Abstract
The telomere and centromere are two specialized structures of eukaryotic chromosomes that are essential for chromosome stability and segregation. These structures are usually characterized by large tracts of tandemly repeated DNA. In mouse, the two structures are often located in close proximity to form telocentric chromosomes. To date, no detailed sequence information is available across the mouse telocentric regions. The antagonistic mechanisms for the stable maintenance of the mouse telocentric karyotype and the occurrence of whole-arm Robertsonian translocations remain enigmatic. We have identified large-insert fosmid clones that span the telomere and centromere of several mouse chromosome ends. Sequence analysis shows that the distance between the telomeric T2AG3 and centromeric minor satellite repeats range from 1.8 to 11 kb. The telocentric regions of different mouse chromosomes comprise a contiguous linear order of T2AG3 repeats, a highly conserved truncated long interspersed nucleotide element 1 repeat, and varying amounts of a recently discovered telocentric tandem repeat that shares considerable identity with, and is inverted relative to, the centromeric minor satellite DNA. The telocentric domain as a whole exhibits the same polarity and a high sequence identity of >99% between nonhomologous chromosomes. This organization reflects a mechanism of frequent recombinational exchange between nonhomologous chromosomes that should promote the stable evolutionary maintenance of a telocentric karyotype. It also provides a possible mechanism for occasional inverted mispairing and recombination between the oppositely oriented TLC and minor satellite repeats to result in Robertsonian translocations.
Keywords: centromere, chromosome, evolution, telomere
Eukaryotic chromosomes contain telomeres and centromeres, both being essential for faithful chromosome segregation during cell division. The telomere comprises functionally important small tandem repeats and is a cap-like structure that protects the ends of chromosomes from degradation and aberrant fusion with other chromosome ends (1). The centromere, on the other hand, is usually located along the chromosome arm and is essential for mitotic spindle capture and checkpoint control, sister chromatid cohesion and release, and cytokinesis (2, 3). The centromere of higher eukaryotes typically consists of tandemly repeated DNA arrays that are devoid of genes. In some organisms, such a tandem repeat structure is absent, as seen in the point centromeres of the budding yeast and the ectopic centromeres (or neocentromeres) that form in nonrepetitive regions of the genome (4).
The karyotype of the mouse genus, Mus, is made up of 19 pairs of telocentric autosomes and a telocentric X chromosome. Here, we define a telocentric chromosome as having no obvious short arm at a cytogenetic level. The Y chromosome contains a short distal arm and can be defined as being acrocentric. Molecular cytogenetic studies have placed the mouse centromeric minor satellite DNA 10–1,000 kb away from the end of the telocentric short arm (p-arm) (5–7). However, what remains unknown are the precise DNA sequence and molecular organization of the mouse telocentric p-arms or, indeed, those of other eukaryotic telocentric chromosomes.
Previous attempts by others at isolating the p-arm telomeric and subtelomeric sequences of the mouse chromosomes have largely been unsuccessful because of the tandem repeat nature of the DNA residing in these regions and difficulties associated with anchoring these sequences to individual chromosomes. We have identified fosmid clones generated for the C57BL/6 mouse genome project (8), containing large randomly sheared inserts that bridge the gap between the p-arm telomere and centromere. The study of these clones has enabled us to establish the true molecular nature of the mouse telocentric p-arm regions and have allowed us to propose possible mechanisms involving karyotype maintenance and evolution.
Results
Identification of Fosmid Clones with Telomeric and Minor Satellite DNA.
Previous studies using pulsed-field gel electrophoresis (PFGE) (5, 6) and stretched chromatin fiber FISH (7) have suggested that the mouse “short-arm” telomere [p-telomeric (p-tel)] was situated in close proximity to the centromeric mouse minor satellite DNA. In an attempt to bridge the gap between the p-tel and the centromere, we used the fosmid end-sequence clone library created from the public C57BL/6 mouse genome project (8). This library provides a relatively unbiased representation of the C57BL/6 mouse genome, because the DNA inserts were isolated from randomly sheared genomic DNA, and the fosmid clones were maintained in a single-copy vector in bacterial cells to minimize recombination and rearrangement between tandemly repeated DNA.
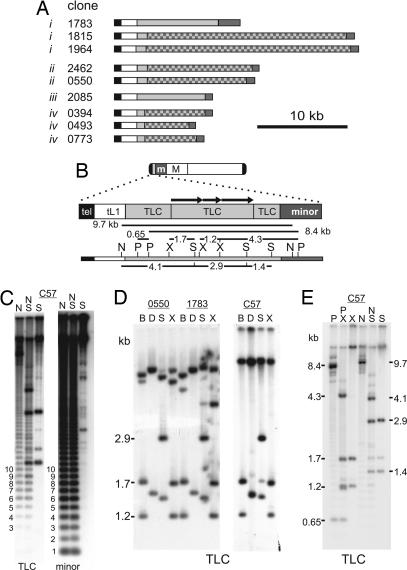
A telomere repeat (TTAGGG)10 and a mouse minor satellite monomer unit were used to blast search the end-sequence library. Twelve fosmid clones were identified with both telomere and minor satellite hits, suggesting that the telomere and centromere were within 40 kb (Fig. 1A). We sequenced the ends of these fosmid clones to confirm their identity (Table 1, which is published as supporting information on the PNAS web site). Three clones, #0773, #1977, and #1022, contained the same insert, because they displayed identical start positions for each end; therefore, only clone #0773 is shown in Fig. 1A. Of the 12 fosmid clones, one was found to have no telomere sequence and was eliminated from this study. For the remaining clones, the TTAGGG repeat was conserved and <500 bp in length.
