Abstract
Accumulation of abnormally hyperphosphorylated tau (P-tau) in the form of tangles of paired helical filaments and/or straight filaments is one of the hallmarks of Alzheimer's disease (AD) and other tauopathies. P-tau is also found unpolymerized in AD. Although the cognitive decline is known to correlate with the degree of neurofibrillary pathology, whether the formation of filaments or the preceding abnormal hyperphosphorylation of tau is the inhibitory entity that leads to neurodegeneration has been elusive. We have previously shown that cytosolic abnormaly hyperphosphorylated tau in AD brain (AD P-tau) sequesters normal tau (N-tau), microtubule-associated protein (MAP) 1, and MAP2, which results in the inhibition of microtubule assembly and disruption of microtubules. Here, we show that polymerization of AD P-tau into filaments inhibits its ability to bind N-tau and as well as the ability to inhibit the assembly of tubulin into microtubules in vitro and in the regenerating microtubule system from cultured cells. Like AD P-tau, the in vitro abnormally hyperphosphorylated recombinant brain N-tau binds N-tau and loses this binding activity on polymerization into filaments. Dissociation of the hyperphosphorylated N-tau filaments by ultrasonication restores its ability to bind N-tau. These findings suggest that the nonfibrillized P-tau is most likely the responsible entity for the disruption of microtubules in neurons in AD. The efforts in finding a therapeutic intervention for tau-induced neurodegeneration need to be directed either to prevent the abnormal hyperphosphorylation of this protein or to neutralize its binding to normal MAPs, rather than to prevent its aggregation into filaments.
Keywords: abnormal hyperphosphorylation of tau, microtubule assembly, microtubule-associated proteins, neurofibrillary degeneration, paired helical filaments
A common feature of the dementia disorders that are known as tauopathies [which include Alzheimer's disease (AD)1 and frontotemporal dementia with Parkinsonism-linked to chromosome 17 (FTDP-17)] is the accumulation in the brain neurons of abnormally hyperphosphorylated tau (P-tau), mostly polymerized into tangles of paired helical filaments (PHFs) and/or straight filaments (SF) (1). Besides hyperphosphorylation, tau in PHF/SF has been shown to be glycated (2), truncated (3), and immunoreactive with an Ab to a stable 4-hydroxy-2-nonenal (HNE)-lysine adduct (4). The number of neurofibrillary tangles is known to correlate directly with the degree of dementia in AD patients (5–7). Also, the recent discovery of the cosegregation of specific mutations in the tau gene with disease in some pedigrees of FTDP-17 has confirmed that the tau pathology can be a primary cause of neurodegeneration and dementia (8–10). Up to 40% of the abnormally hyperphosphorylated tau in AD brain (AD P-tau) is in the cytosol (11–13). Understanding whether this AD P-tau or its polymer, PHF/SF, initiates the neurodegeneration is critical for developing a rational therapeutic treatment of AD and related tauopathies.
The AD P-tau sequesters normal tau (N-tau), microtubule-associated protein (MAP) 1, and MAP2, and it disassembles microtubules and self-assembles into PHFs (14–17). The binding between AD P-tau and MAPs seemed to be of hydrophobic nature because it is stimulated at 200 mM NaCl and inhibited at 2 M NaCl; also, it is completely inhibited by ≈0.5% Triton X-100 (15, 18). AD P-tau can sequester the six isoforms of tau with different affinity; fetal tau binds the least of the six isoforms (19). AD P-tau is able to destroy the microtubules formed with all of the tau isoforms (19). Recombinant human brain tau on in vitro phosphorylation to a stoichiometry of 4–6 mol sequesters N-tau and disassembles microtubules. On further hyperphosphorylation to ≈10 mol of phosphate per 1 mol of protein, tau self-assembles into bundles of PHF/SF (17, 20). Dephosphorylation of AD P-tau as well as PHF converts them into normal-like protein, which promotes assembly and stabilizes microtubules (14, 21, 22). The FTDP-17 mutated taus behave as more favorable substrates than N-tau for hyperphosphorylation by brain protein kinases and self-assemble into tangles of PHF/SF (20). Because of the presence of P-tau in different aggregation states, it was not known whether the toxic entity was the misfolded modified protein, the oligomer, or the filaments.
In this article, we describe that, upon in vitro polymerization into PHF/SF, AD P-tau and in vitro abnormally hyperphosphorylated recombinant human brain tau441 lose their inhibitory behavior and become inert. Also, PHFs isolated from AD brains behave as inert in their interaction with tubulin and microtubules and do not bind N-tau, and, when added to tubulin and N-tau, they do not inhibit microtubule assembly. Similar results were obtained when using a regenerating microtubule system from cultured cells. These findings suggest that AD P-tau from the cytosol is the entity in the neurons that is responsible for the microtubule disruption and that a therapeutic intervention for tau-induced neurodegeneration that can inhibit tau hyperphosphorylation or neutralize its binding to normal MAPs is the most promising.
Results
AD P-Tau, but Not PHF, Sequesters N-Tau and Inhibits Microtubule Assembly.
