Abstract
Background
Quercetin, the predominant flavonoid, has been reported to lower the risk of several cancers. This flavonoid found in onion, grapes, green vegetables, etc. has been shown to possess potent antiproliferative effects against various malignant cells. This study was designed to investigate its effects on insulin-like growth factors (IGFs) and their binding protein-3 (IGFBP-3) proteins secretion and also apoptosis induction in the human prostate cancer cell line, PC-3.
Methods
We evaluated the secretion of IGF-I, -II and IGFBP-3 in quercetin treated cells by immunoradiometric (IRMA) method. Apoptosis was studied in quercetin treated cells by TUNEL and DNA fragmentation. Protein expressions of Bcl-2, Bcl-xL, Bax and caspase-3 were studied by western blot.
Results
At a dose of 100 μM concentration, we observed increased IGFBP-3 accumulation in PC-3 cells conditioned medium with a dose dependent increase with 2 fold over a base line, and significantly reduced the both IGF-I and IGF-II levels. Apoptosis induction was also confirmed by TUNEL assay. Bcl-2 and Bcl-xL protein expressions were significantly decreased and Bax and caspase-3 were increased.
Conclusion
These results suggest that the decreased level of IGFs could be due to the increased levels of IGFBP-3, because of the high binding affinity towards IGFs, thereby decreasing the cell proliferation. The increased level of IGFBP-3 was associated with increased pro-apoptotic proteins and apoptosis in response to quercetin, suggesting it may be a p53-independent effector of apoptosis in prostate cancer cells.
Background
Much attention has focused on the growth hormones antagonists from the natural sources for the treatment of prostate cancer are being developed to block the activity of IGFs or to promote the activity of IGFBP-3; these agents may offer additional ways of stimulating apoptosis in malignant cancer cells. Quercetin, a flavonoid found in onion, grapes, green vegetables, etc. has been shown to possess potent antiproliferative effects against various malignant cells [1-3]. Recent report indicated that quercetin inhibited the proliferation of rat prostate cancer cells by interacting with IGF-I signaling pathway [4]. IGFBP-3 is a member of a family of specific high affinity binding proteins that modify the mitogenic actions of IGFs by regulating their access to the IGF-I receptor (IGFRI). There is still some ambiguity in determining the role of IGFBP-3 in regulating IGF actions, with studies showing both enhancement and inhibition of IGFs mitogenic effects [5,6]. However, IGFBP-3 has been shown to have IGFRI-independent anti-proliferative and proapoptotic effects. IGFBP-3 is expressed in many tissues including prostate [7] and others have demonstrated its growth inhibitory effects in human prostate cancer cells [8,9] as well as in IGFRI-null fibroblasts [10]. More recently, it has been suggested that the anti-proliferative effects of IGFBP-3 may be mediated via an induction of apoptosis. Indirect evidence has come from reports that an increase in IGFBP-3 expression is associated with the induction of apoptosis [11-15]. High levels of recombinant nonglycosylated IGFBP-3 can induce apoptosis in MCF-7 breast carcinoma cells, although under some conditions these levels of exogenous IGFBP-3 may induce apoptosis indirectly by sequestering anti-apoptotic IGFs from the IGFRI [14]. However, no induction of apoptosis was observed in Hs578T breast carcinoma cells exposed to exogenous IGFBP-3 alone [16], although in this study IGFBP-3 augmented the cellular response to apoptotic stimuli. Studies by Rajah et al [12] in prostate carcinoma cells and IGFRI-negative mouse fibroblasts showed an induction of apoptosis by both exogenous and endogenous IGFBP-3 and provide the strongest evidence of an IGFRI-independent pro-apoptotic role for IGFBP-3 in cancer cell growth. IGFBP-3 production was induced by potent growth inhibitory and pro-apoptotic agents including transforming growth factor β1 [TGF-β1; 14], retinoic acid [17], and tumor necrosis factor-a [18] with evidence that these agents mediate their cellular effects through IGFBP-3. Furthermore, IGFBP-3 is an established target of the tumor suppressor p53 [19,20].
The Bcl-2 family has been identified as an important regulator of mammalian cell death with both anti-apoptotic (e.g. Bcl-2 and Bcl-xL) and pro-apoptotic (e.g. Bax and Bad) members [21]. Members of this family regulate susceptibility to apoptosis, whereby over expression of Bcl-2 or Bcl-xL in relation to Bax promotes survival, but over expression of Bax accelerates cell death. Bax over expression in prostate cancer cells leads to induction of apoptosis [22]. These Bcl-2 related proteins play a role in the cell proliferation and apoptosis of prostate cancer [23], suggesting changes in the ratio of Bcl-2-related proteins may be an important determinant in the response of prostate cancer cells to apoptotic stimuli. Our previous study showed that quercetin reduce, IGF-I protein secretion in conditioned media, while IGFBP-3 was induced in prostate cancer cells [24]. In this study, the effect of quercetin on the induction of apoptosis in human prostate cancer cells, which is a p53 negative cell line, was examined.