Fig. 1.
Sequence structure of the telocentric regions of mouse chromosomes. (A) List of fosmid clones identified with (TTAGGG)n (black) and minor satellite (dark gray) repeats on either end. All regions were sequenced apart from the checkered boxes. The white and light-gray boxes define a truncated LINE-1 (tL1) and a telocentric tandem repeat (TLC), respectively. i–iv denote four possible groupings of chromosomal loci represented by the different fosmid clones. (B) Consensus domain map of the telocentric region of mouse chromosomes. Shown on top is a typical mouse telocentric chromosome showing the positions of centromeric minor satellite and pericentric major satellite DNAs, m and M, respectively. Below is shown the restriction map of clone #1783 for enzymes used in C–E; BspHI and DraI are not shown. The map corresponds with that obtained by using genomic DNA digestion (see below) and therefore represents a consensus mouse telocentric map. Arrows in the telocentric region denote internal duplications. (C) Duplicate C57 genomic DNA blots probed with TLC or minor satellite under low-stringency conditions. For both probes, a laddering pattern of 120-bp periodicity that corresponds to minor satellite is seen with NdeI (N) and/or SpeI (S) or PstI (not shown). (D) Fosmid clones #0550 and #1783 and C57BL/6 (C57) genomic DNA were digested with BspHI (B), DraI (D), SpeI (S), and XbaI (X) and hybridized with the TLC probe (1783-44). Internal restriction fragments within the TLC array ranged in size from 1.2 to 2.9 kb and were observed for both fosmid and genomic DNA. (E) Genomic C57 DNA probed with TLC revealed an 8.4-kb PstI (P) fragment bridging the TLC and minor satellite arrays, whereas a 9.7-kb NdeI (N) fragment links up the tL1, TLC, and minor satellite repeats (refer to restriction map in B). Other restriction enzymes used were XbaI (X) and SpeI (S).
A Highly Conserved Truncated Long Interspersed Nucleotide Element 1 (tL1) Element Is Present in Every Fosmid Clone.
Fosmid end-sequencing indicated that for each of the clones, the telomeric repeat sequence led directly into a 1,780-bp 5′-tL1 retrotransposable DNA element. We used primer walking to sequence through the tL1 in each clone. This initial characterization indicated that the clones could be divided into two groups, one showing a telomere→tL1→minor satellite order, whereas the other a telomere→tL1→novel tandem repeat (see below) →minor satellite order (Fig. 1A).
Multiple sequence alignments of the tL1 repeats from the different fosmid clones showed an extremely high degree of conservation across all of the clones (Fig. 6, which is published as supporting information on the PNAS web site). Across the 1,780-bp sequence, there were only up to four base substitutions between the clones, representing a sequence identity of 99.8–100%. Sequence comparison with full-length mouse L1 repeats showed that tL1 belonged to the L1Md-A2 family and involved a 5′ truncation through ORF2. The remaining 1,104 bp of ORF2 contained no stop codons, suggesting a recent insertion event. Interestingly, when the mouse genome was searched with the tL1 sequence, a high level of homology of 98.5–98.9% between some genomic L1s was seen. The top 12 matching L1 sequences were present at different chromosome locations, with 10 of the 12 sequences being full length, further suggesting that the tL1 originated from a relatively young member of the L1 family.
A Newly Discovered Repeat (TLC) Is Present Between the Telomere and Centromere.
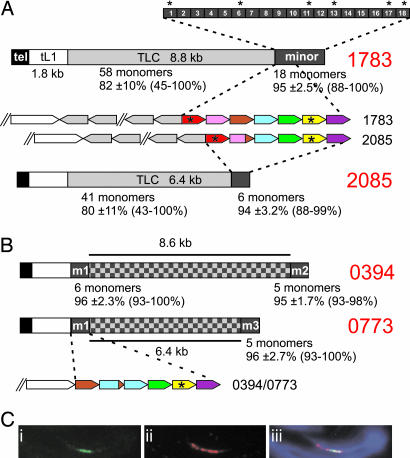
Sequencing between the tL1 and the minor satellite array revealed a tandem repeat with a monomer unit of 146 bp in some of the fosmid clones. This repeat, which we have called TLC, is found in three of the four possible groupings of chromosomal loci represented by the different telomere-minor satellite-containing fosmids (Fig. 1A). Complete sequence analysis of a full-length 8.8-kb TLC array was carried out by using clone #1783. A consensus restriction site BfaI, present in >50% of monomers, was used as base 1 in the TLC-monomer analysis. Of the 58 monomers within this clone, 29 were 146 bp in length. Pair-wise comparisons of each monomer combination showed a mean identity of 82 ± 10% with a range between 45% and 100%. The entire 8.8-kb TLC array in clone #1783 displayed a region of ≈4.7 kb that showed internal duplication of 1.0- and 1.8-kb fragments (Fig. 1B). In contrast, the flanking sequences were predominantly made up of monomeric units of the TLC repeat.
