Abstract
Transplantation of cell suspensions containing olfactory ensheathing cells (OECs) has been reported to remyelinate demyelinated axons in the spinal cord with a Schwann cell (SC)-like pattern of myelination. However, questions have been raised recently as to whether OECs can form SC-like myelin. To address this issue we prepared SCs and OECs from transgenic rats in which a marker gene, human placental alkaline phosphatase (hPAP), is linked to the ubiquitously active promoter of the R26 gene. SCs were prepared from the sciatic nerve and OECs from the outer nerve-fiber layer of the olfactory bulb. Positive S100 and p75 immunostaining indicated that >95% of cells in culture displayed either SC or OEC phenotypes. Suspensions of either SCs or OECs were transplanted into an X-irradiation/ethidium bromide demyelinating lesion in the spinal cord. We observed extensive SC-like remyelination following either SC or OEC transplantation 3 weeks after injection of the cells. Alkaline phosphatase (ALP) chromagen reaction product was associated clearly with the myelin-forming cells. Thus, cell suspensions that are enriched in either SCs or OECs result in peripheral-like myelin when transplanted in vivo.
Keywords: Olfactory ensheathing cell, Schwann cell, alkaline phosphatase, myelin, axonal repair, demyelination
INTRODUCTION
Normally, SCs are excluded from entry into the CNS during development, but there are numerous pathological situations in which they invade the CNS and myelinate axons (reviewed by Franklin and Blakemore, 1993). Additionally, several studies have demonstrated successful histological remyelination by transplanted SCs (e.g. Blakemore and Crang, 1985; Barron-Van Evercooren et al., 1992). Moreover, remyelination by transplanted SCs can improve the electrophysiological properties of spinal cord axons (Honmou et al., 1996; Kohama et al., 2001). Several reports suggest that transplantation of OECs, which display astrocyte-like and SC-like properties in culture (Pixley, 1992; Au et al., 2002; Au and Roskams, 2003), into demyelinated CNS results in remyelination with a peripheral SC-like morphology (Franklin et al., 1996; Imaizumi et al., 1998). Moreover, remyelination has been reported following transplantation of human SCs (Kohama et al., 2001) and human OECs (Barnett et al., 2000; Kato et al., 2000) into demyelinated spinal cords of immunosuppressed rats. Xenotransplantation of porcine OECs also results in remyelination of demyelinated spinal cords in nonhuman primates (Radtke et al., 2003).
A recent study failed to observe myelination in vitro in a co-culture experiment with dorsal root ganglion neurons and immunoselected (p75+) OECs under culture conditions that allows myelination by SCs (Plant et al., 2002). These investigators raise the important question of whether transplanted OECs might induce or enhance the migration of endogenous SCs into the transplantation site (Brook et al., 1998). To address this issue, we prepared cell suspensions of SCs derived from peripheral nerve and OECs from the olfactory bulb of alkaline phosphatase-expressing transgenic rats (Kisseberth et al., 1999). The hPAP marker gene is linked to the ubiquitously active R26-gene promoter, and stable expression of hPAP has been demonstrated in neural precursor cells, both in culture and after transplantation into the CNS (Mujtaba et al., 2002; Han et al., 2002). We report that transplantation of cell suspensions enriched in either SCs or OECs (p75+ and S100+) derived from hPAP-transgenic rats can be identified readily in vivo and are associated with myelin formation. The extensive remyelination by identified SCs and OECs indicates that, under appropriate conditions in vivo, both cell types can form myelin in the spinal cord.
OBJECTIVE
The objective of this study is to determine whether OECs derived from the olfactory bulb form myelin when transplanted into the persistently demyelinated spinal cord. To achieve this we prepared OECs and SCs from transgenic rats that express hPAP and from green fluorescent protein (GFP)-expressing mice and studied the morphological and electrophysiological properties of the axons 1–6 weeks after transplantation.
METHODS
Lesion induction and transplantation
A focal demyelinated lesion was created in the dorsal column of the spinal cord of 12-week-old rats with X-irradiation and ethidium bromide injection (X–EB). X-irradiation depletes endogenous oligodendrocyte progenitors (Hinks et al., 2001), and the ethidium bromide is toxic and kills cells in the injection zone. Rats were anesthetized by I.P. injection of ketamine (75 mg kg−1) and xylazine (10 mg kg−1), and a 40 Gy surface dose of X-irradiation delivered through a 2×4 cm opening in a lead shield (4-mm thick) to the spinal cord using a Siemens Stabilipan radiotherapy machine (250 kV, 15 mA, 0.5 mm Cu; 1 mm Al filters, SDD 28 cm, dose rate 220.9 Gy min−1; Siemens AG). The cross-hairs of the collimeter were positioned ∼4 mm rostral to the attachment of the T12 rib (∼T10/T11 spinal-cord segment) to irradiate a 4-cm length of spinal cord. Three days after irradiation, rats were anesthetized as described above and a laminectomy performed to target near the longitudinal center of the irradiation field. Using a drawn-glass microelectrode, injections of 0.5 μl of 0.3 mg ml−1 EB in saline were made at depths of 0.7 mm and 0.4 mm. In some experiments EB was injected without X-irradiation, to examine endogenous repair. Three days after EB injection (X–EB model), 1 μl of DMEM only (Gibco BRL) or a cell suspension containing either 30 000 SCs or OECs per μl was injected into the longitudinal center of the EB–X-induced lesion at two depths (0.7 mm and 0.4 mm; 0.5 μl at each depth). The relatively low number of cells injected allowed us to study more efficiently the cells in the center of the lesion. Chari and Blakemore (Chari and Blakemore, 2002) estimate that repopulation of the X–EB lesion by endogenous progenitor cells outside of the irradiation zone can occur at a rate of ∼0.5 mm week−1 during the first month. The lesion zone induced by EB injection was 6–8 mm long. Thus, the rostral and caudal margins of the lesions were ∼1.6 mm from the edge of the irradiation field. Both control and cell-transplanted rats were immunosuppressed with cyclosporin (10 mg kg−1 day−1, s.c.; Sandoz Pharmaceuticals) 1 day before injection into the spinal cord and each day thereafter.