As quercetin is more readily consumed worldwide, it is worth investigating the effects of quercetin on prostate cancer. While, IGFs are capable of enhancing, proliferation and inhibiting apoptosis in normal and malignant prostate epithelial cells by autocrine/paracrine actions, IGFBP-3 is found to be pro apoptotic. So the present study was aimed to investigate the effect of quercetin on IGFs and IGFBP-3 secretion in PC-3 cells.
Materials and methods
Minimum Essential Medium (MEM), Fetal Bovine Serum (FBS), Trypan Blue and quercetin were purchased from Sigma Chemical Co., USA. Thymidine [3H] was purchased from BRIT, Mumbai. Other chemicals were obtained from Sisco Research Laboratories (SRL), India. All the chemicals used were extra pure and were of culture grade. The androgen-independent prostatic carcinoma PC-3 cell line was procured from National Center for Cell Science (NCCS), Pune, India. Quercetin was dissolved in DMSO. DMSO in culture media never exceeded 0.1% (v/v), the concentration known not to affect the cell proliferation and IGFBP-3 production. Cell viability was tested by Trypan Blue exclusion method. PC-3 cells were plated at 1 × 105 cells per well in 12-well plates in MEM containing 5% FBS. The growth inhibitory effect of quercetin was studied using Thymidine [3H] incorporation. For IGF-I, -II and IGFBP-3 assays, 1 × 106 cells were treated with quercetin (25, 50, 75 and 100 μM) in conditioned medium containing 0.1% Bovine serum albumin for 24 h and 48 h. The doses were selected based on of our previous studies [24].
Cell proliferation
Cell proliferation was assessed by Thymidine incorporation method [25]. During the final 4th h of quercetin treatment, 1 μCi/well [3H] thymidine-containing medium was added and incubated. Monolayers were rinsed twice with ice-cold saline and fixed with 1 ml/well ice-cold methanol-acetic acid mixture at 4°C for a minimum of 2 h. Cells were solubilized in 0.5 ml of SDS and 250 μl of each lysate was mixed with the scintillation fluid, before counting.
Analysis of conditioned media for IGF-I, -II and IGFBP-3 secretion
IGF-I, -II and IGFBP-3 secretion in conditioned media of control and quercetin treated PC-3 cells were quantitated by immunoradiometrically (IRMA). Cells were seeded at 1 × 106 cells in each petri dishes and treated with quercetin for 24 h and 48 h. Conditioned media were collected 24 h and 48 h later and analyzed for IGF-I, -II and IGFBP-3. The results were expressed as ng/ml.
Western blotting of Bcl-2, Bcl-xL, Bax and caspase-3
Cells were plated on 75 cm2 flasks at density of 2 × 106 cells per flask, allowed to attach overnight and then exposed to quercetin for 24 h. Control cells were exposed to DMSO for 24 h. After the cells were washed three times with 33ice-cold PBS, then total proteins were extracted by adding 200 μl of cold lysis buffer (50 mM Tris-HCl, pH 7.4; 1 mM sodium Fluoride (NaF); 150 mM NaCl; 1 mM EDTA; 1 mM phenylmethylsulphonyl fluoride (PMSF) and 10 mg/ml leupeptin) to the cell pellets. After 30 min on ice the cell debris was pelleted by centrifugation at 10 000 × g for 30 min at 4°C. The proteins were quantified by Lowry's method [26]. The samples (50μg of total protein) were mixed with 5X sample buffer, containing 0.3 M Tris-HCl (pH 6.8), 25% 2-mercaptoethanol, 12% sodiumdodecyl sulphate (SDS), 25 mM EDTA, 20% glycerol, and 0.1% bromophenol blue. The mixtures were boiled at 95°C for 5 min, and subjected to 10–15% SDS-PAGE (polyacrylamide gel electrophoresis) at a constant current of 20 mA. Following electrophoresis, proteins on the gel were electro-transferred onto a PVDF membrane (Sigma, U.S.A.) in transfer buffer composed of 25 mM Tris-HCl (pH 8.9), 192 mM glycine and 20% methanol. The membranes were blocked with 20 mM Tris-HCl (pH 7.4), 125 mM NaCl, 0.2% Tween 20, 3% bovine serum albumin (BSA), and 0.1% sodium azide. The membranes were then immunoblotted with mouse monoclonal anti-human Bcl-2, Bcl-xL, Bax, caspase-3 and β-actin antibodies (generous gift from Dr. Ion V. Deaciuc, University of Louisville, Kentucky, USA). The membranes were washed and were incubated with horseradish peroxidase-labelled antimouse Rabbit IgG antibody at a dilution of 1:1000. The bands were developed using ECL kit (Perkin Elmer, USA) and density of the bands was detected with scanning densitometry.
