Abstract
Reflecting a paradigm shift in clinical neuroscience, many chronic psychiatric illnesses are now hypothesized to result from perturbed neural development. However, most work in this area focuses on schizophrenia. Here, we extend this paradigm to pediatric bipolar disorder (BD), thus demonstrating traction in the developmental psychobiology perspective. To study amygdala dysfunction, we examined neural mechanisms mediating face processing in 22 youths (mean age 14.21 ± 3.11 yr) with BD and 21 controls of comparable age, gender, and IQ. Event-related functional MRI compared neural activation when attention was directed to emotional aspects of faces (hostility, subjects’ fearfulness) vs. nonemotional aspects (nose width). Compared with controls, patients perceived greater hostility in neutral faces and reported more fear when viewing them. Also, compared with controls, patients had greater activation in the left amygdala, accumbens, putamen, and ventral prefrontal cortex when rating face hostility, and greater activation in the left amygdala and bilateral accumbens when rating their fear of the face. There were no between-group behavioral or neural differences in the nonemotional conditions. Results implicate deficient emotion–attention interactions in the pathophysiology of BD in youth and suggest that developmental psychobiology approaches to chronic mental illness have broad applicability.
Keywords: amygdala, faces, functional MRI
Recently, psychology and psychiatry have witnessed a major paradigm shift: virtually all chronic adult mental illnesses are now thought to result from long-term perturbations in neural development. Two lines of research support this perspective: family-based/longitudinal studies and neurobiological studies. Family-based and longitudinal studies implicate developmental perturbations in a range of conditions, including behavior disorders, substance abuse, mood disorders, and psychoses (1–3). However, virtually all research on developmental neurobiology focuses on schizophrenia, where data implicate a neural circuit connecting the dorsolateral prefrontal cortex, striatum, and hippocampus (4, 5). Neurocognitive correlates of schizophrenia, such as deficient working memory, are thought to result from dysfunction in this circuit (6). An important next step is the extension of the developmental neurobiological approach to other mental illnesses and other neural systems associated with information processing and emotion regulation.
For several reasons, bipolar disorder (BD) is an ideal illness in which to expand the emerging developmental paradigm by conducting neurobiologically oriented developmental research. BD causes marked disruption in social, academic, and family function. Major questions persist concerning the boundaries of the condition in children; neurobiological data might ultimately resolve them. Most importantly, research in adult patients and animals implicates a circuit encompassing the amygdala, striatum, and ventral prefrontal cortex (VPFC) in the pathophysiology of BD (7). This circuit has considerable developmental plasticity. The identification of amygdala-based perturbations in children with BD would have profound implications for developmental conceptualizations of chronic mental illness, because it would suggest that perturbations in neural development play a role in diverse mental illnesses, with specific circuits implicated in specific conditions.
Much available research implicates the amygdala in BD. Structural MRI (sMRI) studies in bipolar adults find either increased or unchanged amygdala volume relative to controls (8–10), whereas functional MRI (fMRI) studies find that adults with BD, relative to controls, have either amygdala hyperactivation (11, 37) or hypoactivation (12) in response to facial stimuli. In contrast to adult data, sMRI studies in bipolar children consistently document decreased amygdala volume in patients compared with controls (13–17). This greater consistency in data among children relative to adults raises essential questions about the role of amygdala development in the pathophysiology of BD and in the behavioral and cognitive deficits characteristic of the illness. Although the few fMRI studies in pediatric BD have not revealed functional abnormalities in the amygdala (18, 19), these studies have not used paradigms ideally suited for examining amygdala function.
This study documents the on-line occurrence of cognitive misperceptions in children with BD during face viewing, allowing us to elucidate information-processing perturbations instantiated in an amygdala-striatal-VPFC circuit (20, 21) thought to mediate both emotional face processing (22–25) and emotion regulation (26). These psychological processes are of interest because children with BD have difficulty categorizing facial emotions (27) and regulating both their attention (28) and their affect (29). These deficits may be related: children with BD may mislabel facial emotions because their affective response to a face disrupts emotion categorization; such mislabeling may contribute to inappropriate emotional responses to environmental stimuli, and thus to emotional dysregulation.
Given our previous data documenting face-processing deficits in children with BD (27), we expected to see behavioral and neurophysiological differences between BD youths and controls when they attended to emotional aspects of neutral faces (i.e., when they rated hostility of the face or their fear of the face), but not when they attended to nonemotional aspects of the faces (i.e., rating nose width). Specifically, we hypothesized that, during emotional but not nonemotional tasks, children with BD, compared with controls, would report more negative subjective ratings, have slower reaction times, and have greater activation in the amygdala, striatum, and VPFC.
Results
Participant Demographics and Clinical Characteristics.
