Abstract
Binding of the N-terminal 70-kDa (70K) fragment of fibronectin to fibroblasts blocks assembly of intact fibronectin and is an accurate indicator of the ability of various agents to enhance or inhibit fibronectin assembly. Such binding is widely thought to be to already assembled fibronectin. We evaluated this hypothesis with fibronectin-null mouse fibroblasts plated on laminin-1 in the absence of intact fibronectin. As a proteolytic fragment or recombinant protein, 70K bound fibronectin-null cells specifically in linear arrays that extended outwards from the periphery of spread cells. At early time points, these arrays were similar to those formed by intact fibronectin. 70K arrays formed within 5min following ligand addition at concentrations as low as 5nM, indicating rapid and high affinity binding. Bound 70K was extractable with Triton X-100 or deoxycholate but became insoluble when cross-linked with a membrane-impermeable agent into large SDS-stable complexes. Intact fibronectin, in contrast, became progressively non-extractable in the absence of cross-linking. The detergent-resistant arrays of cross-linked 70K localized to tips of cellular extensions and partially overlapped with α 6 and β 1 integrin subunits at the base of the extensions. α 5 did not localize with 70K arrays, but became progressively co-localized with assemblies of intact fibronectin over time. These results support a model in which the 70-kDa region of fibronectin binds to linearly arrayed cell surface molecules of adherent cells to initiate assembly, display of the arrays is controlled by the integrin that mediates adhesion, and fibronectin-binding integrins promote fibronectin–fibronectin interactions during progression of assembly.
Keywords: Fibronectin, N-terminal 70-kDa fragment, Assembly, Fibrillogenesis, Initiation, Integrin
Abbreviations: FN, fibronectin; 70K, N-terminal 70-kDa region of fibronectin; r70K, recombinant 70K; LPA, lysophosphatidic acid; BSA, bovine serum albumin; FITC, fluorescein isothyocyanate; PBS, phosphate-buffered saline; TBS, tris-buffered saline; FUD, functional upstream domain; PFA, paraformaldehyde
1. Introduction
The fibrillar form of fibronectin (FN) provides information important for cell adhesion, migration, survival and proliferation, and for connective tissue remodeling (Pankov and Yamada, 2002; Sottile and Hocking, 2002). Much is known about the transformation of soluble FN into its fibrillar form (Magnusson and Mosher, 1998; Mao and Schwarzbauer, 2005; Wierzbicka-Patynowski and Schwarzbauer, 2003), but the precise mechanism is yet to be elucidated. On adherent cells, FN assembly is efficiently initiated by interaction of soluble FN with cell surface molecules. Following this initial binding, progression of assembly involves FN–FN interactions, insolubilization of FN and formation of elongating fibrils (Mao and Schwarzbauer, 2005; McKeown-Longo and Mosher, 1983; Peters and Mosher, 1987).
FN is a dimer, with each subunit being composed of three types of modules with some variability due to alternative splicing (Mao and Schwarzbauer, 2005; Pankov and Yamada, 2002). The five N-terminal type I modules are essential for fibrillogenesis (McDonald et al., 1987; McKeown-Longo and Mosher, 1985; Schwarzbauer, 1991; Sottile et al., 1991). The N-terminal 70-kDa catheptic fragment (70K), containing these modules and the adjacent gelatin-binding region, binds to cell monolayers with the same affinity as intact fibronectin and blocks assembly of intact fibronectin, but does not become incorporated into detergent-insoluble extracellular matrix (McKeown-Longo and Mosher, 1985). Bound 70K co-localizes with pre-existing FN fibrils (Chernousov et al., 1985), albeit incompletely (Zhang et al., 1994). 70K also binds to FN that is adsorbed to surfaces and subjected to tension (Zhong et al., 1998). Thus, the N-terminal region of FN has been proposed to mediate binding to stretched or otherwise conformationally altered FN molecules (Hocking et al., 1994, 1996; Wierzbicka-Patynowski and Schwarzbauer, 2003; Zhong et al., 1998). This proposed interaction is incorporated into a model in which FN fibrillogenesis begins by binding of an integrin recognition sequence in FN, e.g., RGD in repeat III10, to an integrin, e.g., α 5β 1(Mao and Schwarzbauer, 2005; Wierzbicka-Patynowski and Schwarzbauer, 2003). Interaction with integrins is hypothesized to promote extension of FN and thereby facilitate interactions of the N-terminal 70K region with other parts of FN (Aguirre et al., 1994; Bultmann et al., 1998; Hocking et al., 1994, 1996).