Flowcytometry analysis
Cells were plated at 1 × 106 cells in a 25 cm2 flasks for 24 h. Cells were then washed with fresh media and treated with 50 and 100 μM concentration of quercetin. 24 h post-treatment, cells were trypsinized, and aliquots of 1 × 106 cells were suspended in 1 ml of fluorochrome solution (50 mg/ml propidium iodide, 1 mg/ml RNase A, 1.5% Triton X–100) for at least 1 h in the dark at 4°C. Cell cycle analysis was performed using a Beckman Vantage flow cytometer.
DNA fragmentation
After 24 h and 48 h of treatment, the DNA was extracted from the cell lysate as follows. Both attached and floating cells were collected, washed with PBS and centrifuged at 1500 × g for 5 min to collect the cell pellet, which was resuspended in 0.5 ml of lysis buffer, transferred to a microfuge tube and incubated for 1 h at 37°C. To this, 4 μl of proteinase K (1 mg/ml) was added and the tubes were incubated at 50°C for 3 h. To each tube, 0.5 ml of phenol-chloroform-isoamyl alcohol (25: 24: 1) was added, mixed and centrifuged at 10,000 × g for 1 min to separate the DNA containing upper aqueous phase. To the resulting aqueous phase, 2 vol of ice-cold absolute ethanol and 1/10 vol of 3 M sodium acetate were added and incubated for 30 min on ice to precipitate DNA. DNA was pelleted by centrifuging at 10, 000 × g for 10 minutes at 4°C, the supernatant was aspirated and the pellet was washed with 1 ml of 70% ethanol. Then the DNA was quantified by UV-visible spectroscopy and electrophoresed in a 1.2% agarose gel containing ethidium bromide.
TUNEL
DNA strand breaks in apoptotic cells were measured by terminal deoxynucleotidyl transferase mediated biotinylated UTP nick end-labeling (TUNEL) using an in situ cell death detection kit from Roche Molecular Biochemicals, Germany. Treated cells were harvested and fixed with 4% paraformaldehyde solution and incubated in a 0.1% Triton permeabilization solution on ice according to the manufacturer's instructions. Cells incubated with the solution without the terminal transferase was used as a negative control.
Statistical analysis
The data were analyzed using the SPSS 7.5 Windows Students version software. For all the measurements, one-way ANOVA followed by Student's Newman Keuls (SNK) test was used to assess the statistical significance of difference between control and quercetin-treated. A statistically significant difference was considered to be at P < 0.05.
Results
Cell proliferation
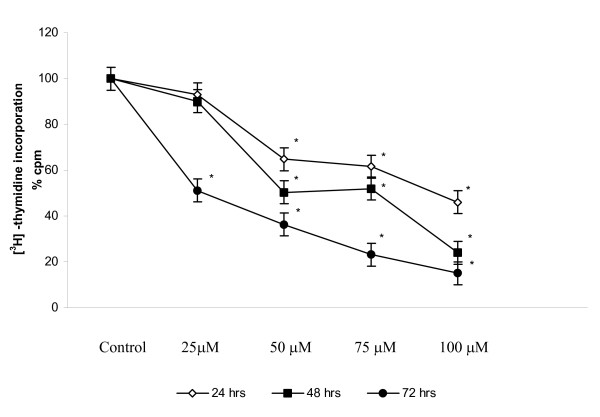
PC-3 cells showed a significant decrease in thymidine [3H] uptake. Fig.1 shows the kinetics of proliferation upto 0 – 72 h quercetin treatments, during which the thymidine uptake was decreased between 3- and 4- fold in quercetin treated cells. Time response data demonstrate 50% growth inhibition at 100 μM for 24 h.
Figure 1.
Effect of quercetin on PC-3 cell proliferation. 3 × 103 cells were plated in 24-well plates. After reaching 70–80% confluence, in the presence of 5% FBS, cells were treated with vehicle or with various concentrations of quercetin (25, 50, 75, and 100 μM) for 24 h, 48 h and 72 h. Proliferation of cells was quantitated by [3H] thymidine incorporation. Values represents % of control. Experiments were performed in triplicate, and SD was less than 10%. denotes the statistical significance at P < 0.05 level between control and quercetin treated using Student's Newman Keuls (SNK).
IGF-I, -II and IGFBP-3 level
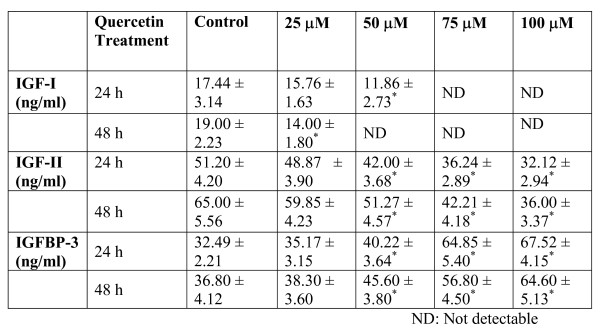
After treatment of quercetin, the secretions of IGF-I,-II and IGFBP-3 levels were measured in conditioned media by immunoradiometric method. The secretion of IGF-I was significantly reduced in conditioned media of quercetin treated PC-3 cells. The IGF-II level was also significantly reduced in conditioned media of quercetin treated PC-3 cells. Interestingly, the secretion of IGFBP-3 by quercetin treated PC-3 cells was significantly increased in quercetin treated conditioned medium of both 24 h and 48 h treated experiments (Table-1).