We have shown (14, 15) that AD P-tau sequesters N-tau. This toxic property requires hyperphosphorylation because, upon dephosphorylation, P-tau loses its ability to sequester N-tau (19). In this study, we investigated whether the assembly state of phosphorylated tau affects its ability to bind N-tau. Thus, increasing amounts (10–100 ng of tau) of AD P-tau and of three preparations of PHF from three different AD brains were dotted in duplicates on nitrocellulose membrane strips. One strip was overlaid with 5 μg/ml N-tau to detect its binding to AD P-tau or PHF, and the second strip was overlaid with BSA to serve for the background levels of Tau-1 immunoreactivity of AD P-tau and PHF. These strips were then developed with mAb Tau-1 to assay the amount of N-tau bound to the P-taus. A third strip was developed with a mixture of three polyclonal phosphoindependent Abs against total tau to determine the total amount of tau per dot, and we used these data to normalize the values determined from the first two strips. The quality of the AD P-tau, a representative PHF preparation, and the N-tau that we used is shown in Fig. 1A. To verify the specificity of the binding, two strips were spotted with N-tau, overlaid with BSA, or overlaid with N-tau. After binding of N-tau, washing, and fixing as described, the bound tau was detected by using Tau-1 Ab, which recognizes normal but not P-tau from AD brain (compare Fig. 1A, AD P-tau and PHF without and with alkaline phosphatase treatment). The strip overlaid with BSA was negative with Tau-1 (Fig. 1B) because both AD P-tau and PHF are phosphorylated at Tau-1 epitope. The strips spotted with N-tau had the same immunoreactivity whether overlaid with BSA or with N-tau (Fig. 1B), showing that N-tau is unable to bind N-tau. The quantitations of the binding are shown in Fig. 1C. AD P-tau was able to bind N-tau, whereas PHF did not bind detectable levels of N-tau, suggesting that polymerized P-tau does not sequester N-tau.
Fig. 1.
Coomassie blue staining, Western blotting, and the binding of N-tau to AD P-tau, PHF, and in vitro hyperphosphorylated N-tau. (A) N-tau (50 ng) and 4-μg aliquots of AD P-tau and PHF were subjected to SDS/PAGE, transferred to poly(vinylidene difluoride) membrane, and immunodetected with Tau-1 Ab. One strip was stained with Coomassie blue (Cb). Before immunostaining, the blots were treated with (+) or without (−) alkaline phosphatase (AP, 100 units/ml) for 3 h at 35°C. (B) Increasing concentrations of AD P-tau and duplicates of three different preparations of PHF, and as a control N-tau (10–100 μg tau content), were spotted on nitrocellulose membrane and overlaid with 5 μg/ml τ4L or with BSA and developed with Tau-1 Ab. To verify that the proteins bound to the membrane, one strip without any overlay was developed with a mixture of three phospho-independent polyclonal Abs to total tau. (C) Quantitations of the tau bound to AD P-tau (■) and to PHF (▴). (D) N-tau was hyperphosphorylated in vitro, as described in ref. 19; one aliquot was probe-sonicated for 1 min to disrupt the tau filaments formed during the incubation period (19). The sonicated sample was centrifuged at 55,000 × g for 20 min, and the pellet was discarded. The nonsonicated and the supernatant of the sonicated samples were dotted in triplicates on three nitrocellulose membrane strips. These strips were processed as described above. The quantitations are given as mean ± SD. ∗, P < 0.005.
We have proposed (14, 15) that AD P-tau inhibits microtubule assembly through sequestration of N-tau. We postulated that PHF, which is unable to bind N-tau, and self-assembled AD P-tau will not inhibit tau-promoted microtubule assembly. To test this hypothesis, we investigated the effect of AD P-tau or PHF on microtubule assembly. Tubulin (2 mg/ml) was mixed with N-tau in the absence or presence of AD P-tau (soluble or previously incubated to induce AD P-tau self-assembly into filaments) or PHF. The addition of AD P-tau but not PHF or self-assembled AD P-tau inhibited tau-promoted microtubule assembly (Fig. 2, compare curves 1–4). These findings suggest that, unlike AD P-tau, PHF formed in AD brain or polymerized in vitro from AD P-tau is not inhibitory to the polymerization of tubulin into microtubules.
Fig. 2.
Effect of AD P-tau and PHF on microtubule assembly. Inhibition of microtubule assembly was studied as described in Materials and Methods. The assembly reaction was carried out by using 2 mg/ml tubulin mixed with 0.1 mg/ml N-tau alone (curve 1), with 0.2 mg/ml AD P-tau (curve 2), with 0.2 mg/ml self-assembled AD P-tau (curve 3), or 0.2 mg/ml PHF (curve 4). Tubulin without any addition was incubated as a control (curve 5). AD P-tau inhibited the microtubule-assembly-promoting activity of N-tau (compare curves 1 and 2), whereas PHF did not show any significant effect (compare curves 1 and 4 as well as 1 and 3, respectively).
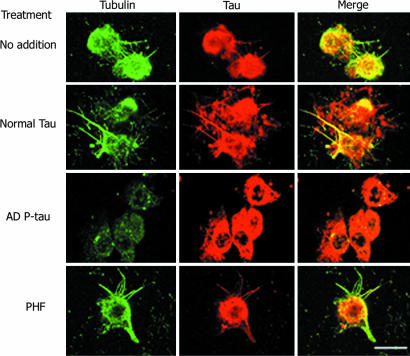
To evaluate the effect of AD P-tau and PHF on the regeneration of microtubule network from a cell, we incubated Triton X-100-extracted 3T3 cells in which the endogenous microtubules had been depolymerized by nocodazole with rat brain cytosol without or with recombinant tau, AD P-tau, or PHF in the presence of 1 mM GTP (Fig. 3). Bundles of microtubules were formed from the cell microtubule-organizing centers when treated with brain cytosol without any addition or with addition of tau or PHF (Fig. 3). We found that only the unpolymerized P-tau inhibited the regeneration of microtubules (Fig. 3). This finding suggests that, even in the presence of endogenous MAPs, only the soluble P-tau disrupts the regeneration of microtubules.