Patients (n = 22) and controls (n = 21) did not differ on age (BD patients, 14.2 ± 3.1 yr; controls, 14.5 ± 2.5 yr), sex (BD males, 45.5%; controls, 52.4%), or IQ (BD patients, 109.3 ± 11.6; controls, 114.3 + 11.4) as measured with the Wechsler Abbreviated Scale of Intelligence (WASI) (30). There was a significantly greater proportion of Caucasians in the BD sample (χ2 = 11.92, P = 0.001). Clinically, 90.9% (n = 20) of patients met criteria for Bipolar I (61); 81.8% (n = 18) had at least one comorbid diagnosis (1.4 ± 1.1). Of all patients, 81.8% (n = 18) were medicated when scanned (2.5 ± 1.8 medications per subject). See Table 1, which is published as supporting information on the PNAS web site.
To evaluate current mood, clinicians administered the Children’s Depression Rating Scale (CDRS) (31) and Young Mania Rating Scale (YMRS) (32). General functioning was measured by using the Children’s Global Assessment Scale (CGAS) (33). The patients’ mean CDRS score was 29.2 ± 9.3, and the mean YMRS score was 9.0 ± 6.1. At the time of scanning, 54.5% (n = 12) of patients were euthymic (CDRS < 40; YMRS < 12). Four patients were depressed (CDRS, >40; YMRS, <12), and six were hypomanic (YMRS, >12 but <26; CDRS, <40). CGAS score (53.8 ± 14.3) indicated that the BD sample was moderately impaired.
Behavioral Data.
Ratings.
A repeated measures ANOVA comparing rating scores (hostility, afraid, nose width) between the two groups showed a significant group × ratings interaction [F(2,82) = 6.08, P = 0.008)]. Post hoc analyses found that patients, compared with controls, rated the neutral faces as significantly more hostile (BD patients, 2.00 ± 0.61; controls, 1.56 ± 0.39; t test value (t) = 2.80, P = 0.008) and themselves as significantly more afraid (BD patients, 2.02 ± 0.88; controls, 1.39 ± 0.38; t = 3.02, P = 0.004) (see Table 2, which is published as supporting information on the PNAS web site). There were no between-group differences on nose width ratings.
Reaction time (RT).
Repeated measures ANOVA of RT during ratings found a significant group × ratings interaction [F(2,40) = 11.36, P < 0.001)]. Post hoc analyses found that patients were significantly slower than controls to rate the faces’ hostility (BD patients, 2,203.59 ± 326.12 ms; controls, 1,754.90 ± 276.08 ms; t = 4.86, P < 0.001) (see Table 3, which is published as supporting information on the PNAS web site). There were no significant between-group differences in RT on afraid ratings or nose-width ratings.
fMRI Data.
Primary between-group contrasts.
The contrasts of interest compared activation during an emotional task (rating hostility on the face or one’s own fear) vs. a nonemotional task (rating nose width or viewing a fixation cross). Thus, the primary contrasts, all with neutral faces, were as follows: afraid rating vs. nose-width rating (“afraid vs. nose”), afraid rating vs. fixation (“afraid vs. fixation”), hostile rating vs. nose width rating (“hostile vs. nose”), and hostile rating vs. fixation (“hostile vs. fixation”) (see Table 4, which is published as supporting information on the PNAS web site).
Afraid vs. nose and afraid vs. fixation.
On the “afraid vs. nose” contrast, patients had significantly greater activation than controls in the left amygdala (t = 4.05, P = 0.001) and bilateral accumbens (left, t = 3.68, P = 0.003; right, t = 2.58, P = 0.037) (Fig. 1 and Table 4). On the afraid vs. fixation contrast, patients had significantly greater activation than controls in the bilateral amygdala (left, t = 3.40, P = 0.008; right, t = 3.03, P = 0.019), left accumbens (t = 3.04, P = 0.018), and left putamen (t = 3.45, P = 0.016) (Table 4).
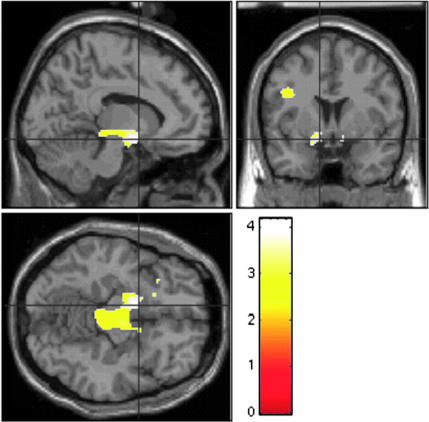
Fig. 1.
Greater neural activation in children with BD (n = 22) vs. controls (n = 21) when rating their fear of neutral faces. Figure displays voxels where patients exhibited greater activation than controls on the afraid vs. nose contrast for neutral faces. For visual presentation, the threshold is set at P < 0.01 uncorrected. Peak voxels are in the left amygdala (x = −14, y = −2, z = −10), left accumbens (x = −10, y = 4, z = −12), and right accumbens (x = 12, y = 4, z = −10). Figure displays, clockwise from lower left, axial, sagittal, and coronal views. Scale = t values of pairwise comparisons of BDs vs. controls.
Hostile vs. nose and hostile vs. fixation.
