Abstract
Although current demands for therapeutic mAbs are growing quickly, production methods to date, including in vitro mammalian tissue culture and transgenic animals, provide only limited quantities at high cost. Several tumor-associated antigens in tumor cells have been identified as targets for therapeutic mAbs. Here we describe the production of mAb BR55-2 (IgG2a) in transgenic plants that recognizes the nonprotein tumor-associated antigen Lewis Y oligosaccharide overexpressed in human carcinomas, particularly breast and colorectal cancers. Heavy and light chains of mAb BR55-2 were expressed separately and assembled in plant cells of low-alkaloid tobacco transgenic plants (Nicotiana tabacum cv. LAMD609). Expression levels of plant-derived mAb (mAbP) were high (30 mg/kg of fresh leaves) in T1 generation plants. Like the mammalian-derived mAbM, the plant mAbP bound specifically to both SK-BR3 breast cancer cells and SW948 colorectal cancer cells. The Fc domain of both mAbP and mAbM showed the similar binding to FcγRI receptor (CD64). Comparable levels of cytotoxicity against SK-BR3 cells were also shown for both mAbs in antibody-dependent cell-mediated cytotoxicity assay. Furthermore, plant-derived BR55-2 efficiently inhibited SW948 tumor growth xenografted in nude mice. Altogether, these findings suggest that mAbP originating from low-alkaloid tobacco exhibit biological activities suitable for efficient immunotherapy.
Keywords: breast and colorectal cancer, plant biotechnology, transgenic low-alkaloid tobacco, tumor growth inhibition
Although current demands for therapeutic mAbs are growing quickly, production methods to date, including in vitro mammalian tissue culture and transgenic animals, provide only limited quantities at high cost. Other available systems, such as bacterial and yeast, do not provide specific machinery for protein posttranslational modifications required for an active or partially active mAb.
The use of mAbs in diagnosis and treatment of various carcinomas has increased in recent years. mAbs against tumor-associated antigens have proven effective in cancer treatment, especially in conjunction with classical chemotherapy and radiotherapy (1, 2). By binding to antigen expressed on the surface of cancer cells, mAbs trigger antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity, which kills abnormal cells (3–5). ADCC requires the presence of tumor cells overexpressing the tumor-associated antigen, efficient binding of the mAb to this antigen, and effector cells, e.g., macrophages that recognize mAbs through their Fc receptors. mAb BR55-2 recognizes the Lewis Y oligosaccharide antigen (LeY), which is overexpressed predominantly on breast, lung, ovary, and colon cancers (6–8). Murine mAb BR55-2 (IgG2a) inhibits tumor growth and kills human cancer cells xenotransplanted in nude mice (9). Under physiological conditions, LeY is expressed predominantly during embryogenesis but is restricted to granulocytes and epithelial surfaces in adult tissue (10).
Recently plants have become a prospective replacement bioreactor for currently available production systems to manufacture biopharmaceuticals (11, 12). Moreover, plants offer several advantages as a mAb production system, such as the lack of human pathogens, relatively low-cost manufacturing, and ease of production scale-up. Our group has recently shown that recombinant mAb can be safely purified from tobacco plants (13).
Previously we successfully expressed the human rabies virus-neutralizing mAb SO57 (14) and the murine anticancer mAb C017-1A (15) in planta. Both plant-derived mAbs (mAbP) showed excellent in vivo activity similar to that of the parental mAb produced in the mammalian system. However, whereas efficacy of the virus-neutralizing mAb SO57 depends mainly on its activity in binding to virus antigens (16), mAbs for use in cancer immunotherapy require both tumor-associated antigen binding activity and interaction with Fc receptors to exert ADCC effector functions.
Here we report the successful expression and assembly of functional LeY oligosaccharide-specific mAb BR55-2 in transgenic tobacco plants with low alkaloid content (LAMD609). The mAb fusion to the KDEL signal sequence helped to retain the protein inside the endoplasmic reticulum (ER), thus enhancing mAb assembly in plant cells (17). Consequently, it helped to increase the final mAb yields from the plant production system. No significant differences in biological activities suitable for efficient immunotherapy were observed between the mAbP and the mAb BR55-2 obtained from the mammalian system (mAbM). Our results clearly indicate that plants can be used as an excellent source of fully active mAbs.
Results
Generation of Transgenic Plants Expressing Lewis Y-Specific mAb BR55-2.