Preparation of OECs and SCs from hPAP-transgenic rats and GFP-expressing mice
hPAP-transgenic Fischer rats (250–300 g, female) were a gift from Dr. Eric P. Sandgren (University of Wisconsin). GFP-expressing mice [C57BL/6-TgN(ACtbEGFP)] were obtained from Jackson Laboratories Inc. OECs from rat and mouse olfactory bulbs were prepared in nearly identical manners. Animals were sacrificed under sodium pentobarbital anesthesia (60 mg kg−1 i.p.). The bulbs were removed and transferred to ice-cold Ca2+- and Mg2+-free CSS. The meninges were removed, and the outer nerve-fiber layer (ONL) of the rostral 2/3 of the bulb was minced finely with a pair of scalpel blades and incubated in a mixture of 0.4 U ml−1 collagenase A and 0.3 U ml−1 collagenase D (from Clostridium histolyticum, Roche), 0.05 U ml−1 papain (Worthington) and 2 mM cysteine (Sigma) in 20 ml CSS at 37°C for 25 minutes. The enzymatic incubation was stopped by DMEM supplemented with 10% FCS and the tissue was washed twice in DMEM by centrifuging at 300×g for 5 minutes. Tissue was dissociated mechanically by gentle trituration using fire-polished Pasteur pipettes with successively smaller diameters. Media volume was increased to 20 ml and undissociated pieces of tissue were allowed to settle for 2 minutes before transferring the supernatant and centrifuging. To reduce fibroblasts and meningeal cells, rat-cell suspensions were preplated for 2 hours on uncoated culture flasks for 2 hours before washing off unattached cells and centrifuging to remove dead cells and debris. Mouse cells were preplated for 75 minutes.
SCs were isolated from either rat or mouse sciatic nerves as described previously (Lankford et al., 2002). Briefly, sciatic nerves were desheathed, minced, incubated for 45 minutes in DMEM with 10 mg ml−1 of both collagenase A and collagenase D (Roche), pelleted, incubated an additional 15 minutes in CSS with 2.5 mg ml−1 trypsin, triturated though a fire-polished, siliconized pasture pipette, and washed twice with DMEM and 10% fetal bovine serum. All transplanted cells were washed 3 times in DMEM before transplantation.
Histological characterization of transplanted cells
A sample of cells remaining after transplantation procedure was plated on poly-L-lysine coated 8-well-chamber slides at a concentration or 10 000–30 000 cells per well and maintained in DMEM with 10% FBS and penicillin/streptomycin in an incubator at 37°C, 5% CO2. After 24 hours, cells were washed with DMEM, fixed with ice-cold methanol for 10 minutes and stained overnight at 4°C with antibodies directed against: p75 (rabbit 1:1000, Chemicon); S-100 (either mouse 1:1100, Chemicon or rabbit 1:400, Dako); and GFAP (monoclonal SMI221 1000, Sternberger) and visualized with secondary antibodies Alexifluor 595 goat-anti-rabbit and Alexifluor 488 goat-anti-mouse (1:1000) for 4 hours at room temperature. Slides were counterstained for 1 hour with Hoechst (1:1000) and cover-slipped with mounting media (Dako).
Histological processing
Three weeks after transplantation, the rats were anesthetized deeply with ketamine (75 mg kg−1) and xylazine (10 mg kg−1), and perfused through the heart. The spinal cords were fixed for 24 hours in 4% paraformaldehyde in 0.14 M Sorensen's phosphate buffer, pH 7.4. Tissue was washed three times and stored overnight in buffer. The lesions in the spinal cord were separated into rostral and caudal halves, with one half processed for cryostat sectioning and immunohistochemistry, and the other prepared for plastic embedding. Cryostat sections were cut at 20 μm, rinsed four times (10 min each) with 0.1% Triton X-100 in 10 mM phosphate-buffered saline (PBS; pH 7.5). After washing with alkaline phosphatase buffer (0.1 M Tris-HCl, pH 9.5) the tissues were incubated at room temperature in the dark with 1.0 mg ml−1 NBT, 0.1 mg ml−1 BCIP, 5 mM levamisole (Sigma) and 0.1% Tween 20 in alkaline phosphatase buffer for 15 minutes. Slides were examined on a Nikon Eclipse 800 epifluorescent microscope.
Tissue for plastic embedding was fixed overnight in 2% paraformaldehyde/2% glutaraldehyde, cut into 2-mm segments, notched to indicate orientation, post-fixed with 1% osmium (Polysciences, Warrington PA) for 4 hours, and dehydrated and embedded in Epox-812 (Ernest F. Fullam Inc, Latham NY) using standard plastic-embedding protocols. Semithin sections (1 μm) were mounted on slides and counter-stained with methylene blue and azure II (0.5% each in 0.5% borax).