Sequence comparison between TLC and minor satellite showed that the two sequences shared homology ranging in identity between 74% and 77% across the TLC array. The base composition revealed that the TLC repeat is 63–65% AT-rich, which is similar to that of minor satellite DNA (67% AT). In addition, the sequence displayed either a thymidine- or adenosine-rich strand, as observed in minor satellite. TLC and minor satellite divergence was evident from the absence of the centromere protein (CENP)-B box motif that is well conserved in the minor satellite sequences. Furthermore, TLC lacked crosshybridization with minor satellite under moderate stringency conditions (see below); however, crosshybridization occurred when the Southern blots were probed under low stringency conditions (Fig. 1C). Interestingly, the TLC and minor satellite repeat clusters were positioned in opposite orientations (Fig. 2A).
Fig. 2.
Minor satellite monomers show a high level of identity, are rich in CENP-B box motifs, and bind CENP-A. (A) Average identities of pair-wise analyses are shown for each repeat cluster at junction and clone ends, including standard deviation and range. Fosmid clones #1783 and #2085 (derived from different chromosomes) both contain TLC repeats. TTAGGG repeats are shown in black, tL1 in white, TLC repeat in light gray, and minor satellite in dark gray (checkered block has not been sequenced). Pair-wise analysis was performed on a minor satellite array of 2.2 kb (18 monomers) of clone #1783. The first two monomers bordering the TLC-minor junction showed a slightly decreased level of identity with a mean of 92% and 94%, respectively, when compared with the other monomers. Monomer units 3 to 18 have a mean identity of 96%. Rectangular boxes represent the 120-bp monomer unit numbered 1–18 with the monomers containing the conserved CENP-B box motif indicated by asterisks. (B) Fosmid clones #0394 and #0773 (derived from the same chromosome) do not contain any TLC repeat. Cross comparisons of tL1-adjacent and clone-end minor satellite clusters show an average identity of 96 ± 2.1% and 97 ± 2.4% for clones #0394 and #0773, respectively. Minor satellite monomer analysis among clones #1783, #2085, and #0773 at the TLC or tL1 borders showed a high level of identity, where same-colored boxes represent >99% identity. (C) CENP-A occupies a subset of the minor satellite DNA. Chromatin fibers from mouse C57BL/6 MEFs were prepared by mechanical stretching and stained with (i) anti-CENP-A antibody (green), followed by FISH hybridization with (ii) minor satellite DNA (red), and (iii) merged images with DAPI staining (blue).
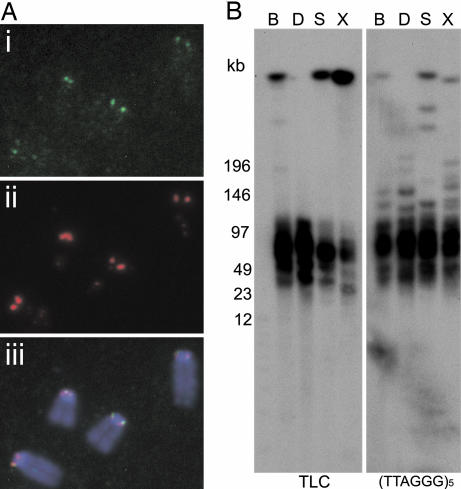
To confirm that the TLC repeat was specific to the telocentric ends of the mouse chromosomes, we performed FISH on C57BL/6 cells using a 3.5-kb (subclone #1783-44) TLC probe under noncrosshybridizing conditions for minor satellite. Positive signals that colocalized with those for the minor satellite were observed on most metaphase chromosomes (Fig. 3A). Individual chromosomes were subsequently identified with chromosome-specific paints. The results demonstrated the presence of the TLC repeat on all of the chromosomes except for the Y chromosome, and barely detectable signals on chromosomes 13 and 18 (data not shown).
Fig. 3.
Juxtapositioning of TLC and telomere repeats. (A) The TLC repeat (i; green) colocalizes with the minor satellite (ii, red) at the p-tel ends of most C57BL/6 mouse chromosomes (stained with DAPI; blue). (iii) Merged images. (B) PFGE analysis of genomic C57BL/6 DNA probed with the TLC and (TTAGGG)5 repeat probes. Cohybridizing fragments ranged in size from 30 to 80 kb for BspHI (B), DraI (D), SpeI (S), and XbaI (X).
Genomic Verification of Fosmid Clones.
Sequencing of ≈3 kb of the telomeric ends of the different fosmid clones across the (TTAGGG)n, tL1, and various lengths of TLC and minor satellite suggested that the clones represented at least four different chromosome ends (Fig. 1A). To confirm that the observed base changes were representative of the mouse chromosomes, we performed PCR and sequenced the products across the tel-tL1 junction of separate monochromosomal hybrids containing mouse chromosome 5, 6, 10, 12, or 14 (9). The PCR region displayed polymorphisms that assisted in the classification of the fosmid clones and their genomic sequences (Fig. 6). Cloned tel-tL1 PCR products from chromosome 5 were found to be identical to fosmid clone #2085, and products from chromosomes 10, 12, and 14 were identical to the #1783, #1815, and #1964 group of fosmids. Interestingly, the sequenced PCR product from chromosome 6 contained one base change and did not have an identical match to any of the fosmid clones. To ascertain whether the PCR products were nonspecifically amplifying nontelomeric L1 sequences, we searched the genome database and found only one unplaced sequence contig with telomere and L1 DNA, with the next highest hit containing six base changes within the 571 bp of L1 sequence for chromosome 5. These changes were never found in the tel-tL1 PCR products.