Protein expression of Bcl-2, Bcl-xL, Bax and caspase-3
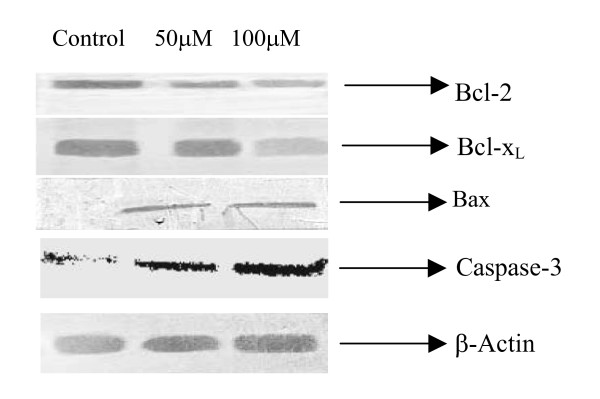
To investigate a possible intracellular mechanism for the observed increase in apoptosis, we examined the protein expression of the apoptotic intermediates of the Bcl-2 family. Quercetin treated cells were analyzed for protein expression of pro-apoptotic Bax and caspase-3, and anti-apoptotic Bcl-2 and Bcl-xL proteins by Western blotting with specific antibodies. Protein levels were quantitated by densitometry and expressed as a percentage of the control. As shown in Fig. 2, a strong 21-kDa band representing Bax protein was seen in quercetin treated lysate, compared with a control. We then examined the Bcl-2 and Bcl-xL proteins expression in quercetin treated prostate cancer cells, we observed significant decrease of Bcl-2 and Bcl-xL proteins (Fig. 2). Western blot analysis was also performed to analyse caspase-3 (active fragment). As shown in figure 2, quercetin increased the levels of caspase-3, suggesting the possible involvement of caspase-3 activation as one of the possible mechanism of apoptosis induction.
Figure 2.
Effects of quercetin on Bcl-2, Bcl-xL, Bax and caspase-3 proteins expression in prostate cancer cell line (PC-3). PC-3 cells were treated with indicated concentrations of quercetin for 24 h. Cell lysates were analyzed by Western blotting. Blots were incubated with anti-Bcl-2, anti- Bcl-xL, Bax, caspase-3 and anti-β actin antibodies.
Apoptosis
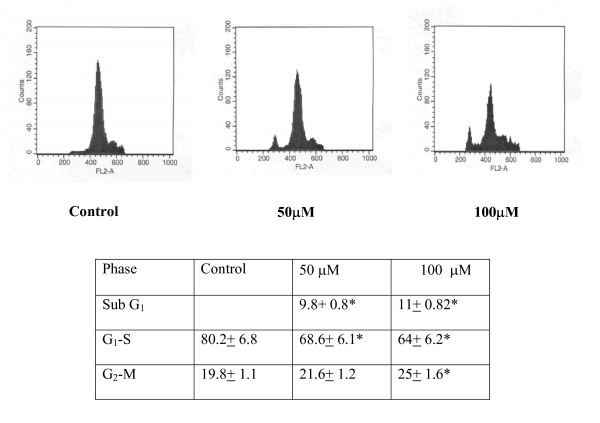
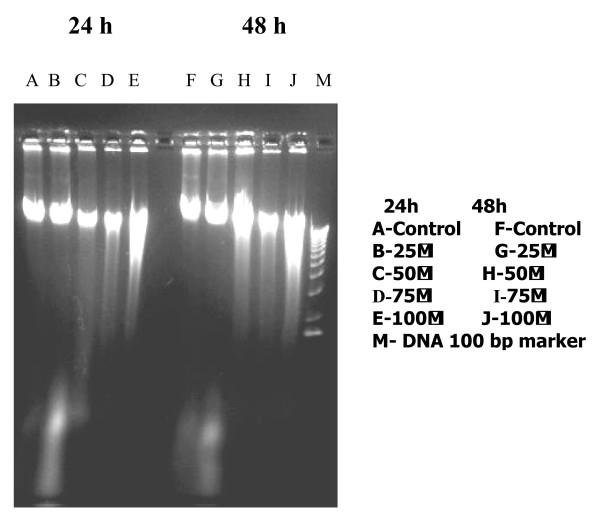
To analyze the mechanism responsible for the growth inhibition of PC-3 cells by quercetin, the effects of quercetin on programmed cell death was examined. A concomitant increase of cells in Sub G1-phase was observed in quercetin treated cells, indicating induction of apoptosis (Fig. 3). The sub G1 phase showed significant increase in the cell number in quercetin treated when compared with control. Significant decrease in the proportions of cells in G and S phases was evident with increase in G2 M phase. DNA agarose electrophoresis for quercetin treated cells showed quercetin treatment caused DNA fragmentation in the PC-3 cells (Fig. 4). Quantitative apoptotic cell death was performed to confirm the induction of apoptosis. Quercetin-caused apoptotic death of PC-3 cells was analysed by TUNEL assay, which showed that 50 and 100 μM treatment of quercetin for 24 h significantly (P < 0.05) increased the percentage of apoptotic cells up to 10 fold compared with that of control (Fig. 5).