Fig. 3.
AD P-tau but not PHF inhibits regeneration of microtubules from 3T3 cells. The extracted cells were incubated with 15% fresh rat brain cytosol in buffer containing 1 mM GTP for 1 h at 37°C, as described in Materials and Methods. Cells were processed for immunofluorescence staining by using DM1-A Ab against tubulin (green) and 134d rabbit polyclonal Ab against tau (red). Only AD P-tau could inhibit microtubule assembly.
Polymerization of in Vitro Hyperphosphorylated N-Tau into Filaments Inhibits Its Binding to N-Tau.
To investigate the effect of polymerization further, we studied the binding of in vitro hyperphosphorylated polymerized or unpolymerized N-tau to N-tau. We have shown (17, 19, 20) that in vitro hyperphosphorylation of tau with brain kinases results in a hyperphosphorylated protein that self-assembles, binds N-tau, and inhibits microtubule assembly. N-tau was in vitro hyperphosphorylated, as described in ref. 19. Under these phosphorylation conditions, ≈12 mol of phosphate per mole of tau are incorporated, tau self-assembles, and ≈50% of the protein becomes insoluble (20). Unlike PHFs isolated from AD brain, tau filaments formed in vitro upon hyperphosphorylation can be disrupted by dilution and sonication. The sample was diluted 10 times in 100 mM Mes buffer (pH 6.7) containing 1 mM EGTA and 1 mM MgCl2, and the filaments were probe sonicated (speed, 6; 50% pulse; Branson) for 1 min. The sonicated P-tau was centrifuged at 55,000 × g for 20 min. The supernatant containing the soluble protein was saved to be used for the binding assays. The amount of tau recovered in the supernatant was very similar to the starting amount (Fig. 1D), suggesting that most of the tau filaments became soluble with this treatment. The P-tau before (mixture of filaments and soluble tau) and after (soluble tau) ultrasonication and centrifugation were dotted in triplicates on nitrocellulose membrane strips. These strips were processed as described above to determine the binding of P-tau to N-tau. The strip overlaid with BSA was negative with Tau-1 (Fig. 1D) because P-tau is phosphorylated at Tau-1 epitope. Sonication of the P-tau was able to disrupt the filaments that formed because almost all tau was recovered in the supernatant (Fig. 1D, total tau). The quantitations of the binding are shown in Fig. 1D Lower. The binding of N-tau to P-tau was increased ≈2.5-fold upon sonication. Together, these results suggest that that P-tau loses its ability to bind N-tau upon polymerization.
AD P-Tau upon Self-Assembly into PHF Loses Its Ability to Bind N-Tau.
To investigate whether the self-assembly of P-tau into filaments inhibits its binding to N-tau, we examined the binding of N-tau to AD P-tau before and after its self-assembly into PHF. AD P-tau self-assembly was induced in vitro, as described in ref. 17. Aliquots were taken from the incubation mixture before the incubation, after 1 h of incubation, and after 2 h of incubation, and they were dotted onto nitrocellulose paper (200 ng of tau per dot). The self-assembly of AD P-tau was confirmed by negative-stain electron microscopy, as described in ref. 14. The nitrocellulose strips were overlaid with 5 μg/ml N-tau and processed as described above to analyze tau binding to AD P-tau. N-tau was bound to AD P-tau when the AD P-tau was dotted unpolymerized (Fig. 4 A). The AD P-tau preparation did not have any filaments before inducing its self-assembly (Fig. 4B). When AD P-tau had self-assembled into tangles of PHF, as confirmed by electron microscopy (Fig. 4C), the binding of N-tau was decreased to <20% of the binding to the unpolymerized AD P-tau (Fig. 4A), although the amount of P-tau per dot was the same (Fig. 4A Inset). These findings show that, upon polymerization, AD P-tau loses its ability to sequester N-tau.
Fig. 4.
Effect of AD P-tau self-assembly on its binding to N-tau. Self-assembly of AD P-tau was induced as described in Materials and Methods. At 0, 60, and 120 min of its self-assembly, aliquots were both examined by negative stained electron microscopy and spotted on nitrocellulose membrane, and overlaid with 5 μg/ml τ4L to determine the binding of N-tau, as described in Fig. 1B. At time 0 min, AD P-tau was nonfibrillized (B), and, after 120 min, bundles of PHF (C) could be detected by electron microscopy. The nonfibrillized AD P-tau bound N-tau and the amount of tau bound decreased with the degree of AD P-tau polymerization (A). (Inset) Nitrocellulose membrane spotted with AD P-tau at different time periods of incubation during the polymerization reaction, then overlaid with nothing, BSA, or N-tau and developed with Tau-1 or Abs against total tau.