Further verification was carried out by PCR amplification across the other repeat junctions (tL1-TLC; tL1-minor satellite; and TLC-minor satellite) using C57BL/6 and monochromosomal hybrid chromosome 5. Sequences from these products corresponded to at least one fosmid clone. In addition, junction sequences were also used in database searches of the C57BL/6 mouse genome (Table 2, which is published as supporting information on the PNAS web site). Each junction sequence showed 100% identity to small unplaced contigs that were not long enough to cover the entire region between telomere and minor satellite DNA.
Next, we used Southern blot analysis of C57BL/6 genomic and fosmid clone DNA to determine and compare the genomic organization of the p-tel domain. Using the 3.5-kb TLC subclone (#1783-44) as a probe under moderate hybridization stringency, we observed the predicted internal restriction fragments of the TLC array (Fig. 1D). Further Southern blot analysis enabled the linking of the TLC array to adjacent repeats (Fig. 1E), as predicted by the restriction map derived from the sequencing of clone #1783 (Fig. 1B).
Terminal restriction fragment analysis was used to physically link the telomere with the TLC array. Restriction enzymes BspHI, DraI, SpeI, and XbaI were chosen because they did not cut within the tL1 repeat. Cohybridizing fragments were observed for both TLC and (TTAGGG)5 probes, ranging in size from 30 to 80 kb (Fig. 3B).
Mouse Minor Satellite Monomers Are Highly Homogeneous Right Up to the TLC or tL1 Junction.
clustalw sequence analysis of 18 minor satellite monomers next to the TLC-minor satellite junction of clone #1783 showed a mean identity of 95 ± 2.5%, with a range of 88–100% (Fig. 2A). The minor satellite monomer identity did not greatly diverge as the TLC junction was approached, with only the first two TLC-proximal minor satellite monomers displaying a relatively small (mean 92% and 94%, respectively) decrease in identity when compared with the other minor monomers. A high level of minor satellite identity was also observed at the TLC junction of clone #2085 (Fig. 2A). In addition, we found a high level of sequence conservation between the two blocks of seven or six TLC-proximal minor satellite monomers for fosmid clones #1783 and #2085 (Fig. 2A).
Minor satellite DNA binds the conserved CENP-B by means of a 17-bp sequence motif known as the CENP-B box. This motif is commonly found in centromeric DNA of many mammalian species and is required for de novo centromere assembly (10). We examined the distribution of the CENP-B boxes within the minor satellite array next to the TLC repeats of clone #1783. Of the 18 minor satellite monomers examined, six contained the consensus CENP-B box (Fig. 2A).
We also examined the mouse minor satellite monomers of the non-TLC-containing fosmid clones. Two clones, #0394 and #0773, derived from the same chromosomal locus were used (Fig. 1A). We sequenced blocks of five or six contiguous minor satellite monomers adjacent to the tL1 repeat (m1, Fig. 2B) and at the minor satellite end of the fosmid clones (m2 or m3). The level of monomer identity in pairwise analysis revealed an average value of 96 ± 2.3%, 95 ± 1.7%, and 96 ± 2.7% for blocks, m1, m2, and m3, respectively. The average identity was additionally examined between tL1-adjacent block (m1) and fosmid-end blocks (m2 and m3) and gave an average identity of 96 ± 2.1% and 97 ± 2.4% for m1/m2 and m1/m3, respectively. These results suggested there is not a significant drop-off in sequence identity as the minor satellite arrays approach the TLC or the tL1 junction. As with the TLC-containing fosmids, CENP-B boxes were also observed in the minor satellite of non-TLC fosmids.
DNA Methylation Extends into the TLC Array.
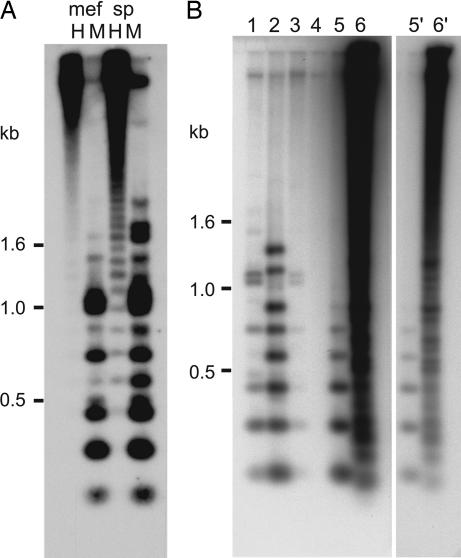
Mouse pericentric DNA is characterized by the methylation of cytosines and a transcriptionally repressed state (11, 12). To examine the methylation status of the TLC repeats, we digested genomic DNA from C57BL/6 spleen and mouse embryonic fibroblast (MEF) cells with MspI and HpaII that cut on average every second monomer. Both DNAs exhibited marked methylation as shown by an increase in fragment sizes in the HpaII digests (Fig. 4A). Interestingly, spleen genomic DNA showed slightly less methylation when compared with MEF DNA.
Fig. 4.