An alternative hypothesis is that tethering of the 70K region to cell surface molecules causes a conformational change in FN and exposes the FN regions involved in FN–integrin and FN–FN interactions. This hypothesis is consistent with findings that the N-terminal modules of FN are available in solution to bind to various molecules (Ingham et al., 1988; Isaacs et al., 1989; Khan et al., 1990), whereas the integrin-binding midpiece is cryptic in solution but is exposed in complexes of FN and substances that bind the N-terminal modules (Ensenberger et al., 2004; Ugarova et al., 1995). A model in which interactions with the N-terminal modules cause a conformational change that opens up the integrin-binding site, rather than vice versa, has a precedent in studies of bacteria–FN interactions (Ozeri et al., 1998). In the case of Streptococcus pyogenes, binding of protein F1 to the N-terminal region of FN enables cellular uptake of bacteria–FN complexes by a process that is inhibitable by RGD peptides, involves integrins and likely contributes to bacterial virulence (Cue et al., 2000; Nyberg et al., 2004; Ozeri et al., 1998). Thus, cellular uptake of S. pyogenes may represent a subversion of the mechanism whereby binding via the N-terminal region of FN exposes the cryptic integrin-binding type III10 repeat. Furthermore, neither monomeric nor dimeric III7–10 fragment binds integrins stably; a trimer is required (Coussen et al., 2002). This finding suggests that cell-associated FN must be assembled into a multimeric form to engage integrins.
FN−/− fibroblasts provide the opportunity to test the two alternative models. If 70K only binds to conformationally altered FN, there should be no binding of 70K to FN−/− cells. If the 70K region binds to molecules at assembly sites that are not FN, 70K should bind to FN−/− cells with the same distribution as intact FN. We report here that 70K does bind to FN−/− cells in a system lacking intact FN and provide evidence that the binding sites have the characteristics of assembly sites. We then probed 70K and integrin localization to discern the role of integrins in assembly. Our results are compatible with a mechanism, whereby assembly is initiated by binding of the 70K portion of FN to linearly arrayed binding sites that are not FN, display of these sites is controlled by integrin-mediated adhesion, and progression of assembly, with concomitant FN–FN interactions, involves translocation of assembling FN tethered to FN-binding integrins.
2. Results
2.1. 70K binds to cells in linear arrays in the absence of intact FN
FN−/− cells assemble exogenous FN in short-term assays when cultured on substrata coated with FN or laminin but not when cultured on vitronectin (Bae et al., 2004). To learn whether the correlation between 70K binding and FN assembly extends to the different effects of the substrates on FN−/− cells, we compared the binding of FN and 70K to cells cultured on FN, vitronectin or laminin. As shown in Fig. 1, all substrates promoted adhesion and spreading of FN−/− cells to a similar extent, FITC–70K only bound to cells adherent to FN or laminin. For cells on laminin, binding of FITC–70K after 1 h was in linear arrays at the cell periphery (Fig. 1). These arrays were perpendicular to the border of the spread cell and similar in length and density to arrays formed by FITC–FN over the same time period (Fig. 1). For cells on FN, some arrays of FITC–FN or FITC–70K were also present on portions of cells other than at the periphery (Fig. 1). Little FITC–FN or FITC–70K bound to FN−/− cells adhered to vitronectin-coated substrate. The experiments in Fig. 1 demonstrate that the adhesive substrate determines the ability of adherent FN−/− cells to bind both 70K and FN. Importantly, the studies of FN−/− cells on laminin indicate that 70K binds to cell surfaces in the absence of intact FN.
Fig. 1.