Discussion
Several neurodegenerative diseases share the characteristic of intracellular and/or extracellular inclusions of protein deposits in the brain as disease hallmarks. It appears as a common feature that the misfolding of certain proteins and their deposits as filaments is tightly associated. Deposits of Aβ as plaques and P-tau as tangles are associated with AD. Also detected in the brain are deposits of tau fibrils in different tauopathies or of mutated tau protein in certain frontotemporal dementias, α-synuclein in Parkinson disease, huntingtin with expanded polyglutamine tracts in Huntington's disease, and prion protein with altered conformations in Creutzfeld–Jacob disease (23–27). It is debated whether the aggregates per se are a cause of neurodegeneration in these diseases, despite the fact that some of these lesions (such as amyloid-β plaques) do not correlate with the severity of the disease. Recently, it has been shown that the formation of inclusion bodies, in the case of Huntington disease, reduces the level of mutant huntingtin and the risk of neuronal death (28). This study shows that polymerization of P-tau into filaments (i.e., PHF/tangles) makes it inert, which, unlike the nonpolymerized AD P-tau, does not bind N-tau and inhibit the microtubule assembly. These findings suggest that inhibition of the hyperphosphorylation of tau rather than its polymerization into PHF/tangles might be promising for therapies for AD and related tauopathies.
Tau promotes the assembly and stabilizes microtubules (29). Tau has been shown to be abnormally hyperphosphorylated in AD and is present in the hyperphosphorylated state in PHF/neurofibrillary tangles (1, 11, 30–32). Microtubules support axoplasmic transport, and, in the tangle-bearing neurons of patients with AD, the microtubule system is disrupted and replaced by PHFs. Microtubules are polymers of tubulin, and it is well known that the degree of tubulin polymerization in a cell has critical consequences on the fate of the cell. Agents that either stabilize or disrupt microtubules can induce apoptosis in many cell types, especially in the neurons, in which the processes are long and the structure and transport are supported by microtubules. Therefore, the presence of P-tau that disrupts microtubules constitutes a threat to the stability of the neurons. Whether tau is a toxic entity for the cells in its polymerized form is a subject of debate. SantaCruz et al. (33), using transgenic mice expressing inducible human four-repeat tau with the P301L mutation, found that the cognitive deficiencies correlate with the appearance of soluble P-tau. Also, they found that, when tau expression was turned off, there was no clearance of the polymerized tau, soluble tau decreased, and there was improvement in cognition, suggesting that the polymerized tau was not sufficient to cause cognitive decline or neuronal cell death. Andorfer et al. (34) showed that, in human tau transgenic mice, although there was widespread neurodegeneration, the PHF-containing neurons seemed “healthy” in terms of nuclear morphology, suggesting that the polymerized protein was probably neuroprotective. A similar conclusion was implied by a morphometric study of brain biopsy specimens from AD and control cases, which found that the decrease in microtubule density was unrelated to PHF accumulation (35). More recently, Khlistunova et al. (36) showed that aggregates of a fragment of tau in cells are toxic and that inhibition of the aggregation decreased toxicity. However, this last study (36) was done with only a fragment of tau, which can behave completely different from the whole molecule, as used in the previous studies.
The portion of tau molecule involved in tau–tau interaction seems to be the microtubule-binding domain (17, 19, 37–39) and of hydrophobic nature (15, 18). The involvement of this tau region in the interaction is suggested because the interaction improves when the second microtubule-binding domain (i.e., four-repeat tau) is present (17). However, tau that lacks the N-terminal region of the molecule does not bind AD P-tau. This result could mean that the N-terminal region is involved in the binding, that the conformation of tau induced by the presence of the N-terminal region is needed for the interaction, or that the fragment that lacks the N-terminal region is involved in a homologous interaction (17, 39). For tau self-assembly, the flanking regions of the microtubule-binding domain are inhibitory of the tau–tau interaction, and phosphorylation neutralizes these inhibitory regions or induces a change of conformation that exposes the region of tau involved in the interaction. The tau conformation involved in its sequestration needs ≈4 mol of phosphate per 1 mol of the protein, whereas tau self-assembly is achieved at ≈10 mol of phosphate per 1 mol of tau (20). The primary sequence of tau influences its conformation and the exposure of its interaction site(s); the presence of the N-terminal inserts, which are highly acidic, and the extra microtubule-binding domain favor both self-assembly and the binding of N-tau to AD P-tau. However, fetal tau (which lacks both the N-terminal inserts and the extra microtubule-binding domain) is sequestered by AD P-tau and self-assembles into filaments (17, 19). The N-terminal region flanking the microtubule-binding domain is required for the tau–tau binding, probably because the conformation of tau without the N-terminal half is unable to bind tau. The change induced in tau by phosphorylation that confers this protein the ability to bind N-tau is not just a charge effect because, when AD P-tau self-assembles, even though the charge does not change, the ability to bind N-tau and inhibit microtubule assembly is lost.
We have shown that the affinity of N-tau for N-tau is minimal (14–16, 19, 20). The affinity of N-tau to AD P-tau cannot be determined because the binding of N-tau to AD P-tau is nonsaturable (15). We previously showed that, when 0.7 mg/ml N-tau was mixed with 0.08 mg/ml AD P-tau, ≈50% N-tau cosedimented with AD P-tau, whereas the sedimentation of 0.08 mg/ml AD P-tau alone was undetectable. These results suggest that the binding of N-tau to AD P-tau is higher than that of AD P-tau to AD P-tau. We can speculate that the neurofibrillary degeneration is initiated with a small fraction of N-tau that is converted into AD P-tau due to a phosphorylation imbalance. Under this condition of high N-tau/AD P-tau ratio, the latter preferably binds N-tau. As the disease progresses and the concentration of AD P-tau increases, AD P-tau probably binds AD P-tau, forming the filaments.