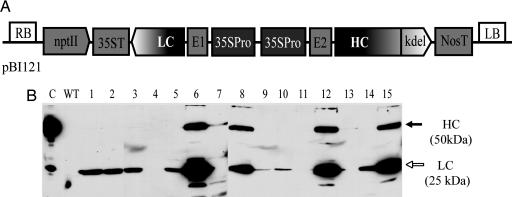
cDNA of heavy chain (HC) γ and light chain (LC) χ of mAb BR55-2 (9) were cloned from the hybridoma-producing murine IgG2a LeY oligosaccharide-specific antibody and placed into the pBI121 binary vector (Clontech), yielding pRB59-2 (Fig. 1A). The direction of LC and HC expression cassettes using the cauliflower mosaic virus 35S promoter (35SPro) containing a duplicated upstream enhancer region (18) for both genes was inverted. The untranslated region from alfalfa mosaic virus RNA4 (E1) or tobacco etch virus (E2) was placed before the LC and HC coding sequences, respectively. Of 36 putative tobacco transformants regenerated on medium with kanamycin, 16 showed integration of HC and LC genes in the genomic DNA (data not shown) and the presence of both proteins in leaf extract (Fig. 1B), as confirmed by PCR and Western blot analyses. Quantitative ELISA indicated expression levels of assembled mAb at 16 and 17 mg/kg of fresh leaves in lines 8 and 6, respectively (data not shown). Line 8, which expressed among the highest detectable amounts of mAb, was self-crossed to obtain homozygous line. Expression and assembly of full-size mAb in randomly selected T1 progeny of line 8 were confirmed by Western blot and quantitative ELISA (data not shown). Average mAb expression levels in this line were similar to those observed in the parental plant (T0 generation), except for plant line 15, which expressed more (31 mg/kg) in fresh leaves.
Fig. 1.
Expression of mAb BR55-2 in T0 transgenic tobacco plants. (A) Schematic representation of pRB59-2 plant expression plasmid. The region between RB and LB (RB and LB, right and left borders of Agrobacterium Ti plasmid, respectively) was transferred into tobacco genomic DNA by A. tumefaciens. 35SPro, the cauliflower mosaic virus 35S promoter with duplicated enhancer region; E1 and E2, untranslated leader sequence of alfalfa mosaic virus RNA4 or tobacco etch virus, respectively; LC and HC, coding sequence of LC and HC of BR55-2 murine IgG2a, respectively; 35ST and NosT, terminator of cauliflower mosaic virus 35S gene and nopaline synthase, respectively; kdel, ER retention signal; nptII, nopaline synthase cassette conferring resistance to the antibiotic kanamycin. (B) Western blot of total protein extract from regenerated tobacco plants transformed with pRB59-2 plasmid (lanes 1–15). Blots were detected with anti-mouse HC+LC-specific antibodies conjugated with horseradish peroxidase. Lane C, 20 ng of mAb BR55-2 purified from hybridoma; lane WT, protein extract from nontransgenic tobacco. Filled and open arrows indicate HC and LC of mAb BR55-2, respectively.
Purification of Plant-Expressed mAb BR55-2.
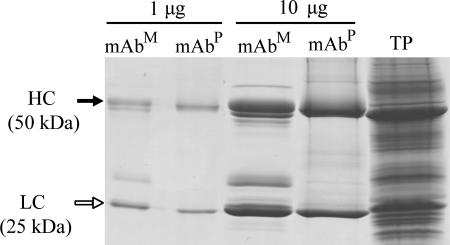
Antibody was purified from leaves harvested from 10- to 12-week-old T0 plants grown in a greenhouse. Protein A column purification yielded an average of 3 mg of mAb BR55-2 per kilogram of fresh leaves. SDS/PAGE analysis of the purified antibody revealed two major bands in the eluate (50-kDa HC and 25-kDa LC) (Fig. 2) and a few additional faint bands, which were of plant origin as confirmed by immunostaining analysis (data not shown). The purity of mAbP was at least 90%.
Fig. 2.
SDS/PAGE of the mAb BR55-2 purified from plants. Two amounts of purified mAbs (1 and 10 μg) of mAbM or mAbP were loaded on the gel and stained with Coomassie brilliant blue R250. Filled and open arrows indicate HC and LC of mAb BR55-2, respectively. TP indicates total protein extract from plant leaf.
Specific Binding of mAbP BR55-2 to LeY-Overexpressing Cancer Cells.
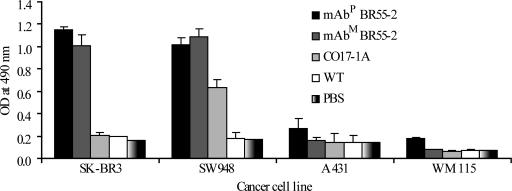
SK-BR3 human breast cancer and SW948 colorectal carcinoma cells, which both overexpress LeY, were used as target cells in an ELISA to assess the ability of mAbP BR55-2 to recognize the LeY. A431 human adenocarcinoma and WM115 human melanoma cells were used as negative controls. Like the mAbM, mAbP BR55-2 had significantly higher absorbance to SK-BR3 and SW948 cells as compared with controls (Fig. 3). Absorbance was low in all cancer cells incubated with protein extract from nontransgenic plant (WT) or with PBS. EpCAM-specific antibody CO17-1A (19) showed high absorbance only on SW948 cells (Fig. 3).