Field-potential recording in vivo
One, 3 and 6 weeks after transplantation, the rats were deeply anesthetized with isoflurane and spinal cords of normal, control, demyelinated (X–EB and EB alone), SC-transplanted and OEC-transplanted rats were exposed and covered with a modified Krebs' solution containing (in mM): 124 NaCl, 26 NaHCO3, 3.0 KCl, 1.3 NaH2PO4, 2.0 MgCl2, 10 dextrose, 2.0 CaCl2. Six animals were used for each of the above groups. Field-potential recordings of compound action potentials were obtained at a rectal temperature of 36 ± 1°C with glass microelectrodes (2–10 MΩ, 1 M NaCl) positioned just off the midline at depths of ∼100 μm in the dorsal columns. Signals were amplified with high-input impedance amplifier. The axons were activated by electrically stimulating the dorsal columns with bipolar, Teflon-coated stainless-steel electrodes cut flush and placed lightly on the dorsal surface of the spinal cord at a fixed point caudal in the lesion zone. Constant-current stimulation pulses were delivered through stimulus-isolation units and the timing device. The recording microelectrode was repositioned in rostral steps to attain conduction velocity measurements. To eliminate synaptic responses, the spinal cord was irrigated with a modified Krebs' solution containing (in mM): 124 NaCl, 26 NaHCO3, 3.0 KCl, 1.3 NaH2PO4, 6.0 MgCl2, 10 dextrose without Ca2+. All variances represent standard error (± S.E.M.). Differences between groups were assessed by ANOVA to identify individual group differences.
RESULTS
Characterization of SCs and OECs in culture
Aliquots of SC and OEC suspensions prepared for transplantation from hPAP-transgenic rats were maintained in culture for ∼24 hours to allow adhesion to coverslips. At this time, the cells were prepared for either immunostaining or ALP chromagen reaction. Results from an OEC preparation are shown in Fig. 1. These short-term cultured cells were immunostained for either p75 (Fig. 1A) or S100 (Fig. 1B) and the nuclei Hoechst stained. The majority of the cells (≥ 95%) were p75+ and S100+, which is characteristic of both SCs and OECs (Au et al., 2002). After this short time in culture, the cells had few processes which become manifest with longer culture times (Kafitz and Greer, 1999). The purpose of studying the phenotype of these cells after very short-term culture was to approximate the phenotypic characteristics and purity of the cells at the time of in vivo injection. These data indicate that the method of preparing the cells yielded relatively enriched cultures. An example of ALP chromagen staining of an OEC culture (3 days in culture) from hPAP-transgenic rats is shown in Fig. 1C. The chromagen reaction product was observed as diffuse staining throughout the cytoplasm, and both spindle shaped and flattened cells were seen.
Fig. 1.
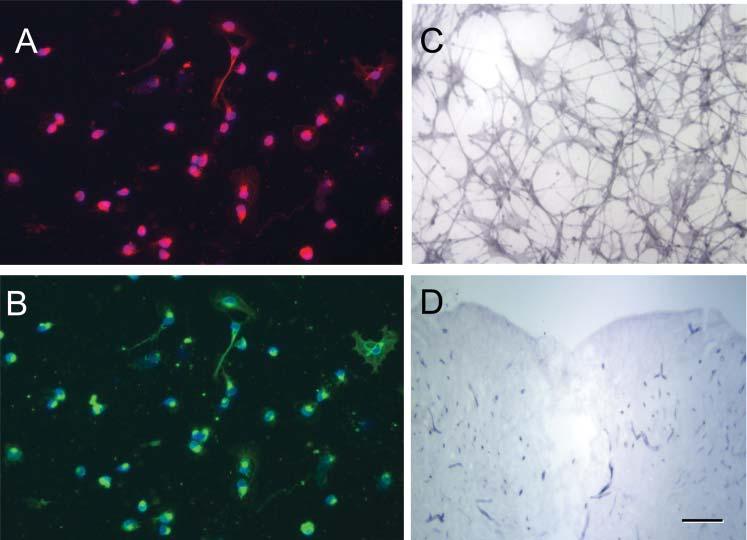
Phentotypic characterization of OECs and hPAP expression in culture. (A,B) Dissociated OECs were p75+ (red) and S100+ (green). The nuclei were stained with the Hoechst method and >95% of cells were p75+. (C) ALP-reaction product can be seen in OECs that were maintained in culture for 5 days. (D) A coronal section through a demyelinated lesion (X–EB) site after ALP chromagen reaction. Note that aside from minor staining in blood vessels, the central lesion site showed little reaction product. Scale bar: 20 μm in A-C; 300 μm in D.
Remyelination by transplanted hPAP-SCs and OECS
Three weeks after transplantation of either hPAP-SCs (n=3) or hPAP-OECs (n=3), rats were perfused and prepared for histological analysis. The spinal cords were blocked through the center of the transplantation zone and adjacent coronal slabs (1 mm) were prepared. One tissue slab was prepared for plastic embedding to obtain semi-thin sections and the other was prepared for frozen sections for ALP histochemistry. Results from SC and OEC transplanted rats are shown in Fig. 2 and Fig. 3, respectively in low-power and high-power micrographs of methylene blue/azure stained semi-thin plastic embedded sections after cell transplantation. The lesion site can be seen clearly as a lighter-stained region within the dorsal funiculus (Fig. 2A, Fig. 3A). Higher-power micrographs show numerous myelinated profiles in the transplantation site (Fig. 2B, Fig. 3B). The presence of hPAP in the transplantation zone is evident by the intense blue of the chromagen reaction (Fig. 2C,D; Fig. 3C,D). Note that in Fig. 2D and Fig. 3D (arrows) that macrophages in the lesion zone do not display an intense reaction product. Enlargement of the plastic embedded (Fig. 2E, Fig. 3E) and ALP reacted (Fig. 2F, Fig. 3F) sections reveal the presence of remyelinated axons, and ALP-chromagen associates with the myelinated axons. Many of these myelin profiles for both SC and OEC transplantation are associated with cell bodies that have large cytoplasmic and nuclear domains, which is characteristic peripheral myelin.