In the hostile vs. nose contrast, patients had significantly greater activation than controls in the left amygdala (t = 3.44, P = 0.006), left accumbens (t = 2.81, P = 0.025), left putamen (t = 3.13, P = 0.026), and left VPFC (t = 3.21, P = 0.032) (Fig. 2 and Table 4). In the hostile vs. fixation contrast, patients had significantly greater activation than controls in the bilateral amygdala (left, t = 3.22, P = 0.011; right, t = 2.93, P = 0.021) and bilateral putamen (left, t = 3.11, P = 0.029; right, t = 2.87, P = 0.049) (Table 4).
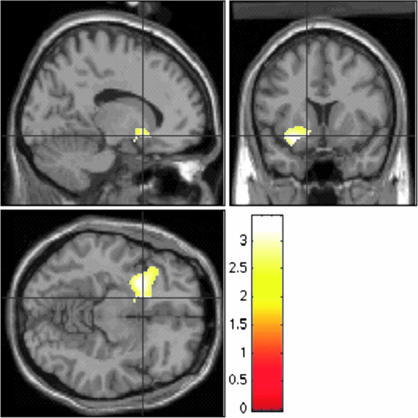
Fig. 2.
Greater neural activation in children with BD (n = 22) vs. controls (n = 21) when rating the hostility of neutral faces. Figure displays voxels where patients exhibited greater activation than controls on the hostile vs. nose contrast for neutral faces. For visual presentation, the threshold is set at P < 0.01 uncorrected. Peak voxels are in the left amygdala (x = −22, y = 4, z = −18), left accumbens (x = −14, y = 12, z = −10), left putamen (x = −24, y = 8, z = −10), and left VPFC (x = −32, y = 20, z = −16). Figure displays, clockwise from lower left, axial, sagittal, and coronal views. Scale = t values of pairwise comparisons of BDs vs. controls.
Post hoc contrasts.
Within-group contrasts on emotional tasks.
To confirm that the between-group differences reflected limbic hyperactivation during the emotional tasks in patients but not controls, we performed within-group comparisons. In patients, the afraid vs. nose contrast revealed significant activation in the left amygdala (t = 4.32, P = 0.001), left nucleus accumbens (t = 2.76, P = 0.028), and bilateral VPFC (left, t = 3.87, P = 0.007; right, t = 2.90, P = 0.05) (Table 5, which is published as supporting information on the PNAS web site). A similar analysis in controls revealed activation only in the right VPFC (t = 3.64, P = 0.01). In the hostile vs. nose contrast, patients had activation in the bilateral amygdala (left, t = 4.09, P = 0.001; right, t = 2.57, P = 0.04), left accumbens (t = 2.81, P = 0.025), left caudate (t = 2.98, P = 0.031), and left putamen (t = 2.98, P = 0.036) (Table 5). The same contrast in controls revealed no activation.
Between-group contrasts on nonemotional tasks.
To ascertain whether neural activation differed between the two groups on the nonemotional control tasks, we compared patients and controls on the nose width vs. fixation contrast and found no between-group differences.
Associations with Behavioral Data.
Covarying for RT and ratings.
Given group differences in RT on hostile vs. nose and hostile vs. fixation, follow-up contrasts used RT as a covariate. All between-group differences remained significant. Specifically, patients had significantly greater activation than controls in the left amygdala (t = 3.71, P = 0.003), accumbens (t = 2.54, P = 0.044), and putamen (t = 2.86, P = 0.047) on the hostile vs. nose contrast, and in the left amygdala (t = 2.47, P = 0.05) and putamen (t = 2.80, P = 0.05) in the hostile vs. fixation contrast.
Given group differences in ratings, follow-up contrasts used ratings as a covariate. All of the regions of interest (ROIs) that showed significant between-group differences in the afraid vs. nose and afraid vs. fixation contrasts remained significant when controlling for ratings. On the hostile vs. nose contrast, the left amygdala (t = 3.44, P = 0.006) and VPFC (t = 3.21, P = 0.032) remained significant when covaried for ratings, and, on the hostile vs. fixation contrast, the bilateral amygdala (left, t = 3.22, P = 0.011; right, t = 2.93, P = 0.021) difference remained significant.
Correlation of neural activation with ratings.
Because patients rated the faces as more hostile and fear-producing than did controls, for each group, we examined correlations between ratings and activation at the peak voxel of each ROI that had revealed significant between-group differences. In controls, there were no significant correlations between ratings and activation; in the patients, hostile ratings correlated with activation of the left amygdala on the hostile vs. nose rating (r = .51, P = 0.01). Between-group comparison of the correlation between left amygdala activation and the hostile vs. nose rating within each sample using a Fisher r-to-z calculation found the correlation in the BD sample to be significantly greater than that in controls (z = 2.32, P = 0.02) (Fig. 3, which is published as supporting information on the PNAS web site).
Relationships between mood, medication, comorbidity, and activation.