TLC repeats are methylated and found in closely related Mus species. (A) HpaII–MspI digestion of genomic C57BL/6 DNA from MEF and spleen showed a strong 0.15-kb TLC laddering in the MspI digest from DNA of both tissues and a weaker laddering signal in the HpaII digest of the spleen DNA. (B) Cross-species conservation of the TLC repeat in the Mus genus. Five micrograms of genomic DNA from each strain or species were digested with MspI. Lanes 1–6 represent C57BL/6, M. musculus castaneus, M. musculus/domesticus, M. pahari, M. spretus, and M. caroli. A shorter exposure for lanes 5 and 6 is shown (5′ and 6′).
Conservation of TLC in Other Mouse Species.
Centromeric DNA is often characterized by its high degree of sequence divergence between species. To determine whether the TLC repeat was conserved in closely related mouse species, we digested equal amounts of genomic DNA with MspI and probed the Southern blot with TLC (Fig. 4B). Varying intensities of a typical laddering pattern of 0.15 kb corresponding to that of TLC repeat arrays was observed for C57BL/6, Mus musculus castaneus, Mus musculus/domesticus, and Mus spretus. No bands were detected with Mus pahari, whereas Mus caroli showed a very strong hybridization signal with an irregular laddering pattern.
Discussion
The Mouse Proximal Telomere Is Truly Telocentric.
We show that the telomere and centromere sequences of the different mouse chromosome ends studied are separated by a distance ranging from 1.8 to 11 kb. We demonstrate that one or two types of sequences separate the telomere and centromere: a tL1 sequence found on all fosmid clones, and a 146-bp TLC tandem repeat found on most fosmids. One group of fosmids (all derived from the same chromosomal locus) contained only the 1.8-kb tL1 but no TLC repeat, bringing the centromere and telomere to within 1.8 kb of each other.
To demonstrate the telocentric location of the TLC repeats, we used FISH on metaphase chromosomes. Within the limits of detection, all of the mouse chromosomes, apart from chromosomes 13, 18, and Y, show the TLC repeats at the telocentric end. PCR amplification and Southern blot analyses of genomic DNA further confirm that the fosmid clones are stable and representative of the genome. To verify that the TLC repeat is present at the ends of the chromosomes and not at interstitial sites, we performed terminal restriction fragment analysis with TLC and (TTAGGG)5 repeat probes. Comigrating fragments with sizes ranging from 30 to 80 kb are observed by using four different restriction enzymes. The sizes of the presumed terminal restriction fragments are within the mouse telomere size range from previous PFGE (13) and Q-FISH studies (14) using TTAGGG probes. Additional evidence supporting the fosmids being representative of the mouse telomeric ends comes from the fact that the telomeric hexamers are in correct orientation with respect to the ends of the chromosomes, and that the sequenced hexameric repeats show no significant degeneration, as would be expected if the repeats resided at interstitial genomic sites.
Extensive Sequence Homogenization Between Nonhomologous Chromosomes Extends from the Telomere to the Centromere.
Tandemly repeated sequences at the centromeric and pericentromeric regions are known to undergo homogenization via intra- and interchromosomal crossing-over events (15, 16). Similarly, the subtelomeric ends of metacentric chromosomes contain tracts of tandem repeat DNA that rapidly exchange between chromosomes (17). Acrocentric and telocentric chromosomes are unique in structure, because they contain two distinct chromosomal loci in close proximity. We now present DNA sequence information that bridges the gap between these two structures on the mouse telocentric chromosomes. We demonstrate that the DNA from the p-tel region through to minor satellite is in the same orientation and has a very high level of homology (>99% across blocks of DNA) from the different chromosome ends (Fig. 2 and Figs. 6 and 7, which are published as supporting information on the PNAS web site). This level of sequence conservation indicates a high prevalence of exchanges between nonhomologues leading to extensive sequence homogenization. A previous study has also demonstrated that the monomers of the pericentric mouse major satellite are highly homogeneous (18), suggesting that homogenization extends into the pericentric satellite domain. It is possible that such frequent interchromosomal exchanges of the telocentric regions is facilitated by the close association of the mouse telomeres observed during meiosis (19).
Unusually High Level of Minor Satellite Sequence Homogeneity at the Array Periphery.
In humans, centromeric α-satellite monomer sequence identity varies between 70% and 100% (20). These monomers are often arranged into higher-order repeat units that show a significantly greater degree of sequence identity. The α-satellite monomers in these higher-order units commonly contain the conserved CENP-B-binding motif, CENP-B box (21). As the α-satellite boundary is approached, the level of sequence identity decreases. An example of a well-studied α-satellite junction region is found in Xp11 (22). Here, the level of α-satellite identity drops off from 98–99% down to 70%, together with the frequency of CENP-B boxes (23).
A previous study using stretched fiber immuno-FISH analysis on a minichromosome in different somatic cell backgrounds showed that the minor satellite DNA partially colocalized with the kinetochore protein CENP-A (24). Here, we have performed immunostretched fiber FISH on C57BL/6 cells and shown that CENP-A occupies over half of the minor satellite domain (Fig. 2C), providing direct evidence that minor satellite is the primary site for nucleating kinetochore assembly. Furthermore, we demonstrate that the minor satellite array is orientated in the same direction on all of the fosmid clones, confirming a similar finding of a previous report based on the use of chromosome orientation FISH (25). We have shown that, unlike human α-satellite, mouse minor satellite monomers at the boundary of TLC or tL1 show a high level of sequence homology (mean identity of 95–96% between monomers) and a high prevalence of CENP-B boxes. Such a high level of minor satellite identity extending across the minor satellite array into the periphery is consistent with a mechanism of rapid interchromosomal sequence homogenization across the entire telocentric region.