Fig. 2.
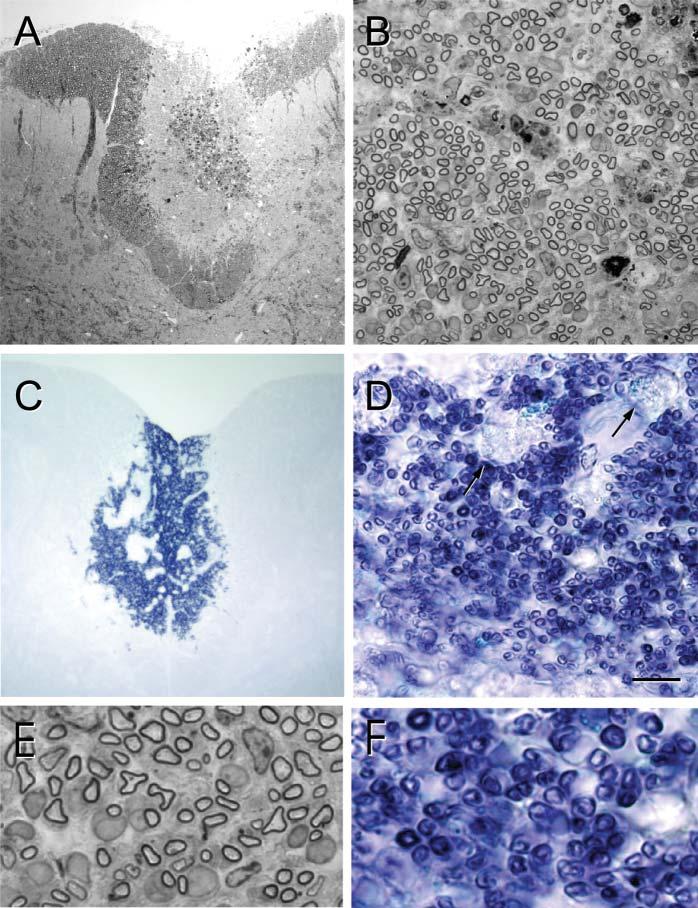
Remyelination after transplantation of hPAP-SCs into an X–EB lesion of the dorsal funiculus. (A) Low-power, plastic-embedded-section of the dorsal spinal cord 3 weeks after hPAP-SC transplantation. Note the centrally positioned lesion site (lighter region). (B) Higher power micrograph shows extensive myelinated profiles throughout the transplantation site. (C) In adjacent, frozen sections reacted for ALP, blue reaction product can be observed in the transplantation site. (D) Higher power of this area shows numerous blue profiles that are characteristic of myelinated axons. Note that the reaction product is minimal in macrophages (arrows). (E) Higher-power images of plastic-embedded sections show myelinated axons, many of which are associated with large cytoplasmic and nuclear surrounds that are characteristic of SC myelination. (F) These profiles are associated with hPAP reaction product. Scale bar: 200 μm in A,C; 25 μm in B,D; 7 μm in E,F.
Fig. 3.
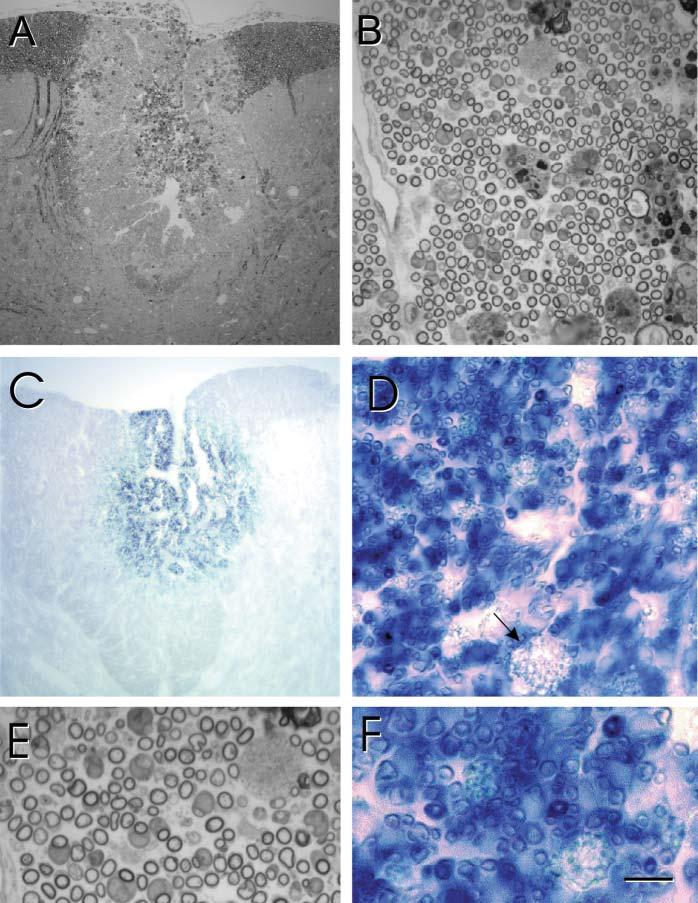
Remyelination after transplantation of hPAP-OECs into an X–EB dorsal funiculus lesion. (A) Low-power, plastic-embedded section of the dorsal spinal cord 3 weeks after hPAP-OEC transplantation. (B) A higher power micrograph shows extensive myelinated profiles throughout the transplantation site. (C) In adjacent, frozen sections reacted for ALP, blue reaction product can be observed in the transplantation site. (D) Higher power of this area shows numerous blue profiles that are characteristic of myelinated axons and similar to that observed following SC transplantation (Fig. 2). (E) Higher power images of plastic-embedded sections shows myelinated axons, many of which are associated with large cytoplasmic and nuclear surrounds characteristic of peripheral myelination. (F) These profiles are associated with hPAP reaction product. Scale bar: 200 μm in A,C; 25 μm in B,D; 7 μm in E,F.