Given the heterogeneity of our patients with regard to mood status during scanning, comorbid diagnoses, and treatment, we conducted a series of ANOVAs and bivariate correlational analyses to compare activation within subgroups of the patients at the peak voxels in the ROIs. We found no differences in activation between euthymic and noneuthymic patients, those with and without comorbid anxiety disorders, or those with and without comorbid attention deficit hyperactivity disorder (ADHD). Also, there were no significant correlations between neural activation and current mood. Finally, we found no significant correlations between activation and the number, or classes, of medications patients were taking. Moreover, examination of fMRI response in the four medication-free subjects revealed similar response patterns to medicated subjects. However, given the small sample sizes in these post hoc analyses, one must interpret them cautiously.
Discussion
An emerging paradigm views chronic mental illnesses as developmental perturbations in specific neural circuits. Consistent with prior studies in adult BD, in pediatric BD, we found functional aberrations in an amygdala-striatal-VPFC circuit (34). We found hyperactivation of this circuit specifically when patients attended to the emotion that they perceive on a face, or to their emotional response to a face, but not when they attended to a nonemotional facial feature.
Because we acquired behavioral measures while scanning subjects, we were able to specify the context in which limbic dysfunction occurs in BD. Routinely acquiring behavioral measures in clinical fMRI studies, as is done in cognitive neuroscience studies on control subjects, will improve investigators’ ability to interpret neuroimaging data.
We expected to find between-group differences in behavior because we had found previously (28) that children with BD demonstrate aberrant behavior and neurophysiology in emotional, but not nonemotional, contexts. Clinical researchers have debated the pros and cons of using fMRI paradigms that yield differences in behavior between patients and controls (35, 36). We suggest that paradigms with between-group behavioral differences yield particularly informative neuroimaging results; to the extent that the behavioral differences are relevant to patients’ symptoms, group differences in neural activation may be relevant to the pathophysiology of those symptoms (35). Of course, it is important to use event-related designs and statistical corrections to ensure that behavioral differences do not confound the fMRI data interpretation.
When subjects’ attention was directed to emotional aspects of neutral faces, the faces elicited more negative attributions in patients than in controls, as well as more limbic activation. Moreover, the magnitude of the negative attribution predicted the degree of amygdala hyperactivation in patients, but not in controls. Neural hyperactivation in patients, compared with controls, was seen in the bilateral amygdala, accumbens, putamen, and left VPFC during emotional tasks (ratings of hostility or fearfulness). Our data provide particularly strong evidence implicating the left amygdala in pediatric BD, because between-group comparisons, within-group analyses, and correlations between blood oxygen level-dependent (BOLD) signal and behavioral data all found an association between increased left amygdala activity and negative perceptions of neutral faces in patients. Studies in bipolar adults (11, 37) have also found exaggerated amygdala response to faces. Furthermore, studies in controls indicate that fluctuating attentional task demands modulate amygdala activation while subjects process emotional stimuli (20, 38). Our results indicate that amygdala hyperactivation in pediatric BD may result from dysregulation of this emotion–attention mechanism, because patients engage the amygdala more than do controls only when attention is directed to the emotional components of a neutral stimulus.
Available data indicate that children with BD have deficient social skills (39) and difficulty labeling facial expressions (27). In the current study, youths with BD interpreted neutral facial expressions as being significantly more hostile and fear-inducing than did controls. Social competence requires proficient face processing, and the over-identification of anger on neutral faces is associated with affective aggression and irritability (40). Because the latter are common in pediatric BD, further study of face processing in these patients may help elucidate the causes of functional impairment and suggest potential therapeutic targets.
Our study is an initial step in specifying developmental hypotheses about BD pathophysiology. Longitudinal and family-based studies suggest that many chronic adult psychopathologies reflect the end result of developmental perturbations in psychobiology. In contrast to schizophrenia, where data implicate perturbations in dorsolateral prefrontal-hippocampal function, the current study implicates amygdala-striatal-VPFC circuitry in the pathophysiology of BD. To determine the neural origins of BD, it will be necessary to specify precisely the development of amygdala structure and function in healthy children and those with BD, and to compare amygdala structure and function in children and adults both with and without BD. The inconsistent literature concerning amygdala structure in adults with BD, compared with consistent findings of decreased amygdala volume in pediatric BD, suggest that amygdala dysfunction in BD may vary developmentally. Such developmental variation would be consistent with data in nonhuman primates suggesting that amygdala lesions produce markedly different effects on emotional processes in immature relative to mature organisms (41).
Our study is unable to determine the precise nature of the association between amygdala dysfunction and pediatric BD. Perturbations in amygdala-based circuits may cause BD, arising early in the course of the illness and mediating its progression. Alternatively, amygdala dysfunction could emerge after children develop BD, either as a manifestation of their illness or as a compensatory adaptation. Longitudinal studies of young nonaffected children at-risk for BD could disambiguate these possibilities, while also delineating the possible effects of intervening life factors, genotype, and medication use. In addition to face processing, neuroimaging paradigms could involve processing of other emotional stimuli, memory for emotional information, and the regulation of behavioral responses to emotional stimuli, all functions mediated by the amygdala (42–44). Finally, amygdala dysfunction may underlie a range of illnesses beyond BD, such as anxiety (45), depression (46), and autism (47). Additional studies in children with other psychopathologies and a larger normative sample will ascertain whether the limbic hyperactivation that we observed is specific to children with BD.