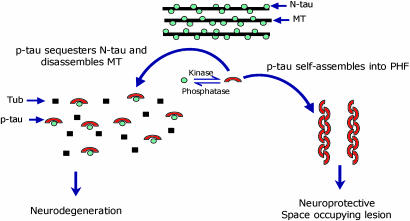
We postulate that abnormal hyperphosphorylation of tau is a key event in neurodegeneration. We showed (14–16, 22) that, unlike N-tau, AD P-tau does not promote but instead inhibits in vitro microtubule assembly by sequestering normal MAPs and that this toxic behavior is due solely to abnormal hyperphosphorylation, because in vitro dephosphorylation converts AD P-tau into a normal-like protein. Also, hyperphosphorylation promotes self-assembly of tau into tangles of PHF/SF (17). These in vitro findings were corroborated recently (40) in cell culture in which treatment with okadaic acid induced thioflavin-S-positive P-tau aggregates. Thus, it is possible that, in AD (probably because of a decrease in phosphatase activity; ref. 41) and FTDP-17 (probably because of mutation), tau becomes a more favorable substrate for kinases (20). The resulting P-tau sequesters N-tau, disrupts the microtubule system, and self-assembles into tangles of filaments. These findings led us to propose a mechanism of tau toxicity in which mainly the cytosolic form of P-tau disrupts microtubule network whereas, on polymerization into PHF, this inhibitory activity is lost (Fig. 5). PHF that is accumulated in the cell body, although initially neuroprotective, might become deleterious when this space-occupying lesion in the neuron compromises its normal function. When we compared the inhibition of microtubule assembly, only AD P-tau, and not PHF or filaments assembled from AD P-tau, were able to inhibit the assembly of tubulin into microtubules promoted by N-tau. We postulate that this inhibitory property is due to the ability of AD P-tau to bind N-tau. Supporting our hypothesis, PHF was unable to bind N-tau. Upon polymerization, AD P-tau lost its ability to bind N-tau. Like AD P-tau, the recombinant N-tau could bind N-tau only when hyperphosphorylated but soluble; dissociation of tau filaments into soluble protein restored its ability to bind N-tau. Together, these results strongly suggest that the cytosolic nonfibrillized form of P-tau is inhibitory to the cytoskeleton. Consistent with our findings, the generation of cytosolic P-tau in the absence of any tau filaments was found to be associated with neurodegeneration in Drosophila (42).
Fig. 5.
Proposed mechanism of tau-induced neurodegeneration in AD and related tauopathies.
Materials and Methods
Materials.
Nocadazole, benzyl alcohol, benzyl benzoate, and sodium borohydride were purchased from ICN. All other reagents that were used in this study were purchased from Sigma, unless indicated otherwise. The Wistar rats were purchased from Charles River Laboratories. The following primary Abs were used: DM1A mouse mAb to α-tubulin (1:2,000; Sigma); rabbit polyclonal Ab 134d to total tau (1:5,000); mAb against tau not phosphorylated at Ser-198, -199, or -202 Tau-1 (1:50,000); Alexa Fluor 488-conjugated goat anti-mouse Ab and Alexa Fluor 594-conjugated goat anti-rabbit or rat Ab (1:1,000; Molecular Probes).
Isolation of Recombinant Human Brain Largest Tau Isoform (N-Tau), AD P-Tau, and PHF.
The prokaryotic expression vector pRK172 bearing tau441 was kindly provided by Michel Goedert (Molecular Biology Unit, Medical Research Council, Cambridge, U.K.). The construct was subcloned, and recombinant tau was purified as described in ref. 43, except that the perchloric acid extraction was avoided. The phosphocellulose-purified tau was purified further by Sephacryl S-300 gel filtration at 4°C.
AD P-tau was purified from the cytosol of frozen AD brains (obtained within 6 h postmortem) by phosphocellulose chromatography (13).
PHFs were isolated by dissociation and sieving of the tissue through nylon mesh and by glucose density-gradient centrifugation (44). The isolated PHF/neurofibrillary tangles were probe-sonicated (speed, 4; 50% pulse; Branson) for 15 min. The sonicated PHFs were dialyzed against 50 mM Tris buffer (pH 7). The samples were stored at −80°C until used. To avoid contamination with unpolymerized P-tau, before use, the PHF fraction was overlaid on a 1 M sucrose solution and centrifuged at 30,000 × g for 15 min at 4°C. The supernatant was discarded, and the pellet was resuspended at 1 mg/ml in 100 mM Mes buffer (pH 6.7) containing 1 mM EGTA and 1 mM MgCl2.
In Vitro Hyperphosphorylation and Assembly of Recombinant Human Brain Tau441 into PHF/SF and then Its Dissociation into the Soluble Protein.