Fig. 3.
Specific binding of mAbP and mAbM BR55-2 to LeY-overexpressing cancer cells. A total of 1 μg/ml purified mAbM and mAbP BR55 was incubated on 96-well ELISA plates seeded with LeY-positive SK-BR3 and SW948 cells or LeY-negative A431 and WM115 cells (106 per well). CO17-1A, EpCAM antigen-specific mAb; WT, 1 μg of total protein extract from nontransgenic tobacco.
Interaction of mAb BR55-2 Fc with FcγRI Receptor (CD64).
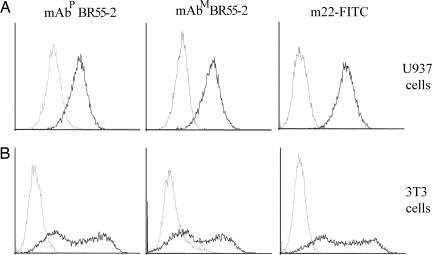
Binding of mAbP and mAbM BR55-2 to U937 cells expressing Fc receptors was analyzed by flow cytometric assay and found to be very similar for both mAbs (Fig. 4). Use of normal human serum to block the interaction between CD64 and the Fc domain of IgGs reduced the number of cells bound by both mAbP and mAbM (Fig. 4A, gray line). mAb m22-FITC bound the IFN-γ-treated U937 cells, indicating that the cells express CD64, whereas the isotype-matched negative control mAb did not (Fig. 4A Right, black and gray lines, respectively). Similar analysis using 3T3 cells transfected to express CD64 revealed comparable binding by mAbP and mAbM (Fig. 4B Left and Center, black line). The wide distribution pattern with two main peaks of bound cells was observed for both mAbP and mAbM (Fig. 4B, black lines), with the second peak for mAbP BR55-2 slightly more shifted to the right compared with mAbM BR55-2 (Fig. 4B Left and Center, black line). The two-peak pattern was similar to that of the m22-FITC mAb CD64-specific positive control (Fig. 4B Right). None of the mAbs bound to mock-transfected cells (Fig. 4B, gray line).
Fig. 4.
Flow cytometric analysis of binding activity of mAbP and mAbM BR55-2 to the FcγRI receptor. (A) U937 cells treated with IFN-γ to stimulate Fc receptor expression were incubated with mAbP or mAbM BR55-2 or CD64-specific mAb m22-FITC. Binding activity of mAbP (Left), mAbM (Center), and m22-FITC (Right) to the activated cells expressing CD64 were analyzed by flow cytometry. Black and gray lines indicate binding of mAbs before or after blocking treatment of human serum, respectively (Left and Center) or indicate cells treated with m22-FITC or an mAb isotype control (Right). (B) 3T3 cells were transfected with CD64 cDNA and treated with mAbP BR55 (Left), mAbM BR55 (Center), or mAb m22-FITC (Right). Black and gray lines indicate transfected or mock-transfected cells bound by each mAb, respectively.
In Vitro Cytotoxicity of mAbP BR55-2.
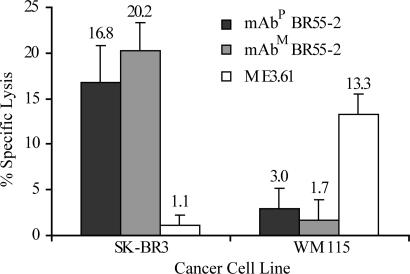
ADCC assay revealed similar cytotoxic activity of both mAbP and mAbM BR55-2 against SK-BR3 breast cancer cells (16.8 ± 4.0% and 20.2 ± 3.1% specific lysis, respectively) but no significant killing of WM115 melanoma cells (3.8 ± 2.2% and 1.7 ± 2.1% specific lysis, respectively), which do not overexpress LeY on the surface (Fig. 5). Melanoma-specific control mAb ME3.61 showed cytotoxicity against WM115 cells but not against SK-BR3 cells (13.3 ± 2.2% and 1.1 ± 1.0% specific lysis, respectively).
Fig. 5.
ADCC of SK-BR3 human breast adenocarcinoma or WM115 human melanoma cells mediated by mAbP or mAbM BR55-2. mAb ME3.61 specific for WM115 cells was used as a control. Percent specific lysis of cancer cells was calculated as described in Materials and Methods.
Suppression of Tumor Growth in Mice by mAbP BR55-2.