A primary limitation of our study is that, because most patients were medicated, we were unable to determine the extent to which our results were associated with BD or, instead, with medication effects. The relevant literature is limited; because of ethical limits on medication discontinuation, it is difficult to obtain data in unmedicated children with BD. Indeed, 3 of 4 fMRI and 16 of 17 structural MRI studies in children with BD include medicated patients (48–50). In adults with BD, one study found that, compared with unmedicated patients, medicated patients had increased dorsolateral prefontal cortex and anterior cingulate activation during a Stroop task (51). In contrast, two studies of medicated and unmedicated bipolar adults (52, 53) and one study in children with BD (54) found more marked differences in activation between unmedicated patients and controls than between medicated patients and controls, suggesting that medication may lead to type II, rather than type I, errors. Data in adults with BD (37) or depression (55) also suggest that lithium, antipsychotics, and antidepressants may normalize neural activation in response to facial expressions. Our post hoc analyses did not find associations between neural activation and medication, although these analyses were limited in power. Clearly, more studies are needed to differentiate abnormalities associated with BD from those associated with medication; such studies could include unmedicated patients or unmedicated children at risk for BD.
Another limitation is the high rate of comorbidity in our patients, although this rate is typical of that seen in other samples of children with BD (56). When we compared patients with and without comorbid anxiety or attention deficit hyperactivity disorder, we did not find between-group differences in neural activation. However, small sample sizes limit the interpretation of these negative results. Further, given that hyperactivation of the amygdala, along with attentional bias to emotional stimuli, has been demonstrated in other childhood mood disorders (45–47, 57, 58), it is important to determine whether the current results are specific to pediatric BD. As previously noted, we anticipate that different psychopathologies will be associated with dysfunction in different neural circuits.
The disparity in racial composition between our patient and control samples is another limitation. However, two factors lead us to believe that this difference is unlikely to have confounded our behavioral or neural results. First, our face stimuli were racially diverse and randomized across subjects. Second, the racial composition of the stimuli was closer to that of the patients than of the controls. Studies find that subjects have greater amygdala activation when they view faces of individuals from other races compared with individuals from their own race (59, 60). Therefore, one might expect the difference in racial composition between patients and controls in our sample to have increased amygdala activation in controls, relative to patients, whereas the effect that we found was in the opposite direction.
Finally, whereas half of our patients were euthymic, the other half were depressed or hypomanic. When we compared euthymic vs. noneuthymic patients, we did not find between-group differences in neural activation. Also, we did not find a relationship between illness severity and neural functioning. Again, caution should be used when interpreting these negative results because of the small sample sizes. Replication of our current results with a larger sample size will help to elucidate the impact of medication, comorbidity, and mood on the behavioral and neurological responses to faces in children with BD.
In sum, our results demonstrate abnormal emotion–attention interactions in pediatric BD that are associated with negative attributions to neutral faces and increased activation in the amygdala and ventral striatum. These findings and prior studies of adults with BD support the view of chronic adult mental disorders as the end result of developmental perturbations in specific neural circuits.
Materials and Methods
Inclusion/Exclusion Criteria.
BD (n = 22) and control (n = 21) subjects were recruited as described (28, 54). The National Institute of Mental Health (NIMH) Institutional Review Board approved the study. Parents and children gave written informed consent/assent. BD inclusion criteria required subjects ages 9–17 to meet DSM-IV (61) criteria for BD, based on the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL) (62); criteria required a history of at least one hypomanic or manic episode meeting full duration criteria (≥4 days), marked by elevated and expansive mood and at least three other criterion “B” symptoms (63). Control subjects and a parent also completed the K-SADS-PL to ensure that the subject had no psychiatric history.
Exclusion criteria included IQ < 70, pervasive developmental disorder, psychosis that interfered with study compliance, unstable medical illness, substance abuse within 2 months, and, for controls, psychiatric illness in a first-degree relative.
Behavioral Task.
Participants viewed 32 faces (8 happy, angry, fearful, and neutral) selected from standardized sets (ref. 64; www.uphs.upenn.edu/bbl/pubs/downloads/nptasks.shtml; www.macbrain.org/faces/index.htm). In this analysis, we included only neutral face trials because individuals with mood disorders tend to misperceive such faces as negative (65, 66) and to maximize our ability to detect between-group differences in emotion–attention interactions.
We used the rapid event related paradigm of Friston et al. (67) and Zarahn and Slifstein (68). On each trial, subjects used a button box (MRI Devices, Waukesha, WI) to rate the displayed face on one of three 5-point scales: (i) the threat-level of the face (“How hostile is the face?”); (ii) their fearful response to the face (“How afraid are you?”); or (iii) a nonemotional facial feature (“How wide is the nose?”). Fixation trials and a passive viewing condition were included as control trials and to facilitate data analysis. Each face or fixation cross was displayed for 4,000 ms, followed by a 750- to 1,250-ms intertrial interval. Visual stimuli were displayed on Avotec Silent Vision Glasses (Stuart, FL).