Recombinant human brain tau441 was hyperphosphorylated by using 100,000 × g brain extract from a 20-day-old rat as the source of protein kinases as described in refs. 17, 19, and 20. The reaction was carried out at 35°C in 60 mM Hepes (pH 7.4), 8 mM MgCl2, 5 mM EGTA, 2 mM ATP, 2 mM DTT, 20 nM calyculin A, 1 mM 4-(2-aminoethylamino)-benzenesulfonyl fluoride (AEBSF, a Ser protease inhibitor), and from 0.1–0.5 mg/ml tau protein and 0.25–0.5 mg/ml brain extract. The brain extract was prepared fresh before the phosphorylation reaction. After 2 and 8 h of incubation, NaF (17 mM) and ATP (2 mM), respectively, were added. After 24 h incubation, ≈12 mol of phosphate are incorporated per 1 mol of tau, the protein self-assembles into tangles of PHF/SF, and ≈50% of the hyperphosphorylated protein becomes insoluble (17, 19–20). The reaction was terminated by diluting the protein 10 times in 100 mM Mes buffer (pH 6.7) containing 1 mM EGTA and 1 mM MgCl2, and the filaments were sonicated by probe sonication (speed, 6; 50% pulse; Branson) for 1 min. The sonicated P-tau was centrifuged at 55,000 × g for 20 min. The supernatant containing the soluble protein was used for assaying its binding to N-tau.
Self-Assembly of Tau into Filaments.
The self-assembly of AD P-tau was carried out by incubating 0.4 mg/ml protein in 100 mM Mes buffer (pH 6.9) containing 2 mM EGTA, 0.5 mM MgCl2, 1 mM AEBSF, 2 mM DTT, 20 nM calyculin A, and 17 mM NaF. After incubation for 120 min at 35°C, 10 μl of the incubated sample was applied on a 300 mesh carbon-coated grid and negatively stained with 2% phosphotungstic acid, as described in refs. 14 and 45.
Binding of N-Tau to P-Tau.
The binding of N-tau to P-tau was studied by a dot-overlay assay (14, 46). For the dot-overlay assay, AD P-tau or PHFs (10–100 ng of tau) were spotted on nitrocellulose, which was blocked with 5% fat-free dry milk in 100 mM Mes buffer (pH 6.7) for 1 h. After blocking, the membrane was overlaid with N-tau (5 μg/ml) for 3 h. The membrane was then washed and fixed with 0.5% formaldehyde; the aldehyde groups were neutralized with 9% glycine in 50 mM Tris buffer (pH 7.4), containing 150 mM NaCl (TBS); and the tau bound was detected with Tau-1 Ab (47). This Ab recognizes tau when it is unphosphorylated at Ser-195, -198, -199, and/or -202 (1, 48). Samples in which overlay with N-tau was substituted with BSA were used to deduct any background binding of Tau-1 to AD P-tau or PHF. A third strip of the membrane was used to detect total tau with a mixture of phosphoindependent polyclonal Abs against tau: 134d (49), 111e (50), and 92e (51) to normalize the data. Two other strips were spotted with N-tau and overlaid with BSA or N-tau to detect unspecific binding and detected with Tau-1 Ab. N-tau was unable to bind N-tau.
Western Blot Analysis and Protein and Tau Assays.
Protein concentration was estimated by the modified Lowry's method, as described by Bensadoun and Weinstein (52). Sample preparation and immunoblots were carried out as described in ref. 30. The levels of AD P-tau and PHF were determined by the radioimmuno-slot-blot method of Khatoon et al. (53) by using the following mixture of polyclonal Abs against total tau: 134d, 92e, and 111e.
Inhibition of Microtubule Assembly.
N-Tau (0.1 mg/ml) was mixed at 4°C with purified bovine brain MAP-free tubulin (2 mg/ml) and 1 mM GTP, all in polymerization buffer (100 mM Mes, pH 6.7/1 mM EGTA/1 mM MgCl2) in a final volume of 60 μl. After rapid mixing, the samples were pipetted into quartz microcuvettes and equilibrated at 37°C in a thermostatically controlled Cary 1 recording spectrophotometer. The turbidity of the reaction mixture was continuously monitored at 350 nm. For the inhibition experiments, N-tau was mixed with 0.2 mg/ml AD P-tau (soluble or previously incubated to induce AD P-tau self-assembly into filaments) or PHF before incubating with tubulin. The products of the assembly reaction were viewed by negative-stain electron microscopy, as described in refs. 14 and 45.
Regeneration of Microtubule Network from Cultured Cells.
Mouse 3T3 cells were cultured on poly-d-lysine-coated eight-well culture slides and extracted with 0.2% Triton X-100, followed by 10 μM nocodazole to destroy endogenous microtubules, as described in ref. 54. The extracted cells were then incubated with 15% freshly prepared rat brain cytosol containing 1 mM GTP without or with 100 μg/ml tau alone or previously mixed with 0.2 mg/ml AD P-tau or PHF for 1 h at 37°C. Cells were then fixed with 0.3% glutaraldehyde/0.5% Nonidet P-40 for 10 min (55) and double-labeled with DM1A mAb to α-tubulin and rabbit Ab 134d to tau. Bound Abs were detected with a mixture of Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 594-conjugated goat anti-rabbit IgG. The slides were mounted in antifade reagent (Molecular Probes) and examined with an Eclipse E800 epifluorescent microscope (Nikon). Images were captured and processed with a PCM 2000 confocal system (Nikon).