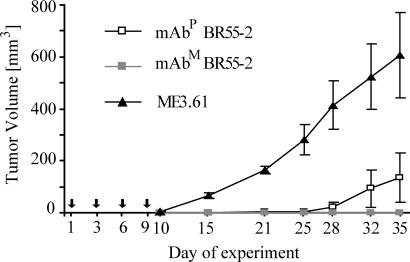
Nude mice were xenografted with SW948 colorectal cancer cells and injected with mAbM BR55-2 or mAb ME3.61 (positive and negative controls, respectively) or with mAbP BR55-2 (Fig. 6). No visible tumor growth was observed up to 25 days after injection of cancer cells in any mouse that received either mAbP or mAbM BR55-2. In mice treated with control mAb ME3.61 the first signs of tumor development appeared at 10 days after tumor cell injection and grew rapidly thereafter, with a mean of 607 mm3 by 35 days. At 28 days, a slightly higher mean tumor volume was detected in the mAbP-treated mice group compared with that of mAbM-treated mice. At 35 days, mean tumor volumes of both mAbP- and mAbM-treated mice were significantly less (P < 0.002 and P < 0.001, respectively) than that of the control group.
Fig. 6.
Tumor growth suppression in nude mice by mAbP BR55-2. BALB/c nu/nu mice were inoculated with 106 SW948 colorectal cancer cells and one dose (100 μg) of mAbP or mAbM BR55-2. The control group received melanoma-specific murine mAb ME3.61. Thereafter all mice were injected with three additional doses of each mAb every 3 days for a total mAb dose of 400 μg. Arrows indicate the day of mAb treatment. Tumor volumes were recorded at 10, 15, 21, 25, 28, 32, and 35 days after initial inoculation with cancer cells. Data are given as mean ± SD.
Discussion
In this study we explored the possibility of producing in planta a fully functional mAb that recognizes LeY present on a certain type of cancer cells. Our data clearly indicate that the plant-derived anti-LeY mAb BR55-2 (mAbP) has similar biological activities suitable for efficient cancer immunotherapy (including in vitro binding specificity of cancer cells, FcγRI receptor binding activity, ADCC activity, and in vivo efficacy in tumor inhibition) to that of the mAbM. Both genes for the HC and LC were organized in a binary plasmid as inverted expression cassettes to reduce the chance of recombination and/or silencing from the repeated promoter sequence. The mAb was expressed in tobacco (Nicotiana tabacum cv. LAMD609) with low alkaloid content to facilitate purification of the mAb. More than 50% of T0 transgenic plants contained both HC and LC genes and correspondent proteins (HC and LC) as confirmed by Western blot analysis. The expression levels of mAb in these plants reached 16–17 mg/kg in fresh leaf material. Some plants of T1 progeny produce an even higher amount, reaching up to 31 mg/kg of fresh tissue as confirmed by quantities sandwich ELISA. This is the highest expression level we have obtained in comparison to the one reported previously for antiviral (14) or colorectal cancer-specific mAbs (15.) That might be because of the use of strong 35S promoter containing a duplicated upstream enhancer region (18) to drive the expression of both HC and LC genes and/or insertion of nontranslated viral leaders (tobacco etch virus and alfalfa mosaic virus) in front of the corresponding coding sequences. In addition, the ER retention signal KDEL (Lys–Asp–Glu–Leu) peptide was fused at the C terminus of the HC to enhance stability and increase overall accumulation of the protein (17, 20).
The assembled mAb BR55-2 was isolated from pooled leaf material of all T0 plants with a simple two-step purification process and was used for subsequent biological functional analyses. Based on the mean expression levels of mAb in primary transformants (6 mg/kg of fresh leaf), we obtained 50% recovery of recombinant mAb from plant tissue. These results show that regulatory elements in expression cassettes can significantly improve the overall yields. The use of low-alkaloid tobacco plants is feasible for efficient mAb production.
A binding assay of plant and mAbM BR55-2 with four different cancer cell lines (SK-BR3, SW948, A431, and WM115) showed similar specificity in recognizing the LeY oligosaccharide antigen present on SK-BR3 (breast cancer) and SW948 (colorectal cancer), whereas mAb CO17-1A specific for the GA733-2 antigen recognized only SW948 cells. These results are consistent and correlate with the abundant presence of LeY on both SK-BR3 and SW948 cells (6–8), whereas GA733-2 antigen was present at high density on SW948 but not on SK-BR3 or other cell lines studied (21, 22). These data indicate that mAbP BR55-2 is highly specific in recognizing LeY.