Distribution of GFP-expressing OECs and SCs in the lesion
The distribution of transplanted OECs and SCs was also studied using GFP-expressing cells from a GFP-expressing transgenic mouse (Akiyama et al., 2002). Fig. 4A1 shows the presence of GFP-expressing SCs within a lesion site in a coronal section. An overlay with a conventional light-microscopy image of the spinal cord shows the position of the transplanted cells in the lesion site. Sagittal sections through the lesion site following transplantation of either SCs (Fig. 4B) or OECs (Fig. 4C) indicate that the transplanted cells are primarily confined to the lesion site. As with the hPAP transplantation experiments, adjacent sections prepared for plastic embedding indicated remyelination within the lesion zone (data not shown).
Fig. 4.
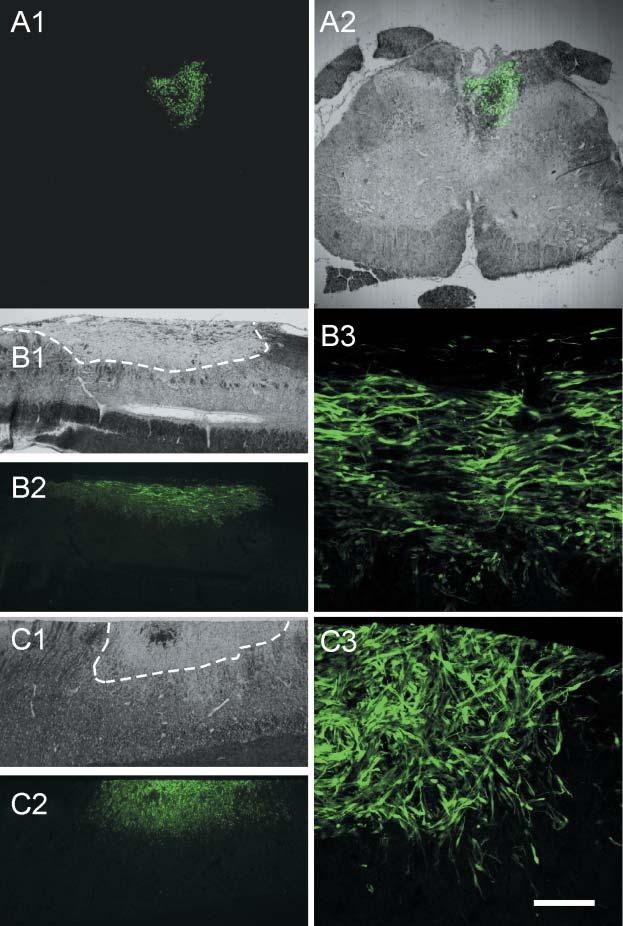
Distribution of GFP-expressing mouse SCs and OECs transplanted into an X–EB dorsal funiculus lesion. (A1) Coronal section of the spinal cord from a rat that was transplanted with SCs from GFP-expressing mice 3 weeks after transplantation. (A2) Superimposition of GFP fluorescent and DIC images. Note that donor SCs that express GFP are localized only in the dorsal funiculus. (B) Sagittal sections through the lesion site showing the distribution of transplanted SCs. The lesion site is within the stippled area in B1. The transplanted cells extend throughout the lesion site (B2). Higher-power micrograph (B3) shows clusters of transplanted cells. (C) Distribution of OECs in the lesion site. C1 outlines the lesion site, and distribution of cells in low and higher power micrographs are shown in C2 and C3, respectively. Scale bar: 500 μm in A1,A2; 1 mm in B1,B2,C1,C2; 50 μm in B3,C3.