Figure 3.
Effects of quercetin on cell cycle distribution in PC-3 cells. PC-3 cells were treated with quercetin at doses of 50 and 100 μM for 24 h in a 25 cm2 flasks. After treatment 1 × 106 cells were trypsinized and suspended in 1 ml of fluorochrome solution (50 mg/ml propidium iodide, 1 mg/ml RNase A, 1.5% Triton X-100) for at least 1 h in the dark at 4°C. Cell cycle analysis was performed using a Beckman Vantage flow cytometer, and quantitation of cell cycle distribution was performed using Multicycle software (Phoenix Flow Systems, San Diego, CA). Significant decrease in the proportions of cells in G1-S and increase in G2-M-phase, were evident after exposure to quercetin for 24 h. A concomitant increase of cells in SubG1-phase was observed in quercetin treated cells, indicating induction of apoptosis. The experiments were repeated thrice, *-denotes the statistical significance at P < 0.05 level between control and quercetin treatment groups using Student's Newman Keuls (SNK) test.
Figure 4.
A photograph of an UV light-illuminated agarose gel containing total cellular DNA; lanes were loaded with DNA preparations made from 24 h and 48 h quercetin treated PC-3 cells with different concentrations.
Figure 5.
Effect of quercetin on induction of apoptosis in PC-3 cells. The percentage of apoptotic cells was determined and summarized. Generation of free 3'-OH DNA fragments was determined by TUNEL assay. Each value is mean ± SEM. Experiments were carried out in triplicates. *-represents statistical significance between control Vs quercetin treatment groups at P < 0.05 level using Student Newman-Keuls (SNK) test.
Discussion
The insulin like growth factors (IGFs) plays an increasingly recognized role in the autocrine/paracrine regulation of cancer cells. There is now accumulating evidence that IGFBP-3 has an important anti-proliferative and pro-apoptotic role in the regulation of cancer cell growth [27]. However, although it has been described, as an effector of anticancer cancer agents-induced apoptosis, moreover there are no reports available in the role IGFBP-3 in quercetin-induced apoptosisin prostate cancer cells. This study examined the effects of quercetin on the induction of apoptosis and possible mediating role of IGFBP-3. The increased endogenous production ofIGFBP-3 by quercetin was associated with the induction of apoptosis in this human prostate cancer cell line. This induction was observed under serum-free conditions, suggesting possible pro-apoptotic role of IGFBP-3 in this cells. There are reports suggesting that IGFBP-3 as effector of apoptosis inducer in PC-3 cells [7,8,12]. This effect was observed in cells not expressing functional p53 protein, so it was independent of p53. This supports the work by Rajah et al. [13] who demonstrated the proapoptotic function of IGFBP-3 in p53 negative prostate carcinoma cells (PC-3) as well as IGFRI deficient mouse fibroblasts.
The induction of apoptosis by quercetin was associated with changes in both pro-apoptotic and anti-apoptotic members of the Bcl-2 family as well as caspase-3. The ratio of pro-apoptotic Bax protein to anti-apoptotic proteins, Bcl-2 and Bcl-xL is a crucial determinant of both cellular susceptibility to apoptosis [21]. Normal prostate epithelium expresses both Bax and Bcl-2 [22,23], the levels of which may be regulated by androgen and estrogen and other factors. IGFBP-3 expression might be one of the modulator of the ratio of apoptotic proteins by up-regulating the pro-apoptotic protein, Bax and down-regulating Bcl-2 and Bcl-xL proteins in quercetin treated PC-3 cells. There is evidence that anti-apoptotic signaling through the IGFRI is associated with changes in expression of Bcl-2 and Bcl-xL [28,29].
By autocrine/paracrine actions IGFs can increase the cell turnover and the susceptibility of malignant cells. It was already reported that IGFs inhibits the programmed cell death [30]. Experiments using animal and cell culture have shown that the antiapoptotic activity of IGF-I is counterbalanced by the activity of IGFBP-3, which may have a direct and independent-stimulating action on apoptosis [31]. The predictive value of IGF-I may be useful in screening for cancer. For example, the ratio of IGF-I to prostate specific antigen may be a better predictor of the development of prostate cancer than the antigen alone [32].