In vitro binding activity of the FcγRI receptor (CD64) to mAbP and mAbM indicated that the Fc binding activity was similar for both mAbs. The interaction between CD64 and the Fc of the mAbP and mAbM was blocked by human IgG to almost the same extent, suggesting that mAb BR55-2 specifically bound the Fc receptors expressed on U937 cells. The binding assay with 3T3 cells expressing only CD64 further confirmed that both mammalian and plant mAbs bind specifically to CD64. The wide distribution of cell number bound by mAb m22-FITC (Fig. 4B Right) to 3T3 cells showed that the CD64 expression level was somewhat heterogeneous among the transfected cells. The pattern of cell distribution was quite similar among all three mAbs (Fig. 4B). We did not observe any differences between mAbP and mAbM in binding to CD64, which is consistent with the results of the experiment using human serum to block mAbP and mAbM binding to U937 cells. The similar specific binding activity of the mAbP and mAbM BR55-2 to CD64 (FCγRI) assumes importance because CD64 interaction with Fc of the mAb is essential for IgG-dependent phagocytic and ADCC activities (23–25). Moreover, it is known that FcγRI is the essential receptor for antibody therapy in a mouse melanoma model (26).
As expected from the Fc receptor binding experiment results, mAbP BR55-2 activity in ADCC was not significantly different from parental mAbM, suggesting that murine macrophages used as effector cells could bind both antibodies with similar efficiency. In the nude mice experiment, both mAbP and mAbM fully protected mice from tumor growth up to 25 days. However, after 25 days the mAbP-treated mice showed some increase in tumor growth, which could be the result of a shorter half-life of plant mAbP, which itself could be due to the type of glycosylation (high-mannose) associated with the addition of the ER retention KDEL motif (13). On the other hand, this fucose-deficient type of glycosylation of mAbP BR55-2 did not perturb the in vitro interaction between the Fc of the mAb and the Fc receptor (27, 28). This change in glycosylation also did not alter ADCC with murine effector cells of mAbP, which is in agreement with reports from other groups who showed that lack of fucose on the human IgG1 had no effect on binding to human FcγRI (28). Moreover, the removal of carbohydrates resulted in a 15- to 20-fold reduction of the IgG1 binding to FcγRIII receptor (27), suggesting that the carbohydrates are important in the function of the FcγRIII.
In this article we have presented a complex, well defined approach to elucidate the biological functions of mAbM and mAbP. The differences in glycosylation pattern between mAbM and mAbP remain to be further resolved.
Altogether, our data clearly demonstrate that plant mAbP BR55-2 shows excellent biological activity similar to that of the parental mAbM isolated from the hybridoma system. mAbP recognizes the LeY on both SK-BR3 and SW948 cancer cells and displays comparable activity in activating macrophages in ADCC. Comparison of mAb Fc receptor interaction confirmed equal specificity of the mAbP and mAbM. Plant-derived BR55-2 antibody exhibits biological activities suitable for efficient cancer immunotherapy.
Materials and Methods
Cell Culture.
SK-BR3 human breast cancer, SW948 colorectal carcinoma, A431 human epidermal carcinoma, WM115 human melanoma, U937 human lymphoma, and 3T3 mouse fibroblast cells were obtained from the American Type Culture Collection and maintained according to the supplier's instructions. The hybridoma producing mAb BR55-2 (our laboratory collection) was maintained in DMEM supplemented with 10% FBS. Mouse macrophages were isolated from CBA mice (The Jackson Laboratory) and cultured as described (29).
Cloning of LC and HC of mAb BR55-2 into the Plant Vector.
Cloning and other DNA manipulations were carried out according to standard methods (30). Escherichia coli JM109 competent cells (Promega) were used for plasmid transformation.
Total RNA was extracted from hybridoma cells producing murine IgG2a mAb BR55-2 (anti-LeY) by using an RNeasy Mini kit (Qiagen, Valencia, CA). mRNA was reverse-transcribed into cDNA by using oligo(dT) primers and avian myeloblastosis virus reverse transcriptase (Promega). cDNA encoding LC and HC genes of mAb BR55-2 were amplified from the cDNA by PCR by using murine κ and IgG2a-specific primers, respectively. The LC gene was amplified by using BR55L forward (5′-TCATGAAGTTGCCTGTTAGGCTTTTGGTGCTGAT-3′) and BR55L reverse (5′-TCTAGACTAACACTCATTCCTGTTGAAGCT-3′) oligonucleotides containing BspHI and XbaI sites in the 5′ region, respectively. The HC gene was amplified by using BR55H forward (5′-ACCATGGACTTGGGGCTCAGCTTGATT-3′) and BR55H reverse (5′-TCTAGATCAAAGTTCATCTTTACCCGGAGTCCGGGAGAAGCT-3′) oligonucleotides containing NcoI and XbaI sites in the 5′ region, respectively. The BR55H reverse primer contains sequence-encoding KDEL amino acids as an ER retention signal in the 5′ region. The LC and HC genes were cloned under the control of the 35S promoter from cauliflower mosaic virus with a duplicated upstream enhancer region (18) into plasmid pBI525 (31) or pRTL2 (32), respectively. The LC and HC expression cassettes were transferred as HindIII–EcoRI and HindIII fragments, respectively, into the plant binary vector pBI121 (Clontech), yielding plasmid pRB59-2. The binary plasmid obtained was transferred to Agrobacterium tumefaciens strain LAB4404.