Conduction properties of demyelinated axons and axons remyelinated by SCs and OECs
Just prior to preparation of histological sections, in vivo recordings were obtained from the dorsal columns of the spinal cords using glass microelectrodes. Fig. 5A shows field-potential responses recorded from the normal dorsal columns following local stimulation of the dorsal column (see Methods). The responses consist of an early and a late negativity (Fig. 5A1, arrows). When the surface of the spinal cord was washed with Krebs' containing no Ca2+ and high Mg2+ (6.0 mM), the late negativity was did not occur but the early negativity remained (Fig. 5A2). This indicates the synaptic origin of the late negativity, and that the early negativity is a fiber volley (i.e. compound action potential) of the dorsal column axons. Superimposed traces recorded at three distances (1 mm separation between each site) are shown in Fig. 5B in control, X–EB lesion alone, and SC-transplanted and OEC-transplanted spinal cords, three weeks after lesion induction or cell transplantation. The mean conduction velocity for control rats was 16.93 ± 1.02 m sec−1 (S.E.M.) (Fig. 5C). Conduction velocity in the X–EB animals was 1.27 ± 0.14 m sec−1, 1.07 ± 0.13 m sec−1 and 0.86 ± 0.13 m sec−1, at 1, 3 and 6 weeks post-lesion induction, respectively, indicating the persistence of demyelination over 6 weeks in the X–EB model. However, if focal X-irradiation, which disrupts progenitor cells in the irradiated field, was eliminated and only EB injected, conduction velocity was reduced 1 week after injection of EB (0.82 ± 0.27 m sec−1), but progressively increased at 3 weeks (4.09 ± 0.49 m sec−1) and 6 weeks (8.6 ± 0.13 m sec−1) post-EB injection. This agrees with observations that without X-irradiation endogenous remyelination occurs in rat spinal cord after chemical demyelination (Graca and Blakemore, 1986; Felts and Smith, 1992). Following transplantation of either SCs or OECs, conduction velocity increased at 3 (7.86 ± 1.65 m sec−1 and 6.24 ± 1.79 m sec−1, respectively) and 6 weeks (10.84 ± 1.2 m sec−1 and 7.80 ± 0.57 m sec−1, respectively) post-transplantation. P values are presented in the legend to Figure 5.
Fig. 5.
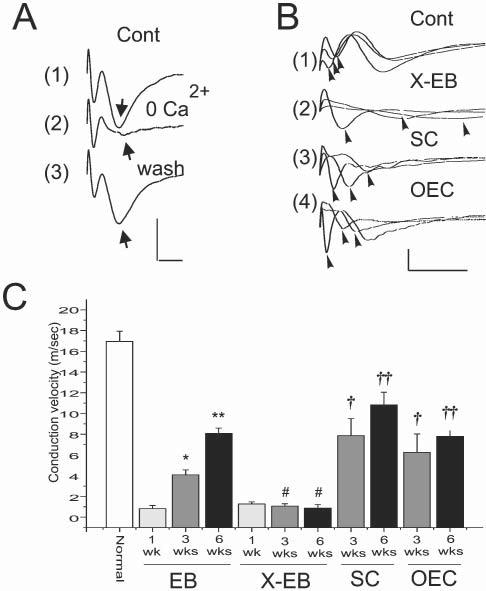
Conduction velocity of demyelinated and remyelinated axons, recorded in vivo. (A) Compound action potentials recorded from the dorsal column (near the midline and within 100 μm of the surface) with a glass microlectrode in vivo following stimulation of the dorsal column surface near the midline. Note the early and late negativities. Washing the surface of the spinal cord with a Ca2+-free, high (6 mM) Mg2+ Krebs' solution (2) reversibly (3) eliminates the second negativity (arrow), indicating its synaptic nature. Thus, the first negativity corresponds to conducting dorsal column axons. (B) Superimposed compound action potentials recorded at 1.0 mm increments longitudinally along the dorsal columns in normal (1), X–EB lesion (2) and 3 weeks after transplantation of either SCs (3) or OECs (4). (C) Conduction velocity (error bars indicate S.E.M.) of dorsal column axons from control animals (normal), and 1, 3 and 6 weeks after EB injection without prior X-irradiation, after X–EB lesion induction, and 3 and 6 weeks after SC and OEC transplantation into the X–EB lesion. * P<0.1, ** P<0.01, †P <0.05, †† P<0.05, # not statistically significant. Scals bars: A, 2 mV, 1 msec; B, 1 mV; 5 msec.
CONCLUSIONS
Transplantation of either OECs or SCs from hPAP transgenic rats and eGFP transgenic mice into the X–EB demyelinated spinal cord of adult rats results in extensive, peripheral-like remyelination by the donor cells.
Conduction velocity is reduced in the X–EB lesion for up to 6 weeks, but is improved equally by either OEC or SC-remyelinated axons 3 weeks after induction of the lesion.
DISCUSSION
SCs and OECs prepared from either hPAP-expressing transgenic rats or GFP-expressing mice formed myelin upon transplantation into demyelinated spinal cord of rats. The pattern of myelination was similar between SCs and OECs in that many of the myelin profiles were associated with a single myelin-forming cell with a large cytoplasmic and nuclear domain. Importantly, the remyelinated axons showed a strong ALP chromogen reaction product that was associated with individual axons and myelin-forming cells. This reaction product was not present to an appreciable extent in normal tissue and in X–EB lesion zones without transplanted cells. Moreover, macrophages in the lesion did not react for ALP, further indicating the specificity of the reaction for donor cells derived from hPAP transgenic rats. Because both our SC and OEC preparations were >95% p75+ and S100+, and because they displayed similar amounts of myelination, it is likely that both cell types are responsible independently for the observed remyelination. The extensive myelination of an area of persistent demyelination by hPAP-donor SCs and OECs identified in this study demonstrates that the transplanted cells are unlikely to induce sufficient migration of endogenous SCs from the periphery to account for the remyelination.
An important caveat when interpreting SC-like myelination by OECs is the possibility of SC contamination of OEC cultures by SCs. As pointed out by Plant et al. (Plant et al., 2002), glial preparations from carefully isolated meninge- and spinal root-free spinal cord have been reported to be contaminated by SCs (unpublished data by Wood, as cited in Plant et al., 2002). One possibility is that SCs in nerves innervating large CNS vessels might be a source of SC contamination (Plant et al., 2002). We view this as unlikely in our OEC preparations, because 1–3 days after plating 95% of cells in culture were p75+ and S100+. This high purity of cells without significant expansion in culture is inconsistent with contamination from SCs associated with CNS vessels. Moreover, the extent of remyelination by equal numbers of transplanted SCs and OECs was similar, which further indicates that contamination of OEC preparation by a small number of SCs did not account for the remyelination by the transplanted OECs.
