferrari G. V., Canales M. A., Shin I., Weiner L. M., Silman I., Inestrosa N. C. Biochemistry. 2001;40:10447–10457. doi: 10.1021/bi0101392. [DOI] [PubMed] [Google Scholar]
- 17.Glick D., Ben Moyal L., Soreq H. Genetic Variation in Butyrylcholinesterase and the Physiological Consequences for Acetylcholinesterase Function. London: Martin Dunitz; 2003. pp. 55–67. [Google Scholar]
- 18.Alvarez A., Alarcon R., Opazo C., Campos E. O., Munoz F. J., Calderon F. H., Dajas F., Gentry M. K., Doctor B. P., De Mello F. G., Inestrosa N. C. J. Neurosci. 1998;18:3213–3223. doi: 10.1523/JNEUROSCI.18-09-03213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dvir H., Harel M., Bon S., Liu W. Q., Vidal M., Garbay C., Sussman J. L., Massoulie J., Silman I. EMBO J. 2004;23:4394–4405. doi: 10.1038/sj.emboj.7600425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazit E. FASEB J. 2002;16:77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 21.Tartaglia G. G., Cavalli A., Pellarin R., Caflisch A. Protein Sci. 2004;13:1939–1941. doi: 10.1110/ps.04663504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makin O. S., Atkins E., Sikorski P., Johansson J., Serpell L. C. Proc. Natl. Acad. Sci. USA. 2005;102:315–320. doi: 10.1073/pnas.0406847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belbeoc’h S., Massoulie J., Bon S. EMBO J. 2003;22:3536–3545. doi: 10.1093/emboj/cdg360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez A., Opazo C., Alarcon R., Garrido J., Inestrosa N. C. J. Mol. Biol. 1997;272:348–361. doi: 10.1006/jmbi.1997.1245. [DOI] [PubMed] [Google Scholar]
- 25.Porat Y., Mazor Y., Efrat S., Gazit E. Biochemistry. 2004;43:14454–14462. doi: 10.1021/bi048582a. [DOI] [PubMed] [Google Scholar]
- 26.Inouye H., Sharma D., Goux W. J., Kirschner D. A. Biophys. J. 2006;90:1774–1789. doi: 10.1529/biophysj.105.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wurth C., Guimard N. K., Hecht M. H. J. Mol. Biol. 2002;319:1279–1290. doi: 10.1016/S0022-2836(02)00399-6. [DOI] [PubMed] [Google Scholar]
- 28.Chiti F., Taddei N., Bucciantini M., White P., Ramponi G., Dobson C. M. EMBO J. 2000;19:1441–1449. doi: 10.1093/emboj/19.7.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aharoni A., Gaidukov L., Khersonsky O., McQ G. S., Roodveldt C., Tawfik D. S. Nat. Genet. 2005;37:73–76. doi: 10.1038/ng1482. [DOI] [PubMed] [Google Scholar]
- 30.Vitkup D., Sander C., Church G. M. Genome Biol. 2003;4:R72. doi: 10.1186/gb-2003-4-11-r72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soreq H., Zakut H. Pharm. Res. 1990;7:1–7. doi: 10.1023/a:1015867021628. [DOI] [PubMed] [Google Scholar]
- 32.Loewenstein-Lichtenstein Y., Schwarz M., Glick D., Norgaard Pedersen B., Zakut H., Soreq H. Nat. Med. 1995;1:1082–1085. doi: 10.1038/nm1095-1082. [DOI] [PubMed] [Google Scholar]
- 33.Basun H., Nilsberth C., Eckman C., Lannfelt L., Younkin S. Dementia Geriatr. Cogn. Disord. 2002;14:156–160. doi: 10.1159/000063605. [DOI] [PubMed] [Google Scholar]
- 34.Arndt U., Kaltwasser J. P., Gottschalk R., Hoelzer D., Moller B. Ann. Hematol. 2005;84:159–166. doi: 10.1007/s00277-004-0950-z. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W. G., Liu S. H., Cao X. M., Cheng Y. X., Ma X. R., Yang Y., Wang Y. L. Leuk. Res. 2005;29:3–9. doi: 10.1016/j.leukres.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Aiuti A., Ficara F., Cattaneo F., Bordignon C., Roncarolo M. G. Curr. Opin. Allergy Clin. Immunol. 2003;3:461–466. doi: 10.1097/00130832-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Herzog R. W., Arruda V. R. Exp. Rev. Cardiovasc. Ther. 2003;1:215–232. doi: 10.1586/14779072.1.2.215. [DOI] [PubMed] [Google Scholar]
- 38.De Rosa R., Garcia A. A., Braschi C., Capsoni S., Maffei L., Berardi N., Cattaneo A. Proc. Natl. Acad. Sci. USA. 2005;102:3811–3816. doi: 10.1073/pnas.0500195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meshorer E., Biton I. E., Ben-Shaul Y., Ben-Ari S., Assaf Y., Soreq H., Cohen Y. FASEB J. 2005;19:910–922. doi: 10.1096/fj.04-2957com. [DOI] [PubMed] [Google Scholar]
- 40.Ellman G. L., Courtney K. D., Andres V., Jr., Feather-Stone R. M. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 41.Grisaru D., Pick M., Perry C., Sklan E. H., Almog R., Goldberg I., Naparstek E., Lessing J. B., Soreq H., Deutsch V. J. Immunol. 2006;176:27–35. doi: 10.4049/jimmunol.176.1.27. [DOI] [PubMed] [Google Scholar]
- 42.LeVine H., III Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeVine H., III Arch. Biochem. Biophys. 2002;404:106–115. doi: 10.1016/s0003-9861(02)00246-1. [DOI] [PubMed] [Google Scholar]
- 44.Glaser F., Pupko T., Paz I., Bell R. E., Bechor-Shental D., Martz E., Ben-Tal N. Bioinformatics. 2003;19:163–164. doi: 10.1093/bioinformatics/19.1.163. [DOI] [PubMed] [Google Scholar]