Acknowledgments
We thank Dr. Ezzat El-Akkad (New York State Institute for Basic Research) for recombinant tau, Dr. M. Goedert for tau plasmids, and Dr. L. I. Binder (Northwestern University, Chicago) for Ab Tau-1. We also thank Janet Murphy and Sonia Warren for secretarial assistance in the preparation and submission of this article. Autopsied brain specimens were provided by the Brain Tissue Resource Center (McLean Hospital, Belmont, MA) under Public Health Service Grant MN/NS31862. This work was supported by the New York State Office of Mental Retardation and Developmental Disabilities, National Institutes of Health Grant AG19158, a grant from the Institute for the Study of Aging of New York, and a pilot project grant from Silberstein Institute for Aging and Dementia at New York University School of Medicine (New York).
Abbreviations
- AD
Alzheimer's disease
- P-tau
abnormally hyperphosphorylated tau
- AD P-tau
abnormally hyperphosphorylated tau in AD brain
- FTDP-17
frontotemporal dementia with Parkinsonism linked to chromosome 17
- MAP
microtubule-associated protein
- N-tau
normal tau
- PHF
paired helical filament
- SF
straight filaments
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Grundke-Iqbal I., Iqbal K., Tung Y.-C., Quinlan M., Wisniewski H. M., Binder L. I. Proc. Natl. Acad. Sci. USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ledesma M. D., Bonay P., Avila J. J. Neurochem. 1995;65:1658–1664. doi: 10.1046/j.1471-4159.1995.65041658.x. [DOI] [PubMed] [Google Scholar]
- 3.Novak M., Kabat J., Wischik C. M. EMBO J. 1993;12:365–370. doi: 10.1002/j.1460-2075.1993.tb05665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda A., Smith M. A., Avila J., Nunomura A., Siedlak S. L., Zhu X., Perry G., Sayre L. M. J. Neurochem. 2000;75:1234–1241. doi: 10.1046/j.1471-4159.2000.0751234.x. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson B. E., Blessed G, Roth M. J. Neurol. Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 6.Alafuzoff I., Iqbal K., Friden H., Adolfsson R., Winblad B. Acta Neuropathol. 1987;74:209–225. doi: 10.1007/BF00688184. [DOI] [PubMed] [Google Scholar]
- 7.Arriagada P. V., Growdon J. H., Hedley-Whyte E. T., Hyman B. T. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 8.Hutton M., Lendon C.L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., et al. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 9.Poorkaj P., Bird T. D., Wijsman E., Nemens E., Garruto R. M., Anderson L., Andreadis A., Wiederholt W. C., Raskind M., Schellenberg G. D. Ann. Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 10.Spillantini M. G., Murrell J. R., Goedert M., Farlow M. R., Klug A., Ghetti B. Proc. Natl. Acad. Sci. USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iqbal K., Grundke-Iqbal I., Zaidi T., Merz P. A., Wen G. Y., Shaikh S. S., Wisniewski H. M., Alafuzoff I., Winblad B. Lancet. 1986;2:421–426. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- 12.Bancher C., Brunner C., Lassmann H., Budka H., Jellinger K., Wiche G., Seitelberger F., Grundke-Iqbal I., Iqbal K., Wisniewski H. M. Brain Res. 1989;477:90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- 13.Köpke E., Tung Y.-C., Shaikh S., Alonso A. del C., Iqbal K., Grundke-Iqbal I. J. Biol. Chem. 1993;268:24374–24384. [PubMed] [Google Scholar]
- 14.Alonso A. del C., Zaidi T., Grundke-Iqbal I., Iqbal K. Proc. Natl. Acad. Sci. USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso A. del C., Grundke-Iqbal I., Iqbal K. Nat. Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 16.Alonso A. del C., Grundke-Iqbal I., Barra H. S., Iqbal K. Proc. Natl. Acad. Sci. USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso A. del C., Zaidi T., Novak M., Grundke-Iqbal I., Iqbal K. Proc. Natl. Acad. Sci. USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruben G. C., Ciardelli T. L., Grundke-Iqbal I., Iqbal K. Synapse. 1997;27:208–229. doi: 10.1002/(SICI)1098-2396(199711)27:3<208::AID-SYN7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Alonso A. del C., Zaidi T., Novak M., Grundke-Iqbal I., Barra H. S., Iqbal K. J. Biol. Chem. 2001;279:37967–37973. doi: 10.1074/jbc.M105365200. [DOI] [PubMed] [Google Scholar]
- 20.Alonso A. del C., Mederlyova A., Novak M., Grundke-Iqbal I., Iqbal K. J. Biol. Chem. 2004;279:34873–34881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- 21.Wang J.-Z., Gong C.-X., Zaidi T., Grundke-Iqbal I., Iqbal K. J. Biol. Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- 22.Wang J.-Z., Grundke-Iqbal I., Iqbal K. Brain Res. Mol. Brain Res. 1996;38:200–208. doi: 10.1016/0169-328x(95)00316-k. [DOI] [PubMed] [Google Scholar]
- 23.Goedert M. Prog. Brain Res. 1998;117:287–306. doi: 10.1016/s0079-6123(08)64022-4. [DOI] [PubMed] [Google Scholar]