The failure to observe myelination by p75-immunopanned OECs (90–95% p75+ and S100+) in a culture preparation led Plant et al. (Plant et al., 2002) to challenge numerous studies in which myelination was observed following in vivo transplantation of OECs (Franklin et al., 1996; Barnett et al., 2000; Kato et al., 2000; Imaizumi et al., 1998). The failure of OECs to form myelin in vitro could be because culture conditions optimal for SCs to form myelin in vitro may be different for OECs. In our in vivo lesion model, progenitors are depleted by X-irradiation and all cells in the dorsal funiculus lesion zone are killed by the EB injection. Thus, an aglial region of several millimeters in length is produced. Virtually no myelination is observed in the lesion zone for at least 6 weeks after lesion induction. However, progenitors outside the irradiation field can migrate into the lesion zone at later times (Chari and Blakemore, 2002). One possibility that might account for the myelination we observe at 3 weeks post-transplant, is that the transplanted cells accelerate the migration of endogenous progenitors. We view this as unlikely given the large number of myelin-forming cells in the transplantation zone that were associated with either hPAP or GFP marker genes. These data indicate strongly that the donor cells were responsible for the remyelination observed.
Our data do not necessarily mean that that the increase in the number of myelinated axons observed following transplantation of either SCs or OECs into other lesion models, such as spinal cord contusion (Takami et al., 2002) and tran-section (Imaizumi et al., 2000a,b), result from remyelinaton by the transplanted cells. In non-X-irradiated lesions, facilitation or recruitment of endogenous cells from either CNS progenitors or migration of peripheral cells occurs (Felts and Smith, 1992). From our electrophysiological results, demyelination by EB alone without X-irradiation is followed by robust remyelination in rats (data not shown) and a significant improvement in conduction velocity over the course of 6 weeks. Clearly, endogenous cells are responsible for this response. It is clear that the size of the lesion in the spinal cord-contusion model is reduced following either SC or OEC transplantation 1 week after lesion induction (Plant et al., 2003) and the number of myelinated profiles is increased (Takami et al., 2002). Moreover, several reports indicate that transplanting OECs into spinal cord-transection (Ramon-Cueto et al., 1998; Ramon-Cueto et al., 2000; Imaizumi et al., 2000a; Imaizumi et al., 2000b) or tract-ablation (Li et al., 1997; Li et al., 1998) models enhances axon regeneration and improves the behavioral outcome. Relatively acute application of transplanted cells might provide a neuroprotective effect and promote the recruitment of endogenous progenitors that contribute to the increase in peripheral-like myelinating profiles. However, a recent study has demonstrated that delaying transplantation of OECs into an ablation lesion of the corticospinal tract results in limited axonal regeneration and improved forepaw reaching behavior (Kevyan-Fouladi et al., 2003). Although the extent to which donor OECs and SCs contribute to neuroprotection, enhancement of axonal outgrowth and remyelination is yet to be fully elucidated when transplanted into the injured spinal cord, our results demonstrate that OECs have the potential to form myelin after transfer in vivo.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (NS10174, NS043432 and DC00210), the National Multiple Sclerosis Society (RG2135), and the Medical and Rehabilitation and Research and Development Services of the Department of Veterans Affairs.
REFERENCES
- Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. Journal of Neuroscience. 2002;22:6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au W, Treloar HB, Greer CA. Sublaminar organization of the mouse olfactory bulb nerve layer. Journal of Comparative Neurology. 2002;446:68–80. doi: 10.1002/cne.10182. [DOI] [PubMed] [Google Scholar]
- Au W, Roskams AJ. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;3:224–236. doi: 10.1002/glia.10160. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Alexander CL, Iwashita Y, Gilson JM, Crowther J, Clark L, et al. Identification of a human olfactory ensheathing cell that can effect transplant-medicated remyelination of demyelinated CNS axons. Brain. 2000;123:1581–1588. doi: 10.1093/brain/123.8.1581. [DOI] [PubMed] [Google Scholar]
- Baron-Van Evercooren A, Gansmuller A, Duhamel E, Pascal F, Gumpel M. Repair of a myelin lesion by Schwann cells transplanted into the adult mouse spinal cord. Journal of Neuroimunology. 1992;40:235–242. doi: 10.1016/0165-5728(92)90139-c. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Crang AJ. The use of cultured autologous Schwann cells to remyelinate areas of persistent demyelination in the central nervous system. Journal of Neurological Science. 1985;70:207–223. doi: 10.1016/0022-510x(85)90088-7. [DOI] [PubMed] [Google Scholar]
- Brook GA, Plate D, Franzen R, Martin D, Moonen G, Schoenen J, et al. Spontaneous longitudinally oriented axonal regeneration is associated with the Schwann cell framework within the lesion site following spinal cord compression injury of the rat. Journal of Neuroscience Research. 1998;54:51–65. doi: 10.1002/(SICI)1097-4547(19980701)53:1<51::AID-JNR6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Chari DM, Blakemore WF. Efficient recolonization of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia. 2002;37:307–313. [PubMed] [Google Scholar]