The experiment had four blocks: one for each of the three rating types, and one for passive viewing. Rating instructions were presented for 3,000 ms before each block. Each block comprised 10 trials (eight of the 32 faces, 2 fixations). Four blocks, one for each task, were grouped into one epoch, and four 40-trial epochs were integrated into one 160-trial run. Block and trial order were randomized.
MRI Data Acquisition.
Whole-brain fMRI data were acquired on a General Electric Signa 3T scanner. After localization and shimming, T2*-weighted images were acquired by using echo-planar single-shot gradient echo imaging [matrix = 64 × 64; repetition time (TR) = 2,000 ms; echo time (TE) = 40 ms; field of view (FOV) = 240 mm; 3.75 × 3.75 × 5 mm voxels; 23 contiguous 5-mm axial slices]. A high-resolution T1-weighted anatomical image was acquired to aid with spatial normalization [180 1-mm sagittal slices; FOV = 256; no. of excitations (NEX) = 1; TR = 11.4 ms, TE = 4.4 ms, matrix = 256 × 256; inversion time (TI) = 300 ms; bandwidth = 130 Hz per pixel, 22 kHz per 256 pixels].
fMRI Data Analysis.
Subjects moving >1.5 mm in any plane were discarded. Analyses were conducted with spm99 software (Wellcome Department of Neurology, University College London). Data were corrected for slice timing, motion corrected, coregistered to the anatomical data, and spatially normalized to T1-weighted template image supplied with spm99.
At the individual subject level, event-related response amplitudes were estimated by using the general linear model (GLM) for each of four event types: subjects rating afraid, hostility, or nose width during neutral face viewing, and fixation. The waveform in the GLM was a rectangular pulse (4-s duration) convolved with the hemodynamic response function specified by spm99. Contrast images were generated for each subject by using pairwise comparisons of the event-related blood oxygen level-dependent (BOLD) responses.
Each contrast image was divided by the subject-specific voxel time series means, yielding values proportional to percentage fMRI signal change (69). Each contrast image was then smoothed with an isotropic Gaussian kernel [full width at half maximum (FWHM) = 11.4] to decrease nonstationarity in the spatial autocorrelation structure introduced by the previous step.
For all group-level analyses, a random-effects model was used to permit population-level inferences (70). To test our a priori hypotheses, we used the Gaussian random field threshold (P < 0.05) in selected ROIs and applied the small volume correction within each region. The ROIs were bilateral amygdala, VPFC, and ventral striatum (i.e., accumbens, putamen, and caudate). Each was defined by using standard anatomical criteria (71) on the canonical structural MRI images provided by spm99 software and then applied to all normalized brains at the group level. Coordinates are in Montreal Neurological Institute (MNI) space. Secondary analyses covaried for RT and ratings in instances where neural activation differed significantly between patients and controls. Bivariate correlational analyses were used to examine associations between ratings and the magnitude of the blood oxygen level-dependent (BOLD) signal at peak voxels in those ROIs showing significant between-group differences.
Acknowledgments
We thank the children and families of patients and controls without whose participation this research would not have been possible. We also acknowledge the staff of the Mood and Anxiety Program at the National Institute of Mental Health (NIMH). This research was supported by the Intramural Research Program of NIMH, National Institutes of Health.
Abbreviations
- BD
bipolar disorder
- VPFC
ventral prefrontal cortex
- fMRI
functional MRI
- RT
reaction time
- ROI
region of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
Data deposition: The neuroimaging data have been deposited with the fMRI Data Center, www.fmridc.org (accession no. 2-2006-121MD).