- 24.Prusiner S. B. Proc. Natl. Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lansbury P. T., Jr., Kosik K. S. Chem. Biol. 2000;7:R9–R12. doi: 10.1016/s1074-5521(00)00068-5. [DOI] [PubMed] [Google Scholar]
- 26.Rochet J. C., Lansbury P. T., Jr. Curr. Opin. Struct. Biol. 2000;10:60–68. doi: 10.1016/s0959-440x(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 27.Wanker E. E. Biol. Chem. 2000;381:937–942. doi: 10.1515/BC.2000.114. [DOI] [PubMed] [Google Scholar]
- 28.Arrasate M., Mitra S., Schweitzer E. S., Segal M. R., Finkbeiner S. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 29.Weingarten M. D., Lockwood A. H., Hwo S.-Y., Kirschner M. W. Proc. Nat. Acad. Sci. USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y.-C., Zaidi M. S., Wisniewski H. M. J. Biol. Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 31.Iqbal K., Grundke-Iqbal I., Smith A. J., George L., Tung Y. C., Zaidi T. Proc. Natl. Acad. Sci. USA. 1989;86:5646–5650. doi: 10.1073/pnas.86.14.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goedert M., Spillantini M. G., Cairns N. J., Crowther R. A. Neuron. 1992;8:159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- 33.SantaCruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M., Guimaraes A., DeTure M., Ramsden M., McGowan E., et al. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andorfer C., Acker C. M., Kress Y., Hof P. R., Duff K., Davies P. J. Neurosci. 2005;25:5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cash A. D., Aliev G., Siedlak S. L., Nunomura A., Fujioka H., Zhu X., Raina A. K., Vinters H. V., Tabaton M., Johnson A. B., et al. Am. J. Pathol. 2003;162:1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khlistunova I., Biernat J., Wang Y., Pickhardt M., von Bergen M., Gazova Z., Mandelkow E., Mandelkow E. M. J. Biol. Chem. 2005;281:1205–1214. doi: 10.1074/jbc.M507753200. [DOI] [PubMed] [Google Scholar]
- 37.Perez M., Arrasate M., Montejo De Garcini E., Munoz V., Avila J. J. Neurochem. 1996;67:1183–1190. doi: 10.1046/j.1471-4159.1996.67031183.x. [DOI] [PubMed] [Google Scholar]
- 38.von Bergen M., Friedhoff P., Biernat J., Heberle J., Mandelkow E. M., Mandelkow E. Proc. Natl. Acad. Sci. USA. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez M., Arrasate M., Montejo De Garcini E., Munoz V., Avila J. Biochemistry. 2001;40:5983–5991. doi: 10.1021/bi002961w. [DOI] [PubMed] [Google Scholar]
- 40.Goldbaum O., Oppermann M., Handschuh M., Dabir D., Zhang B., Forman M. S., Trojanowski J. Q., Lee V. M.-Y., Richter-Landsberg C. J. Neurosci. 2003;23:8872–8880. doi: 10.1523/JNEUROSCI.23-26-08872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong C.-X., Singh T. J., Grundke-Iqbal I., Iqbal K. J. Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 42.Wittmann C. W., Wszolek M. F., Shulman J. M., Salvaterra P. M., Lewis J., Hutton M., Feany M. B. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 43.Singh T. J., Haque N., Grundke-Iqbal I., Iqbal K. FEBS Lett. 1995;358:267–272. doi: 10.1016/0014-5793(94)01445-7. [DOI] [PubMed] [Google Scholar]
- 44.Iqbal K., Zaidi T., Thompson C. H., Merz P. A., Wisniewski H. M. Acta Neuropathol. 1984;62:167–177. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- 45.Wisniewski H. M., Merz P.A., Iqbal K. J. Neuropathol. Exper. Neurol. 1984;43:643–656. doi: 10.1097/00005072-198411000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Kremer L., Dominguez J. E., Avila J. Anal. Biochem. 1988;175:91–95. doi: 10.1016/0003-2697(88)90365-x. [DOI] [PubMed] [Google Scholar]
- 47.Binder L. I., Frankfurter A., Rebhun L. I. J. Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang E., Szendrei G. I., Lee V. M., Otvos L., Jr. Biochem. Biophys. Res. Commun. 1992;187:783–790. doi: 10.1016/0006-291x(92)91264-q. [DOI] [PubMed] [Google Scholar]
- 49.Tatebayashi Y., Iqbal K., Grundke-Iqbal I. J. Neurosci. 1999;19:5245–5254. doi: 10.1523/JNEUROSCI.19-13-05245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso A. del C., Grundke-Iqbal I., Iqbal K. Brain Res. Mol. Brain Res. 1995;31:194–200. doi: 10.1016/0169-328x(95)00051-s. [DOI] [PubMed] [Google Scholar]
- 51.Grundke-Iqbal I., Vorbrodt A. W., Iqbal K., Tung Y.C., Wang G. P., Wisniewski H. M. Mol. Brain Res. 1988;4:43–52. doi: 10.1016/0169-328x(88)90017-4. [DOI] [PubMed] [Google Scholar]
- 52.Bensadoun A., Weinstein D. Anal. Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 53.Khatoon S., Grundke-Iqbal I., Iqbal K. J. Neurochem. 1992;59:750–753. doi: 10.1111/j.1471-4159.1992.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 54.Infante A. S., Stein M. S., Zhai Y., Borisy G. G., Gundersen G. G. J. Cell Sci. 2000;113:3907–3919. doi: 10.1242/jcs.113.22.3907. [DOI] [PubMed] [Google Scholar]
- 55.Black M. M., Slaughter T., Moshiach S., Obrocka M., Fischer I. J. Neurosci. 1996;16:3601–3619. doi: 10.1523/JNEUROSCI.16-11-03601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]