Plant Transformation.
Low-alkaloid tobacco, N. tabacum cv. LAMD609 (Oxford Tobacco Research Station, Oxford, NC), was used for Agrobacterium-mediated transformation according to a published protocol (33) with some modifications. Tobacco leaf explants from in vitro culture were infiltrated with fresh overnight culture of Agrobacterium containing pRB59-2 plasmid. Transformants were regenerated on the plant medium containing kanamycin (100 mg/liter).
Western Blot Assay.
Samples from transgenic plants were collected and analyzed for mAb content. Briefly, two leaf discs (30 mg) of plants were homogenized in PBS supplemented with 0.05% Tween 20 (PBST). Proteins were resolved by SDS/PAGE and either stained by using Coomassie brilliant blue R250 or transferred to a nitrocellulose membrane (Bio-Rad). The membrane was blocked with 5% nonfat milk in PBS and incubated for 1 h at room temperature with goat anti-mouse Fcγ and F(ab′)2 fragment-specific antibodies conjugated to horseradish peroxidase (Sigma) diluted 1:10,000. After washing, reactive protein bands were visualized with chemiluminescent substrate for peroxidase (Pierce).
Purification of Antibody from Plants.
For purification of plant BR55-2 antibody, frozen tobacco plant leaves were homogenized with PBS buffer containing 0.5% Tween 20 and centrifuged at 15,000 × g for 30 min. The supernatant was filtered through a Miracloth (Calbiochem), and solid ammonium sulfate was added to 25% of saturated solution. After 1 h of incubation at 4°C the solution was centrifuged at 15,000 × g for 30 min at 4°C, the precipitate was discarded, and ammonium sulfate was added to the supernatant to 50% saturation. After another 2 h of incubation at 4°C, the solution was centrifuged as before and the pellet was resuspended in PBS to one-fourth of the original volume. Soluble proteins were applied to a protein A column (Amersham Pharmacia), and mAb was eluted according to the manufacturer's recommendations. After overnight dialysis against PBS, mAb was concentrated to 1 mg/ml by using an Amicon Ultra spin column with a 35-kDa cutoff (Millipore, Bedford, MA) and stored at −80°C.
Binding of mAbP BR55-2 to Cancer Cells.
mAbP was analyzed for binding activity to SK-BR3 human breast cancer, SW948 colorectal carcinoma, A431 human epidermal carcinoma, or WM115 human melanoma cells. A total of 106 cells were seeded overnight in 96-well flat-bottom plates (Nalge Nunc International, Rochester, NY). Plates containing cell monolayers were washed twice with PBS and fixed with 0.05% glutaraldehyde in PBS for 20 min at room temperature, and the PBS-washed plate was stored at 4°C in 50 μl of 0.7% glycine or blocked with 1% BSA in PBS for immediate use. After washing with PBS, 100 μl of mAbP, mAbM BR55-2, or melanoma-specific murine mAb ME3.61 (negative control) at a concentration of 1 μg/ml in PBS was applied to the plate, followed by four washes with PBST and addition of 100 μl of anti-mouse Fcγ-specific horseradish peroxidase-conjugated secondary antibody (Sigma) (1:10,000 in PBST). After incubation for 1 h at 37°C, plates were washed four times with PBST and developed with OPD peroxidase substrate (Sigma), and absorbance at 490 nm was determined by using a SpectraMAX 340PC microplate reader (Molecular Devices).
Flow Cytometric Analysis of mAbP BR55-2 Binding to CD64/FcγRI.
U937 human lymphoma cells were treated overnight with 300 units/ml IFN-γ (Boehringer Ingelheim, Biberach, Germany) and incubated for 30 min at 4°C with mAbP or mAbM BR55-2 (10 μg/ml) in PBS containing 1% BSA and 0.02% sodium azide (immunofluorescence buffer, IFB). Goat anti-mouse antibody conjugated to FITC was used to stain the murine mAbs bound to CD64. Cells were washed twice with IFB and analyzed with a FACSCalibur (BD Biosciences). To confirm the specific interaction between Fc of mAbs and CD64, 3T3 fibroblasts were stably transfected as previously described (34) with CD64 cDNA or mock-transfected and incubated for 30 min at 4°C with mAbP or mAbM BR55-2 (10 μg/ml) in IFB buffer. Goat anti-mouse Ab-FITC was then used to detect mAb bound to CD64. After two washes with IFB, cells were analyzed with a FACSCalibur (BD Biosciences). mAb m22-FITC (Medarex, Annandale, NY) was used to confirm CD64 surface expression on both U937 and transfected 3T3 cells.