Spacer Sequences Between the Telomere and Centromere.
The DNA adjacent to the telomeric ends of human chromosomes comprises small 0.5- to 2.4-kb stretches of tandemly repeated DNA (17). In the mouse genome, only 11q and Xq telomeric ends have been completely sequenced, where the adjacent sequence is 200–300 bp of DNA common to both chromosomes followed by B1 and LTR repeats. Our study has shown that the p-tel ends of the mouse telocentric chromosomes contain a common partial L1 (tL1) repeat that is next to TLC or minor satellite tandem repeats.
L1 repeats have been identified at the pericentric regions of human chromosomes. The most detailed example comes from the study of the human Xp, where an age-dependent gradient of L1 repeats has been observed, with evolutionary age decreasing as the higher-order α-satellite repeat array is approached (22, 23). We have shown the presence of a tL1 repeat next to the TTAGGG repeat in at least four of the mouse p-tel ends. The presence of this tL1 may be explained by an initial transposition event to a single subtelomeric chromosome site that then rapidly spreads to other chromosomes by means of interchromosomal translocations. It is interesting that the junction between the TTAGGG repeat and tL1 contains an overlapping AACCC sequence (see Fig. 6). This sequence may have directed the truncation of a full-length L1 by an unequal crossing-over event. At present, it is unclear what function, if any, the tL1 sequence might serve. It is possible that the presence of this sequence serves to demarcate the telomeric and centric chromatin. Further studies, such as involving the identification of chromatin boundary protein elements, will be required to address this possibility.
We have shown that the TLC repeat is located at the telocentric ends of most C57BL/6 mouse chromosomes. The average sequence identity between monomers across a TLC array is ≈82%, which is considerably lower than those seen between the minor or major satellite monomers (95% and 96%, respectively; see ref. 18 and the present study). Sequence comparison between minor satellite and TLC repeats suggests that the two repeats are distantly related, raising the possibility that the TLC repeat might have been derived from minor satellite or a common progenitor sequence. It is unclear why the TLC monomers are less homogeneous compared to those of the minor (or major) satellite. Perhaps, like the tL1 sequence, the TLC domain may merely serve a spacer role between the centromere and telomere regions, in which case the conservation of a strict sequence identity may be less crucial. The identification of at least a small number of fosmids/chromosomes that lack this domain further argues against an essential role of the TLC domain.
Cross-species analysis within the Mus genus indicates that the TLC repeat is present in closely related subspecies and in more distantly related species such as M. spretus. However, this repeat appears absent in M. pahari, which last shared a common ancestor with M. musculus around 6 million years (Myr) ago. It is interesting that M. caroli diverged around 4 Myr ago but shows a very strong hybridization signal, albeit with a different periodicity that may reflect crosshybridization with the predominant 60- and 79-bp centromeric repeat monomers of M. caroli. The M. caroli repeat monomers show an identity ranging from 86% to 92% across 38- to 50-bp regions against the TLC repeat (26).
Telocentric Karyotype Evolution and Robertsonian (Rob) Translocations.
Telocentric chromosomes are not uncommon in mammalian species and are found, for example, in mouse, dog, cattle, sheep, and goats (27). The prevalence of telocentric chromosomes in the Mus genus suggests that this karyotype is relatively stable through evolution. Here, we provide evidence that the various DNA sequences of the telocentric domain exhibit the same polarity that extends from the minor satellite array to the p-tel end. This identical polarity should greatly favor the exchange of the telocentric ends (but not the occurrence of Rob translocations; see below) among nonhomologous chromosomes, thereby providing a mechanism to maintain the stability of the telocentric karyotype (Fig. 5A).
Fig. 5.
Possible mechanisms for interchromosomal exchanges between telocentric chromosomes. (A) Exchange of telocentric arms between nonhomologous chromosomes anywhere (dashed crosses) along the common unidirectional telocentric DNA drives p-tel sequence homogenization and maintains stability of the telocentric karyotype. (B) Occasional mispairing and exchange between sequence-related but oppositely oriented TLC and minor satellite repeat arrays result in the generation of a Rob chromosome and an acentric fragment that is subsequently lost.
The mouse telocentric structure also allows a possible mechanism for the occasional Rob translocation events to take place. This mechanism may involve the mispairing between the sequence-related TLC and minor satellite repeat arrays. Because the arrays for these two repeats are orientated in opposite directions, aberrant recombination between them will give rise to Rob translocations (Fig. 5B). The resulting Rob chromosomes can retain considerable amounts of minor satellite DNA to preserve centromere function. Previous studies have shown that minor satellite is present in varying amounts on Rob chromosomes. This suggests that different exchange sites within the minor satellite array and/or the subsequent loss of minor satellite sequences are possible through processes such as unequal crossing over or looping out unstable inverted minor satellite sequences generated from the translocation events (25, 28–30). Rob translocations constitute the most common chromosomal rearrangement observed in humans. Similar molecular mechanisms have been proposed for the derivation of such translocation events (31). Further studies will be required to determine whether the TLC repeats indeed provide a molecular mechanism for mouse Rob translocation events, and whether possible lessons learned from such mouse studies will assist in the delineation of the human Rob mechanisms.