Recent evidences suggest that silibinin, a commonly occurring flavonoid can inhibit the IGF-I secretions, and increase the level of IGFBP-3 and induce of apoptosis in both in vivo and in vitro model of prostate cancer [33,34]. IGF-I interacts with IGF-IR to stimulate cell growth. Insulin receptor substrate-I (IRS-I) is a key signaling molecule activated by IGFs. Increased IRS-I tyrosine phosphorylation by IGFs is directly correlated with increased activation of the downstream effector molecules phosphatidylinositol 3' kinase and mitogen activated protein kinase, and the growth response to IGFs is mediated by these pathways [35,36]. It was reported that IGFBP-3 reduces the IRS-I tyrosine phorphorylation [34]. The present results demonstrate that quercetin induces IGFBP-3 secretions and that IGFBP-3 reduces the amount of available ligands (IGFs) available for the interaction of IGF-IR. Thus it appears that the antiproliferative action of quercetin in androgen- independent PC-3 cells involves increased IGFBP-3 production and induction of apoptosis. Furthermore, quercetin also deserves study in the context of prostate cancer preventions, because others have recently shown in populations studies that higher circulating IGFs level and/or lowers IGFBP-3 levels are associated with increased risk of prostate cancer [37,38]. It is relevant that the quercetin shown to be nontoxic in humans includes the possibility that this compound may be useful in prostate cancer treatment. There is also evidence that IGFBP-3 can induce apoptosis by itself, as well as potentiate the apoptotic effects of other factors such as ionizing and UV irradiation and chemotherapeutic agents [39-41].
Hence, the present study demonstrates that quercetin is capable of mediating growth inhibition against prostate cancer cells in vitro and is pro-apoptotic. As quercetin is able to induce apoptosis in PC-3 cells, the present findings suggest that quercetin-induced apoptosis could be mediated by the down regulation of IGFs and up regulation of IGFBP-3 in PC-3 cells. Therefore, quercetin can be considered for the evaluation of their in vivo or clinical efficacy against prostate cancer.
Conclusion
This data raise the possibility that IGFBP-3 is the positive regulator of quercetin-induced apoptosis in PC-3 cells as IGFBP-3 is reported to induce apoptosis in various cancer cell lines. The increased level of IGFBP-3 was associated with increased pro-apoptotic proteins and apoptosis in response to quercetin, suggesting it may be mediated via its modulation of the Bax: Bcl-2 protein ratio and hypothesize that IGFBP-3 act as an effector of quercetin induced-apoptosis in prostate cancer cells. The role of IGFBP-3 as an effector of p53-independent apoptotic pathways has particular relevance in the treatment of prostate cancer, where inactivating mutations in the p53 gene occur at high frequency. Hence our data raise the possibility that IGFBP-3 is the positive regulator of quercetin-induced apoptosis and quercetin may have some therapeutic value for prostate cancer.
Note
Table 1 Effect of quercetin on IGF-I, -II and IGFBP-3 secretion by the PC-3 cells. Cells were seeded at a density of 1 × 106/cm2 in 100 mm petri dishes and the medium then replaced with quercetin containing medium. After 24 h and 48 h, media were collected and quantified for IGF-I, -II and IGFBP-3. The results shown are those for five separate experiments. *-denotes the statistical significance at P < 0.05 level between control and quercetin treatment groups using Student's Newman Keuls (SNK) test. ND – Not detectable.
Acknowledgments
Acknowledgements
We would like to thank Dr. Ion V. Deaciuc, (University of Louisville, Kentucky) for Bcl-2 and Bax antibodies. This study was supported by the Council of Scientific and Industrial Research (CSIR), India, grant no. 9/115 (633)/2005-EMR-I dt. 21-3-2005 in the form of Senior Research Fellowship to Mr. M. R. Vijayababu is gratefully acknowledged. The financial assistance by Department of Science and Technology (DST-FIST) is also acknowledged.
Contributor Information
Marati R Vijayababu, Email: vijaybabu77@yahoo.co.in.
A Arunkumar, Email: ta_arun@hotmail.com.
P Kanagaraj, Email: aimskanagaraj@yahoo.co.in.
J Arunakaran, Email: j_arunakaran@hotmail.com.