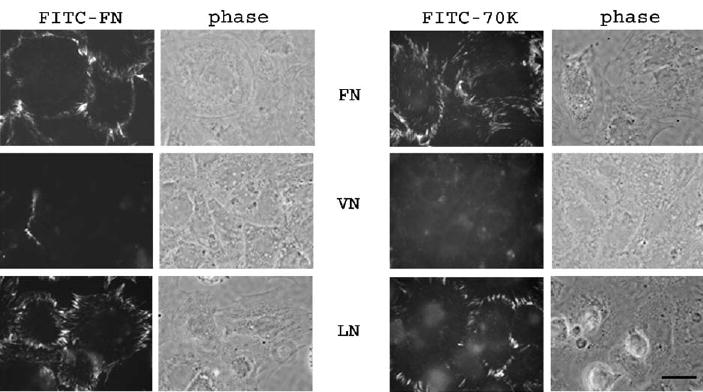
FITC–70K and FITC–FN form linear arrays on FN−/− cells adhered to FN or laminin but not to vitronectin. FN−/− cells were plated for 2 h on coverslips coated with FN, laminin or vitronectin. FITC–FN (20nM) or FITC–70K (40nM) were incubated with the cells for 1h in DMEM containing 400nM LPA and 0.2% BSA. Coverslips were washed, fixed and observed under fluorescence and phase contrast microscopy. Bar=10 μm.
To establish this point more solidly, we examined all components of cultures of FN−/− cells adherent to laminin for the presence of intact FN. Because proteolytic 70K was purified by gelatin affinity chromatography, which could potentially allow co-purification of unproteolyzed intact FN, we carried out parallel experiments with recombinant 70K (r70K) purified by its C-terminal His tag. r70K bound in linear arrays that were indistinguishable from arrays formed by proteolytic 70K (Fig. 2, top right panel). FN−/− cells, which were derived from embryonic stem cells from a FN-knock-out mouse (George et al., 1993) and selected based on their fibroblastic character, have been demonstrated not to secrete FN (Saoncella et al., 1999). In addition, we detected no FN in cell layers with rabbit anti-human 70K antibodies that recognize mouse FN (Fig. 2, top left panel). Cells were cultured in 10% fetal calf serum, but experiments were performed in serum-free medium. After trypsinization and prior to plating for experiments, FN−/− cells were transiently suspended in bovine serum depleted of FN by gelatin affinity chromatography. The depleted serum did not contain FN as ascertained by immunoblotting with rabbit anti-FN antibodies that recognize bovine FN (not shown). In other experiments, FN−/− cells were cultured in defined medium in the absence of fetal calf serum (Sottile et al., 1998) for 3 weeks prior to assaying for 70K array formation and found to also form linear arrays of FITC–70K (not shown). The laminin was mouse laminin-1 derived from EHS tumors. Anti-FN antibodies that recognized mouse FN detected no FN in SDS-PAGE and immunoblotting analysis of laminin (not shown). Thus, we found no evidence of intact FN in the linear array assays.
Fig. 2.

Antibodies to 70K recognize linear arrays only upon addition of exogenous 70K to FN−/− cells. FN−/− or FN+/− cells were plated on laminin-coated coverslips for 2h. Proteolytically derived 40nM 70K (+70K) or recombinant 70K (+r70K) was added to cells for 1 h in DMEM, 0.2% BSA, 400 nM LPA; coverslips were washed, fixed and incubated with rabbit anti-70K antibodies, followed by FITC-conjugated anti-rabbit IgG. Exposure times were set to coverslips that received 70K. Note lack of reactivity on FN−/− cells and punctate reactivity on FN+/− cells in the absence of 70K (−70K). In the presence of 70K, note defined linear arrays at the periphery of FN−/− cells and numerous short streaks or fibrils all over FN+/− cell surfaces (+70K). Bar=10 μm.
To learn the impact of endogenous FN on 70K binding, we compared the pattern of 70K binding to FN−/− cells to that of 70K binding to FN+/− cells. FN−/− or FN+/− cells were plated for 3 h on laminin-coated coverslips in DMEM-BSA in the absence of serum, after which proteolytically derived 70K or recombinant 70K was added for 1 h. Following fixation, coverslips were incubated with rabbit antibodies raised to human 70K followed by FITC-labeled goat anti-rabbit IgG. The rabbit anti-70K antibodies reacted by immunoblotting with human, mouse and bovine FN (not shown). As described above and shown in Fig. 2, FN−/− cells incubated in the absence of 70K had no reactivity with anti-70K antibodies. In contrast, anti-70K antibodies recognized short linear and punctate structures present on FN+/− cells incubated in the absence of exogenous 70K (Fig. 2), indicating early surface deposition of secreted FN by FN+/− cells. In contrast to the restricted distribution of 70K or r70K between cells and substratum as linear arrays at the cell periphery of FN−/− cells, 70K added to FN+/− cells bound in both linear arrays at the cell periphery and in short streaks on the cell body that had the same distribution as the staining for endogenous FN (Fig. 2). Endogenous FN, therefore, allows the formation of additional binding sites on the cell surface that are not present in FN−/− cells cultured on laminin but is not required for 70K binding in linear arrays at the cell periphery.