Materials and Methods
Mouse Fosmid Clones and Subclones.
The female C57BL/6 sheared-insert fosmid library was previously constructed by Kerstin Lindblad-Toh and colleagues, Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, MA. This library was deposited at the BACPAC Resource Center, Children's Hospital Oakland Research Institute, from which replicate fosmid clones were obtained for this study. To facilitate the sequencing of the repetitive fosmid inserts, DNA was randomly sheared using sonication and separated on an agarose gel to excise fragments between 3 and 5 kb. The fragments were purified by using Gelase (Epicentre Technologies, Madison, WI), and the ragged ends were repaired with T4 DNA polymerase and T4 polynucleotide kinase (NEB) and blunt-end cloned into plasmid vector pAlter-1 (Promega). Insert sizes of the subclones were then determined, and paired-sequence reads assisted in the assembly and closure of contigs. Additional sequencing and PCR analyses are in Supporting Text, which is published as supporting information on the PNAS web site.
FISH and Immunocytochemistry.
FISH was performed on C57BL/6 MEF by using standard methods. A 3.5-kb TLC DNA probe (#1783-44) was subcloned from fosmid clone #1783. The minor satellite probe was used from a previous study (32). For medium stringency hybridization and washing, probes were hybridized in 30% formamide/2 × SSC, 37°C, and washed at 1 × SSC, 50°C. For high stringency, hybridization was performed in 50% formamide, 2 × SSC, 37°C, and washing at 60°C, 0.1 × SSC was used. Medium stringency was used for the TLC FISH followed by the Rainbow FISH. Images of metaphase spreads were captured and coordinates recorded by using an England Finder graticule (SPI, West Chester, PA). Individual mouse chromosomes were then identified with a second round of FISH by using Rainbow Star FISH (Cambio, Cambridge, U.K.) per the manufacturer's instructions. Stretched chromatin fiber FISH was performed as described (33). Images were captured by using an Axioplan2 microscope (Zeiss), with a Sensys-cooled charge-coupled device camera (Photometrics, Tucson, AZ) and processed with ip lab software (Scanalytics, Billerica, MA).
Cell Lines and DNA.
MEF cell lines were derived from pooled day 12.5 male and female C57BL/6 mouse embryos. A monochromosomal somatic cell hybrid containing a mouse chromosome 5 in a human background was generated for this study through microcell-mediated fusion of a mouse embryonic stem (ES) cell line containing a targeted CENP-A-GFP fusion product and neomycin resistance gene (32) into the human fibrosarcoma cell line HT1080, using a previously described method (34). Genomic DNA was extracted from the spleen of a 4.5-mo-old C57BL/6 female or pooled day-12.5 embryos for liquid and PFGE blocks using standard molecular methods. PFGE gels were run under the following conditions: 0.9% agarose/6 V/cm, 14°C/0.5 × TBE, pulsed time 2–30 sec, run time 22 h, using a Bio-Rad Chef Mapper. Mouse genomic DNA from different species and strains were obtained from The Jackson Laboratory. Southern blots using the TLC probe were hybridized at 55°C or 60°C in Church buffer and washed in 2 × SSC/0.1% SDS, 55°C or 60°C. The (TTAGGG)5 telomere probe was end-labeled, and blots were hybridized at 50°C in Church buffer and washed in 2 × SSC/0.1% SDS, 50°C.
Supplementary Material
Acknowledgments
We thank Dr. Kerstin Lindblad-Toh (Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, MA) for the mouse fosmid clones, Prof. Phil Avner for mouse monochromosomal hybrid DNAs, and Dr. Tim Littlejohn for assistance with bioinformatics analyses. K.H.A.C. is a Senior Principal Research Fellow of National Health and the Medical Research Council.
Abbreviations
- MEF
mouse embryonic fibroblast
- PFGE
pulsed-field gel electrophoresis
- tL1
truncated long interspersed nucleotide element 1
- CENP
centromere protein
- Rob
Robertsonian
- p-tel
p-telomeric.