References
- Kefford JF. Citrus fruits and processed citrus products in human nutrition. World Rev Nutr Diet. 1996;6:197–249. doi: 10.1159/000391426. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yarnmoto M, Nikaido T. Quercetin arrest leukemic T-cells in late G1 phase of the cell cycle. Cancer Res. 1992;52:6676–6681. [PubMed] [Google Scholar]
- Csokay B, Prajda N, Weber G, Olah E. Molecular mechanisms in the antiproliferative action of quercetin. Life Sci. 1997;60:2157–2163. doi: 10.1016/S0024-3205(97)00230-0. [DOI] [PubMed] [Google Scholar]
- Wang S, DeGroff VL, Clinton SK. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J Nutr. 2003;133:2367–2376. doi: 10.1093/jn/133.7.2367. [DOI] [PubMed] [Google Scholar]
- De Mellow JS, Baxter RC. Growth hormone-dependent insulin-like growth factor (IGF) binding protein both inhibits and potentiates IGF-I-stimulated DNA synthesis in human skin fibroblasts. Biochem Biophys Res Commun. 1988;156:199–204. doi: 10.1016/S0006-291X(88)80824-6. [DOI] [PubMed] [Google Scholar]
- Conover CA. Potentiation of insulin-like growth factor (IGF) action by IGF-binding protein-3: studies of underlying mechanism. Endocrinology. 1992;130:3191–3199. doi: 10.1210/en.130.6.3191. [DOI] [PubMed] [Google Scholar]
- Kaicer E, Blat C, Harel L. IGF-I and IGF-binding proteins: stimulatory and inhibitory factors secreted by human prostatic adenocarcinoma cells. Growth Factors. 1991;4:231–237. doi: 10.3109/08977199109104819. [DOI] [PubMed] [Google Scholar]
- Rajah R, Khare A, Lee PD, Cohen P. Insulin-like growth factor-binding protein-3 is partially responsible for high-serum-induced apoptosis in PC-3 prostate cancer cells. J Endocrinol. 1999;163:487–494. doi: 10.1677/joe.0.1630487. [DOI] [PubMed] [Google Scholar]
- Hong J, Zhang G, Dong F, Rechler MM. Insulin-like growth factor (IGF)-binding protein-3 mutants that do not bind IGF-I or IGF-II stimulate apoptosis in human prostate cancer cells. J Biol Chem. 2002;277:10489–10497. doi: 10.1074/jbc.M109604200. [DOI] [PubMed] [Google Scholar]
- Valentinis B, Bhala A, DeAngelis T, Baserga R, Cohen P. The human insulin-like growth factor (IGF) binding protein-3 inhibits the growth of fibroblasts with a targeted disruption of the IGF-I receptor gene. Mol Endocrinol. 1995;9:361–367. doi: 10.1210/me.9.3.361. [DOI] [PubMed] [Google Scholar]
- Rajah R, Lee KW, Cohen P. Insulin-like growth factor binding protein-3 mediates tumor necrosis factor-alpha-induced apoptosis: role of Bcl-2 phosphorylation. Cell Growth Differ. 2002;13:163–171. [PubMed] [Google Scholar]
- Rajah R, Valentinis B, Cohen P. Insulin-like growth factor binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-β1 on programmed cell death through a p53- and IGF-independent mechanism. J Biol Chem. 1997;272:12181–12188. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- Angelloz-Nicoud P, Binoux M. Autocrine regulation of cell proliferation by theinsulin-like growth factor (IGF) and IGF binding protein-3 protease system in a human prostate carcinoma cell line (PC-3) Endocrinology. 1995;136:5485–5492. doi: 10.1210/en.136.12.5485. [DOI] [PubMed] [Google Scholar]
- Oh Y, Muller HL, Ng L, Rosenfeld R. Transforming growth factor-βinduced cell growth inhibition in human breast cancer cells is mediated through insulin-like growth factor-binding protein-3 action. J Biol Chem. 1995;270:13589–13592. doi: 10.1074/jbc.270.23.13589. [DOI] [PubMed] [Google Scholar]
- Huynh H, Pollak M, Zhang JC. Regulation of insulin-like growth factor (IGF) I and IGF binding protein-3 autocrine loop in human PC-3 prostate cancer cells by vitamin D metabolite 1,25 (OH) 2D3 and its analog EB1089. Int J Oncol. 1998;13:137–143. doi: 10.3892/ijo.13.1.137. [DOI] [PubMed] [Google Scholar]
- Gill ZP, Perks CM, Newcomb PV, Holly JMP. Insulin-like growth factor-binding protein (IGFBP-3) predisposes breast cancer cells to programmed cell death in an non-IGF- dependent manner. J Biol Chem . 1997;272:25602–25607. doi: 10.1074/jbc.272.41.25602. [DOI] [PubMed] [Google Scholar]
- Gucev ZS, Oh Y, Kelley KM, Rosenfeld RG. Insulin-like growth factor binding protein-3 mediates retinoic acid- and transforming growth factor β2-induced growth inhibition in human breast cancer cells. Cancer Res. 1996;56:1545–1550. [PubMed] [Google Scholar]
- Rozen F, Zhang J, Pollak M. Antiproliferative action of tumor necrosis factor-α on MCF-7 breast cancer cells is associated with increased insulin-like growth factor binding protein-3 accumulation. Int J Oncol. 1998;13:865–869. doi: 10.3892/ijo.13.4.865. [DOI] [PubMed] [Google Scholar]
- Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger R, Kley N. Induction of the growth inhibitor IGF-binding protein-3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- Bromfield GP, Meng A, Warde P, Bristow RG. Cell death in irradiated prostate epithelial cells: role of apoptotic and clonogenic cell kill. Prostate Cancer Prostatic Diseases. 2003;6:73–85. doi: 10.1038/sj.pcan.4500628. [DOI] [PubMed] [Google Scholar]
- Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Marani M, Yu J, Nan B, Roth JA, Kagawa S, Fang B, Denner L, Marcelli M. Adenovirus-mediated Bax overexpression for the induction of therapeutic apoptosis in prostate cancer. Cancer Res. 2001;61:186–191. [PubMed] [Google Scholar]
- Catz SD, Johnson JL. BCL-2 in prostate cancer: a minireview. Apoptosis. 2003;8:29–37. doi: 10.1023/A:1021692801278. [DOI] [PubMed] [Google Scholar]
- Vijayababu MR, Kanagaraj P, Arunkumar A, Srinivasan N, Michael Aruldhas M, Arunakaran J. Effects of quercetin on IGF system components in PC-3 cells. XXII symposium of the Society for Reproductive Biology and Comparative Endocrinology (SRBCE) 2004. pp. 30–31. Chennai, India.