References
- 1.Pine D. S., Cohen P., Gurley D., Brook J., Ma Y. Arch. Gen. Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 2.Weissman M. M., Warner V., Wickramaratne P., Moreau D., Olfson M. Arch. Gen. Psychiatry. 1997;54:932–940. doi: 10.1001/archpsyc.1997.01830220054009. [DOI] [PubMed] [Google Scholar]
- 3.Moffitt T. E. Psychol. Rev. 1993;100:674–701. [PubMed] [Google Scholar]
- 4.Lipska B. K., Weinberger D. R. Proc. Natl. Acad. Sci. USA. 1995;92:8906–8910. doi: 10.1073/pnas.92.19.8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keshavan M. S., Diwadkar V. A., Montrose D. M., Rajarethinam R., Sweeney J. A. Schizophr. Res. 2005;79:45–57. doi: 10.1016/j.schres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Keshavan M. S., Sujata M., Mehra A. Schizophr. Res. 2002;59:85–92. doi: 10.1016/s0920-9964(01)00400-5. [DOI] [PubMed] [Google Scholar]
- 7.Manji H. K., Lenox R. H. Biol. Psychiatry. 2000;48:518–530. doi: 10.1016/s0006-3223(00)00929-x. [DOI] [PubMed] [Google Scholar]
- 8.Strakowski S. M., Delbello M. P., Sax K. W., Zimmerman M. E., Shear P. K., Hawkins J. M., Larson E. R. Arch. Gen. Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 9.Altshuler L. L., Bartzokis G., Grieder T., Curran J., Jimenez T., Leight K., Wilkins J., Gerner R., Mintz J. Biol. Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- 10.Brambilla P., Harenski K., Nicoletti M., Sassi R. B., Mallinger A. G., Frank E., Kupfer D. J., Keshavan M. S., Soares J. C. J. Psychiatr. Res. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 11.Yurgelun-Todd D. A., Gruber S. A., Kanayama G., Killgore W. D., Baird A. A., Young A. D. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 12.Lennox B. R., Jacob R., Calder A. J., Lupson V., Bullmore E. T. Psychol. Med. 2004;34:795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- 13.Dickstein D. P., Milham M. P., Nugent A. C., Drevets W. C., Charney D. S., Pine D. S., Leibenluft E. Arch. Gen. Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg H. P., Kaufman J., Martin A., Whiteman R., Zhang J. H., Gore J. C., Charney D. S., Krystal J. H., Peterson B. S. Arch. Gen. Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 15.Delbello M. P., Zimmerman M. E., Mills N. P., Getz G. E., Strakowski S. M. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 16.Chang K., Karchemskiy A., Barnea-Goraly N., Garrett A., Simeonova D. I., Reiss A. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 17.Chen B. K., Sassi R., Axelson D., Hatch J. P., Sanches M., Nicoletti M., Brambilla P., Keshavan M. S., Ryan N. D., Birmaher B., et al. Biol. Psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Chang K., Adleman N. E., Dienes K., Simeonova D. I., Menon V., Reiss A. Arch. Gen. Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 19.Blumberg H. P., Martin A., Kaufman J., Leung H. C., Skudlarski P., Lacadie C., Fulbright R. K., Gore J. C., Charney D. S., Krystal J. H., et al. Am. J. Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 20.Monk C. S., McClure E. B., Nelson E. E., Zarahn E., Bilder R. M., Leibenluft E., Charney D. S., Ernst M., Pine D. S. NeuroImage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 21.McClure E. B., Monk C. S., Nelson E. E., Zarahn E., Leibenluft E., Bilder R. M., Charney D. S., Ernst M., Pine D. S. Biol. Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Blair R. J. Brain Cognit. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 23.Adolphs R., Tranel D. Neuropsychologia. 2003;41:1281–1289. doi: 10.1016/s0028-3932(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 24.Rolls E. T. Philos. Trans. R. Soc. London B Biol. Sci. 1996;351:1433–1443. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- 25.Phillips M. L., Drevets W. C., Rauch S. L., Lane R. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 26.Davidson R. J., Putnam K. M., Larson C. L. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 27.McClure E. B., Treland J. E., Snow J., Schmajuk M., Dickstein D. P., Towbin K. E., Charney D. S., Pine D. S., Leibenluft E. Am. J. Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 28.Rich B. A., Schmajuk M., Perez-Edgar K. E., Pine D. S., Fox N. A., Leibenluft E. Biol. Psychiatry. 2005;58:532–539. doi: 10.1016/j.biopsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Birmaher B., Axelson D., Strober M., Gill M. K., Valeri S., Chiappetta L., Ryan N., Leonard H., Hunt J., Iyengar S., et al. Arch. Gen. Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weschler D. Weschler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 31.Poznanski E. O., Grossman J. A., Buchsbaum Y., Banegas M., Freeman L., Gibbons R. J. Am. Acad. Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Young R. C., Biggs J. T., Ziegler V. E., Meyer D. A. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 33.Shaffer D., Gould M. S., Brasic J., Ambrosini P., Fisher P., Bird H., Aluwahlia S. Arch. Gen. Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 34.Blumberg H. P., Charney D. S., Krystal J. H. Semin. Clin. Neuropsychiatry. 2002;7:243–254. doi: 10.1053/scnp.2002.35220. [DOI] [PubMed] [Google Scholar]
- 35.Casey B. J. Science. 2002;296:1408–1409. doi: 10.1126/science.1072684. [DOI] [PubMed] [Google Scholar]