Footnotes
References
- 1.Chan S. R., Blackburn E. H. Philos. Trans. R. Soc. London B. 2004;359:109–121. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleveland D. W., Mao Y., Sullivan K. F. Cell. 2003;112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 3.Amor D. J., Kalitsis P., Sumer H., Choo K. H. A. Trends Cell Biol. 2004;14:359–368. doi: 10.1016/j.tcb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Amor D. J., Choo K. H. A. Am. J. Hum. Genet. 2002;71:695–714. doi: 10.1086/342730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kipling D., Ackford H. E., Taylor B. A., Cooke H. J. Genomics. 1991;11:235–241. doi: 10.1016/0888-7543(91)90128-2. [DOI] [PubMed] [Google Scholar]
- 6.Narayanswami S., Doggett N. A., Clark L. M., Hildebrand C. E., Weier H. U., Hamkalo B. A. Mamm. Genome. 1992;2:186–194. doi: 10.1007/BF00302876. [DOI] [PubMed] [Google Scholar]
- 7.Garagna S., Zuccotti M., Capanna E., Redi C. A. Cytogenet. Genome Res. 2002;96:125–129. doi: 10.1159/000063028. [DOI] [PubMed] [Google Scholar]
- 8.Waterston R. H., Lindblad-Toh K., Birney E., Rogers J., Abril J. F., Agarwal P., Agarwala R., Ainscough R., Alexandersson M., An P., et al. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 9.Sabile A., Poras I., Cherif D., Goodfellow P., Avner P. Mamm. Genome. 1997;8:81–85. doi: 10.1007/s003359900362. [DOI] [PubMed] [Google Scholar]
- 10.Ohzeki J., Nakano M., Okada T., Masumoto H. J. Cell Biol. 2002;159:765–775. doi: 10.1083/jcb.200207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okano M., Bell D. W., Haber D. A., Li E. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 12.Martens J. H., O'Sullivan R. J., Braunschweig U., Opravil S., Radolf M., Steinlein P., Jenuwein T. EMBO J. 2005;24:800–812. doi: 10.1038/sj.emboj.7600545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kipling D., Cooke H. J. Nature. 1990;347:400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- 14.Zijlmans J. M., Martens U. M., Poon S. S., Raap A. K., Tanke H. J., Ward R. K., Lansdorp P. M. Proc. Natl. Acad. Sci. USA. 1997;94:7423–7428. doi: 10.1073/pnas.94.14.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith G. P. Science. 1976;191:528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- 16.Dover G. Nature. 1982;299:111–117. doi: 10.1038/299111a0. [DOI] [PubMed] [Google Scholar]
- 17.Linardopoulou E. V., Williams E. M., Fan Y., Friedman C., Young J. M., Trask B. J. Nature. 2005;437:94–100. doi: 10.1038/nature04029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vissel B., Choo K. H. Genomics. 1989;5:407–414. doi: 10.1016/0888-7543(89)90003-7. [DOI] [PubMed] [Google Scholar]
- 19.Bass H. W. Cell Mol. Life Sci. 2003;60:2319–2324. doi: 10.1007/s00018-003-3312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudd M. K., Willard H. F. Trends Genet. 2004;20:529–533. doi: 10.1016/j.tig.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Ikeno M., Masumoto H., Okazaki T. Hum. Mol. Genet. 1994;3:1245–1257. doi: 10.1093/hmg/3.8.1245. [DOI] [PubMed] [Google Scholar]
- 22.Schueler M. G., Higgins A. W., Rudd M. K., Gustashaw K., Willard H. F. Science. 2001;294:109–115. doi: 10.1126/science.1065042. [DOI] [PubMed] [Google Scholar]
- 23.Schueler M. G., Dunn J. M., Bird C. P., Ross M. T., Viggiano L., Rocchi M., Willard H. F., Green E. D. Proc. Natl. Acad. Sci. USA. 2005;102:10563–10568. doi: 10.1073/pnas.0503346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng K., de las Heras J. I., Ross A., Yang J., Cooke H., Shen M. H. Chromosoma. 2004;113:84–91. doi: 10.1007/s00412-004-0299-z. [DOI] [PubMed] [Google Scholar]
- 25.Garagna S., Marziliano N., Zuccotti M., Searle J. B., Capanna E., Redi C. A. Proc. Natl. Acad. Sci. USA. 2001;98:171–175. doi: 10.1073/pnas.98.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kipling D., Mitchell A. R., Masumoto H., Wilson H. E., Nicol L., Cooke H. J. Mol. Cell. Biol. 1995;15:4009–4020. doi: 10.1128/mcb.15.8.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardo-Manuel de Villena F., Sapienza C. Genetics. 2001;159:1179–1189. doi: 10.1093/genetics/159.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garagna S., Redi C. A., Capanna E., Andayani N., Alfano R. M., Doi P., Viale G. Cytogenet. Cell Genet. 1993;64:247–255. doi: 10.1159/000133587. [DOI] [PubMed] [Google Scholar]
- 29.Garagna S., Broccoli D., Redi C. A., Searle J. B., Cooke H. J., Capanna E. Chromosoma. 1995;103:685–692. doi: 10.1007/BF00344229. [DOI] [PubMed] [Google Scholar]
- 30.Nanda I., Schneider-Rasp S., Winking H., Schmid M. Chromosome Res. 1995;3:399–409. doi: 10.1007/BF00713889. [DOI] [PubMed] [Google Scholar]
- 31.Choo K. H. Mol. Biol. Med. 1990;7:437–449. [PubMed] [Google Scholar]
- 32.Kalitsis P., Fowler K. J., Earle E., Griffiths B., Howman E., Newson A. J., Choo K. H. A. Chromosome Res. 2003;11:345–357. doi: 10.1023/a:1024044008009. [DOI] [PubMed] [Google Scholar]
- 33.Blower M. D., Sullivan B. A., Karpen G. H. Dev. Cell. 2002;2:319–330. doi: 10.1016/s1534-5807(02)00135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saffery R., Wong L. H., Irvine D. V., Bateman M. A., Griffiths B., Cutts S. M., Cancilla M. R., Cendron A. C., Stafford A. J., Choo K. H. A. Proc. Natl. Acad. Sci. USA. 2001;98:5705–5710. doi: 10.1073/pnas.091468498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.