- Martin JL, Pattison SL. Insulin-like growth factor binding protein-3 is regulated by dihydrotestosterone and stimulates deoxyribonucleic acid synthesis and cell proliferation in LNCaP prostate carcinoma cell. Endocrinology. 2000;141:2401–2409. doi: 10.1210/en.141.7.2401. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–270. [PubMed] [Google Scholar]
- Baxter RC. Signalling pathways involved in antiproliferative effects of IGFBP-3: a review. Mol Pathol. 1997;54:145–148. doi: 10.1136/mp.54.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton JR, Dixit VM, Feldman EL. Type I Insulin-like Growth Factor Receptor Activation Regulates Apoptotic Proteins. J Biol Chem . 1996;271:31791–31794. doi: 10.1074/jbc.271.50.31791. [DOI] [PubMed] [Google Scholar]
- Parrizas M, LeRoith D. Insulin-Like Growth Factor-I Inhibition of Apoptosis Is Associated with Increased Expression of the Bcl-xL Gene Product. Endocrinology. 1997;138:1355–1358. doi: 10.1210/en.138.3.1355. [DOI] [PubMed] [Google Scholar]
- Butt AJ, Firth SM, Baxter RC. The IGF axis and programmed cell death. Immunol Cell Biol. 1999;77:256–262. doi: 10.1046/j.1440-1711.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunnell D, Holly J. Cancer and insulin-like growth factor-I. BMJ. 2000;321:847–848. doi: 10.1136/bmj.321.7265.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djavan B, Bursa B, Seitz C, Soeregi G, Remzi M, Bhaskhah M. Insulin like growth factor-I (IGF-I), IGF-I density and IGF/PSA ratio for prostate cancer detection. Urology. 1999;54:603–606. doi: 10.1016/S0090-4295(99)00280-0. [DOI] [PubMed] [Google Scholar]
- Klein RD, Fischer SM. Black tea polyphenols inhibit IGF-I induced signaling pathway through Akt in normal prostate epithelial cells and Du145 prostate carcinoma cells. Carcinogenesis. 2002;23:217–221. doi: 10.1093/carcin/23.1.217. [DOI] [PubMed] [Google Scholar]
- Singh RP, Dhanalaksmi S, Tyagi AK, Chan DCF, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002;62:3061–3069. [PubMed] [Google Scholar]
- Jackson JG, White MF, Yee D. Insulin receptor substrate-I is the predominant signaling molecule activated by insulin-like growth factor-I, insulin, and interleukin-4 in estrogen receptor-positive human breast cancer cells. J Biol Chem. 1998;273:9994–10003. doi: 10.1074/jbc.273.16.9994. [DOI] [PubMed] [Google Scholar]
- Baserga R. The IGF-I receptor in cancer research. Exp Cell Res. 1999;253:1–6. doi: 10.1006/excr.1999.4667. [DOI] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- Pollak M. Insulin-like growth factor physiology and cancer risk. Eur J Cancer. 2000;36:1224–1228. doi: 10.1016/S0959-8049(00)00102-7. [DOI] [PubMed] [Google Scholar]
- Hollowood AD, Lai T, Perks CM, Newcomb PV, Alderson D, Holly JM. IGFBP-3 prolongs the p53 response and enhances apoptosis following UV irradiation. IntJ Cancer. 2000;88:336–341. doi: 10.1002/1097-0215(20001101)88:3<336::AID-IJC3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Devi GR, Sprenger CC, Plymate SR, Rosenfeld RG. IGFBP-3 induces early apoptosis in malignant prostate cancer cells and inhibits tumor formation in vitro. Prostate. 2002;51:141–152. doi: 10.1002/pros.10068. [DOI] [PubMed] [Google Scholar]
- Lee DY, Yi HK, Hwang PH, Oh Y. Enhanced expression of IGFBP-3 sensitizes the growth inhibiting effect of anticancer drugs in gastric cancer cells. Biochem Biophys Res Commun. 2002;294:480–486. doi: 10.1016/S0006-291X(02)00491-6. [DOI] [PubMed] [Google Scholar]