ADCC.
ADCC assay was performed as described in ref. 35 with modifications. Briefly, macrophages from 8-week-old CBA mice (The Jackson Laboratory) as effector cells (5 × 105 cells per well) were incubated in flat-bottom 96-well ELISA plates (Nalge Nunc International) for 2 h at 37°C in a 5% CO2 atmosphere in 1× MEM (Cellgro, Herndon, VA) supplemented with 10% FBS. Cells were washed twice with warm PBS and incubated with 100 μl of SK-BR3 target cells (104 cells per well), which express high levels of LeY oligosaccharide on the cell surface, and 100 μl of mAbP or mAbM BR55-2, or mAb ME3.61 (50 μg/ml) as a control. Cells on plates were spun down at 400 × g for 5 min and grown for 24 h at 37°C in a 5% CO2 atmosphere. A total of 100 μl of supernatant from each well was moved to a fresh 96-well plate and tested by using the Cytotoxicity Detection kit (Roche, Indianapolis) for lactate dehydrogenase activity released from damaged cells. Absorbance at 492 nm was determined by using a microplate reader (Molecular Devices). Percentage of specific lysis was calculated as follows: [(mAb/effectors/target cell mix − effector cell control) − target cell control]/(high control − target cell control) × 100. Target and effector cell controls were determined as an absorbance from cells incubated with medium alone. High control was assessed in target cells treated with 1% Triton X-100 in medium.
Tumor Growth Inhibition in Vivo.
Colorectal carcinoma SW948 cells (106 cells) were inoculated s.c. into the necks of 6- to 8-week-old BALB/c nu/nu mice (Charles River Laboratories, Wilmington, MA). Three groups of tumor-injected mice (five mice per group) were immediately injected i.p. with 100 μg of mAbP or mAbM BR55-2 or mAb ME3.61 (negative control), followed by three injections of the appropriate mAb every 3 days for a total of 400 μg during 9 days. Tumor growth was recorded at 10, 15, 21, 25, 28, and 35 days after initial injection and calculated based on the three major diameters measured with graduated calipers. At the end of the experiment, mice were killed by CO2 inhalation.
Acknowledgments
We thank Dr. W. Crosby (Plant Biotechnology Institute, Saskatoon, SK, Canada) for pBI525 and J. Carrington (Washington State University, Pullman) for pRTL2. We also thank Thomas Jefferson University/Kimmel Cancer Center research and animal facilities for their support. This work was supported by a grant from U.S. Department of Agriculture to Biotechnology Foundation Laboratories (to H.K.).
Abbreviations
- mAbP
plant-derived mAb
- mAbM
mammalian-derived mAb
- HC
heavy chain
- LC
light chain
- ADCC
antibody-dependent cell-mediated cytotoxicity
- LeY
Lewis Y oligosaccharide antigen
- ER
endoplasmic reticulum.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Stemmler H. J., Kahlert S., Brudler O., Beha M., Muller S., Stauch B., Heinemann V. Clin. Oncol. (R. Coll. Radiol.) 2005;17:630–635. doi: 10.1016/j.clon.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 2.DeNardo S. J. Semin. Nucl. Med. 2005;35:143–151. doi: 10.1053/j.semnuclmed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Steplewski Z., Lubeck M. D., Koprowski H. Science. 1983;221:865–867. doi: 10.1126/science.6879183. [DOI] [PubMed] [Google Scholar]
- 4.Plunkett T. A., Miles D. W. Int. J. Clin. Pract. 2002;56:261–266. [PubMed] [Google Scholar]
- 5.Adams G. P., Weiner L. M. Nat. Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 6.Nichols E. J., Kannagi R., Hakomori S. I., Krantz M. J., Fuks A. J. Immunol. 1985;135:1911.6–1913.6. [PubMed] [Google Scholar]
- 7.Fernandes B., Sagman U., Auger M., Demetrio M., Dennis J. W. Cancer Res. 1991;51:718–723. [PubMed] [Google Scholar]
- 8.Nemoto-Sasaki Y., Mitsuki M., Morimoto-Tomita M., Maeda A., Tsuiji M., Irimura T. Glycoconjugate J. 2001;18:895–906. doi: 10.1023/a:1022252509765. [DOI] [PubMed] [Google Scholar]
- 9.Steplewski Z., Lubeck M. D., Scholz D., Loibner H., McDonald S. J., Koprowski H. In Vivo. 1991;5:79–83. [PubMed] [Google Scholar]