- 36.Nelson C. A., Bloom F. E., Cameron J. L., Amaral D., Dahl R. E., Pine D. Dev. Psychopathol. 2002;14:499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence N. S., Williams A. M., Surguladze S., Giampietro V., Brammer M. J., Andrew C., Frangou S., Ecker C., Phillips M. L. Biol. Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Pessoa L., Kastner S., Ungerleider L. G. Brain Res. Cogn Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 39.Geller B., Bolhofner K., Craney J. L., Williams M., Delbello M. P., Gundersen K. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Dodge K. A., Lansford J. E., Burks V. S., Bates J. E., Pettit G. S., Fontaine R., Price J. M. Child Dev. 2003;74:374–393. doi: 10.1111/1467-8624.7402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amaral D. G., Bauman M. D., Capitanio J. P., Lavenex P., Mason W. A., Mauldin-Jourdain M. L., Mendoza S. P. Neuropsychologia. 2003;41:517–522. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- 42.LeDoux J. E. The Emotional Brain. New York: Simon and Schuster; 1996. [Google Scholar]
- 43.Phillips M. L. Br. J. Psychiatry. 2003;182:190–192. doi: 10.1192/bjp.182.3.190. [DOI] [PubMed] [Google Scholar]
- 44.Calder A. J., Lawrence A. D., Young A. W. Nat. Rev. Neurosci. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- 45.Monk C. S., Nelson E., McClure E. B., Mogg K., Bradley B., Blair R. J., Chen G., Charney D. S., Ernst M., Pine D. S. Am. J. Psychiatry. 2006 doi: 10.1176/ajp.2006.163.6.1091. in press. [DOI] [PubMed] [Google Scholar]
- 46.Thomas K. M., Drevets W. C., Dahl R. E., Ryan N. D., Birmaher B., Eccard C. H., Axelson D., Whalen P. J., Casey B. J. Arch. Gen. Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 47.Baron-Cohen S., Ring H. A., Bullmore E. T., Wheelwright S., Ashwin C., Williams S. C. Neurosci. Biobehav. Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 48.Adler C. M., Delbello M. P., Mills N. P., Schmithorst V., Holland S., Strakowski S. M. Bipolar Disord. 2005;7:577–588. doi: 10.1111/j.1399-5618.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 49.Wilke M., Kowatch R. A., Delbello M. P., Mills N. P., Holland S. K. Psychiatry Res. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Pavuluri M. N., Birmaher B., Naylor M. W. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 51.Strakowski S. M., Adler C. M., Holland S. K., Mills N. P., Delbello M. P., Eliassen J. C. Am. J. Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- 52.Caligiuri M. P., Brown G. G., Meloy M. J., Eberson S. C., Kindermann S. S., Frank L. R., Zorrilla L. E., Lohr J. B. Psychiatry Res. 2003;123:171–182. doi: 10.1016/s0925-4927(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 53.Blumberg H. P., Donegan N. H., Sanislow C. A., Collins S., Lacadie C., Skudlarski P., Gueorguieva R., Fulbright R. K., McGlashan T. H., Gore J. C., et al. Psychopharmacology (Berlin) 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 54.Leibenluft E., Rich B. A., Vinton D., Nelson E., Fromm S., Berghorst L., Joshi P., Robb A., Schachar R., Dickstein D. P., et al. Am. J. Psychiatry. 2006 doi: 10.1176/ajp.2007.164.1.A52. in press. [DOI] [PubMed] [Google Scholar]
- 55.Sheline Y. I., Barch D. M., Donnelly J. M., Ollinger J. M., Snyder A. Z., Mintun M. A. Biol. Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 56.Biederman J., Faraone S. V., Chu M. P., Wozniak J. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:468–476. doi: 10.1097/00004583-199904000-00021. [DOI] [PubMed] [Google Scholar]
- 57.Thomas K. M., Drevets W. C., Whalen P. J., Eccard C. H., Dahl R. E., Ryan N. D., Casey B. J. Biol. Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 58.Pine D. S., Mogg K., Bradley B. P., Montgomery L., Monk C. S., McClure E., Guyer A. E., Ernst M., Charney D. S., Kaufman J. Am. J. Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- 59.Hart A. J., Whalen P. J., Shin L. M., McInerney S. C., Fischer H., Rauch S. L. NeuroReport. 2000;11:2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- 60.Phelps E. A., O’Connor K. J., Cunningham W. A., Funayama E. S., Gatenby J. C., Gore J. C., Banaji M. R. J. Cognit. Neurosci. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- 61.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 62.Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 63.Geller B., Zimerman B., Williams M., Delbello M. P., Bolhofner K., Craney J. L., Frazier J., Beringer L., Nickelsburg M. J. J. Child Adolesc. Psychopharmacol. 2002;12:11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- 64.Ekman P., Friesen W. V. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- 65.Gur R. E., Skolnick B. E., Gur R. C., Caroff S., Rieger W., Obrist W. D., Younkin D., Reivich M. Arch. Gen. Psychiatry. 1984;41:695–699. doi: 10.1001/archpsyc.1984.01790180065008. [DOI] [PubMed] [Google Scholar]
- 66.Donegan N. H., Sanislow C. A., Blumberg H. P., Fulbright R. K., Lacadie C., Skudlarski P., Gore J. C., Olson I. R., McGlashan T. H., Wexler B. E. Biol. Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- 67.Friston K. J., Fletcher P., Josephs O., Holmes A., Rugg M. D., Turner R. NeuroImage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 68.Zarahn E., Slifstein M. NeuroImage. 2001;14:768–779. doi: 10.1006/nimg.2001.0852. [DOI] [PubMed] [Google Scholar]
- 69.Zarahn E., Aguirre G. K., D’Esposito M. NeuroImage. 1997;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]
- 70.Holmes A. P., Friston K. J. NeuroImage. 1998;7:S754. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 71.Szeszko P. R., Robinson D., Alvir J. M., Bilder R. M., Lencz T., Ashtari M., Wu H., Bogerts B. Arch. Gen. Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]