- Felts PA, Smith KJ. Conduction properties of central nerve fibers remyelinated by Schwann cells. Brain Research. 1992;574:178–192. doi: 10.1016/0006-8993(92)90815-q. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Blakemore WF. Requirements for Schwann cell migration within CNS environments: a viewpoint. International Journal Developmental Neuroscience. 1993;11:641–649. doi: 10.1016/0736-5748(93)90052-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Gilson JM, Franceschini IA, Barnett SC. Schwann cell-like myelination following transplantation of an olfactory bulb ensheathing cell line into areas of demyelination in the adult CNS. Glia. 1996;17:217–224. doi: 10.1002/(SICI)1098-1136(199607)17:3<217::AID-GLIA4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Graca DL, Blakemore WF. Delayed remyelination in rat spinal cord following ethidium bromide injection. Neuropathology and Applied Neurobiology. 1986;12:593–605. doi: 10.1111/j.1365-2990.1986.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Han SS, Kang DY, Mujtaba T, Rao MS, Fischer I. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Experimental Neurology. 2002;177:360–375. doi: 10.1006/exnr.2002.7995. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Chari M, O'Leary MT, Zhao C, Keirstead HS, Blakemore WF, et al. Depletion of endogenous oligodentrocyte progenitors rather than increased availability of survival factors is a likely explanation for enhances survival of transplanted oligodendroctye progenitors in X-irradiated compared to normal CNS. Neuropathology and Applied Neurobiology. 2001;27:59–67. doi: 10.1046/j.0305-1846.2001.00303.x. [DOI] [PubMed] [Google Scholar]
- Honmou O, Felts PA, Waxman SG, Kocsis JD. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. Journal of Neuroscience. 1996;16:3199–3208. doi: 10.1523/JNEUROSCI.16-10-03199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Burton WV, Fodor WL, Kocsis JD. Xenotransplantation of transgenic pig olfactory ensheathing cells promotes axonal regeneration in rat spinal cord. Nature Biotechnology. 2000b;18:949–953. doi: 10.1038/79432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Kocsis JD. Transplantation of olfactory ensheathing cells or Schwann cells restores rapid and secure conduction across the transected spinal cord. Brain Research. 2000a;854:70–78. doi: 10.1016/s0006-8993(99)02285-4. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Waxman SG, Greer CA, Kocsis JD. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in demyelinated dorsal column of the rat spinal cord. Journal of Neuroscience. 1998;18:6176–6185. doi: 10.1523/JNEUROSCI.18-16-06176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz KW, Greer CA. The influence of ensheathing cells on olfactory receptor cell neurite outgrowth in vitro. Annals New York Academy of Science. 1998;855:266–269. doi: 10.1111/j.1749-6632.1998.tb10580.x. [DOI] [PubMed] [Google Scholar]
- Kato T, Honmou O, Uede T, Hashi K, Kocsis JD. Transplantation of human olfactory ensheathing cells elicits remyelination of demyelinated rat spinal cord. Glia. 2000;30:209–218. doi: 10.1002/(sici)1098-1136(200005)30:3<209::aid-glia1>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyvan-Fouladi N, Raisman G, Li Y. Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats. Journal of Neuroscience. 2003;28:9428–9434. doi: 10.1523/JNEUROSCI.23-28-09428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Developmental Biology. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- Kohama I, Lankford KL, Preiningerova J, White FA, Vollmer TL, Kocsis JD. Transplantation of cryopreserved adult human Schwann cells enhances axonal conduction in demyelinated spinal cord. Journal of Neuroscience. 2001;21:944–950. doi: 10.1523/JNEUROSCI.21-03-00944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheating transplants. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Regeneration of adult rat corticospinal tract axons induced by transplanted olfactory ensheathing cells. Journal of Neuroscience. 1998;18:10514–10524. doi: 10.1523/JNEUROSCI.18-24-10514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujtaba T, Han SS, Fischer I, Sandgren EP, Rao MS. Stable expression of the alkaline phosphatase marker gene by neural cells in culture and after transplantation into the CNS using cells derived from a transgenic rat. Experimental Neurology. 2002;174:48–57. doi: 10.1006/exnr.2001.7847. [DOI] [PubMed] [Google Scholar]
- Pixley SK. The olfactory nerve contains two populations of glia, identified both in vivo and in vitro. Glia. 1992;5:269–284. doi: 10.1002/glia.440050405. [DOI] [PubMed] [Google Scholar]
- Plant GW, Christensen CL, Oudega M, Bunge MB. Delayed transplantation of olfactory ensheathing glia promotes sparing/regeneration of supraspinal axons in the contused adult rat spinal cord. Journal of Neurotrauma. 2003;1:1–16. doi: 10.1089/08977150360517146. [DOI] [PubMed] [Google Scholar]
- Plant GW, Currier PF, Cuervo EP, Bates ML, Pressman Y, Bunge MB, et al. Purified adult ensheathing glia fail to myelinate axons under culture conditions that enable Schwann cells to form myelin. Journal of Neuroscience. 2002;22:6083–6091. doi: 10.1523/JNEUROSCI.22-14-06083.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke C, Akiyama Y, Brokaw J, Lankford KL, Wewetzer K, Foder WL, et al. Remyelination of the nonhuman primate spinal cord by transplanation of H-transferase transgenic adult pig olfactory ensheathing cells. FASEB Journal Express Article. 2003 doi: 10.1096/fj.03-0214fje. 10.1096/fj.03-021 fje 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing cells. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. Journal of Neuroscience. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. Journal of Neuroscience. 2002;22:6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