- 10.Dettke M., Palfi G., Loibner H. J. Leukocyte Biol. 2000;68:511–514. [PubMed] [Google Scholar]
- 11.Koprowski H. Vaccine. 2005;23:1757–1763. doi: 10.1016/j.vaccine.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko K., Koprowski H. Virus Res. 2005;111:93–100. doi: 10.1016/j.virusres.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Ko K., Tekoah Y., Rudd P. M., Harvey D. J., Dwek R. A., Spitsin S., Hanlon C. A., Rupprecht C., Dietzschold B., Golovkin M., et al. Proc. Natl. Acad. Sci. USA. 2003;100:8013–8018. doi: 10.1073/pnas.0832472100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko K., Wei X., Crooks P. A., Koprowski H. J. Immunol. Methods. 2004;286:79–85. doi: 10.1016/j.jim.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Ko K., Steplewski Z., Glogowska M., Koprowski H. Proc. Natl. Acad. Sci. USA. 2005;102:7026–7030. doi: 10.1073/pnas.0502533102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietzschold B., Gore M., Casali P., Ueki Y., Rupprecht C. E., Notkins A. L., Koprowski H. J. Virol. 1990;64:3087–3090. doi: 10.1128/jvi.64.6.3087-3090.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schouten A., Roosien J., van Engelen F. A., de Jong G. A., Borst-Vrenssen A. W., Zilverentant J. F., Bosch D., Stiekema W. J., Gommers F. J., Schots A., et al. Plant Mol. Biol. 1996;30:781–793. doi: 10.1007/BF00019011. [DOI] [PubMed] [Google Scholar]
- 18.Timmermans M. C., Maliga P., Vieira J., Messing J. J. Biotechnol. 1990;14:333–344. doi: 10.1016/0168-1656(90)90117-t. [DOI] [PubMed] [Google Scholar]
- 19.Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 20.Torres E., Vaquero C., Nicholson L., Sack M., Stoger E., Drossard J., Christou P., Fischer R., Perrin Y. Transgenic Res. 1999;8:441–449. doi: 10.1023/a:1008969031219. [DOI] [PubMed] [Google Scholar]
- 21.Ross A. H., Herlyn D., Iliopoulos D., Koprowski H. Biochem. Biophys. Res. Commun. 1986;135:297–303. doi: 10.1016/0006-291x(86)90976-9. [DOI] [PubMed] [Google Scholar]
- 22.Balzar M., Winter M. J., de Boer C. J., Litvinov S. V. J. Mol. Med. 1999;77:699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- 23.Hazenbos W. L., Gessner J. E., Hofhuis F. M., Kuipers H., Meyer D., Heijnen I. A., Schmidt R. E., Sandor M., Capel P. J., Daeron M., et al. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 24.Barnes N., Gavin A. L., Tan P. S., Mottram P., Koentgen F., Hogarth P. M. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 25.Takai T., Li M., Sylvestre D., Clynes R., Ravetch J. V. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 26.Bevaart L., Jansen M. J., van Vugt M. J., Verbeek J. S., van de Winkel J. G., Leusen J. H. Cancer Res. 2006;66:1261–1264. doi: 10.1158/0008-5472.CAN-05-2856. [DOI] [PubMed] [Google Scholar]
- 27.Radaev S., Sun P. D. J. Biol. Chem. 2001;276:16478–16483. doi: 10.1074/jbc.M100351200. [DOI] [PubMed] [Google Scholar]
- 28.Shields R. L., Lai J., Keck R., O'Connell L. Y., Hong K., Meng Y. G., Weikert S. H., Presta L. G. J. Biol. Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 29.Herlyn D., Powe J., Ross A. H., Herlyn M., Koprowski H. J. Immunol. 1985;134:1300–1304. [PubMed] [Google Scholar]
- 30.Sambrook S., Fritsch E. F., Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 31.Pang S. Z., Nagpala P., Wang M., Slightom J. L., Gonsalves D. Phytopathology. 1992;82:1223–1229. [Google Scholar]
- 32.Carrington J. C., Freed D. D., Leinicke A. J. Plant Cell. 1991;3:953–962. doi: 10.1105/tpc.3.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horsch R. B., Klee H. J., Stachel S., Winans S. C., Nester E. W., Rogers S. G., Fraley R. T. Proc. Natl. Acad. Sci. USA. 1986;83:2571–2575. doi: 10.1073/pnas.83.8.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daly T., Royal R. E., Kershaw M. H., Treisman J., Wang G., Li W., Herlyn D., Eshhar Z., Hwu P. Cancer Gene Ther. 2000;7:284–291. doi: 10.1038/sj.cgt.7700121. [DOI] [PubMed] [Google Scholar]
- 35.Herlyn D., Koprowski H. Proc. Natl. Acad. Sci. USA. 1982;79:4761–4765. doi: 10.1073/pnas.79.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]