2.2. Specificity of linear array formation by FITC– 70K on FN–/– cells
Fig. 3A shows linear array formation in the presence of various potential inhibitors of FN assembly. Unlabeled 70K, 1 μM, completely blocked formation of linear arrays of FITC–70K, indicating specificity of array formation. FUD, a recombinant piece of protein F1 of S. pyogenes with strong affinity for 70K (Ensenberger et al., 2001; Ozeri et al., 1996) and a known inhibitor of FN assembly (Tomasini-Johansson et al., 2001), also blocked linear array formation by FITC–70K. Neither a recombinant piece of FN encompassing repeats III7 through 10 and containing the integrin-binding region of FN, tested at 1μM, nor 1.5mM GRGDSP peptide decreased formation of linear arrays. None of the added potential modulators affected cell attachment or spreading to laminin, as monitored by phase contrast microscopy (Fig. 3A). Parallel experiments quantitatively assessed the effects of potential inhibitors on 125I-70K binding to FN−/− cell monolayers (Fig. 3B). RGDS at 1.5mM did not inhibit specific binding of 125I-70K to FN−/− cell monolayers, whereas 1 μM FUD inhibited 80% of specific binding. These findings indicate that FITC–70K binding to FN−/− cells adherent to laminin is blocked by reagents that mimic or target the N-terminal region of FN but is not disturbed by reagents targeting interactions with the RGD-containing region of FN.
Fig. 3.

FITC–70K linear array formation is inhibited by unlabeled 70K and FUD but not by III7-10 or RGDS peptide. (A) FN−/− cells were plated on laminin-coated coverslips and incubated with 40nM FITC–70K for 1h in the absence or presence of 1 μM 70K, 1 μM FUD, 1μM III7–10 or 1.5mM RGDS peptide. Coverslips were washed, fixed, mounted and visualized by fluorescence microscopy. (B) FN−/− cells were plated on laminin-coated dishes and incubated with 125I-70K for 1h in the absence (NA) or presence of 1 μM FUD, 1.5mM RGDS or 1.5 mM RGES. Monolayers were washed and resuspended in 1N NaOH prior to counting radiolabel. Binding in the presence of 600nM unlabeled 70K (non-specific binding) was subtracted from each condition. Assays were carried out in DMEM, 0.2% BSA and 400nM LPA. Data represent mean±S.D. from four replicate wells pooled from two separate experiments. Bar=10 μm.
2.3. Time course and dose-dependence of linear array formation by FITC–70K
FN−/− cells were plated on laminin-coated coverslips for 2h and then given FITC–70K or FITC–FN. After various time periods, cells on coverslips were washed, fixed and examined by fluorescence microscopy. Fig. 4A shows that FITC–70K or FITC–FN bound in linear arrays within 5min of addition of ligand. At 24h, FITC–FN was deposited as a three-dimensional fibrillar network, whereas FITC–70K remained bound as linear arrays at the cell periphery. FN−/− cells plated on laminin-coated coverslips for 2h were also given increasing amounts of FITC–70K and analyzed by fluorescence microscopy after incubation for 1h. As shown in Fig. 4B, clear arrays could be detected after incubation with concentrations of FITC–70K as low as 5nM. The clarity and intensity of linear arrays increased with increasing concentration of ligand, although concentrations of FITC–70K higher than 40nM did not result in arrays of higher intensity. These results suggest that the binding sites for 70K at the FN−/− cell periphery are saturable and of similar high affinity as that previously described for binding of 70K to FN-secreting cells (Zhang et al., 1994).
Fig. 4.

Time course and concentration-dependence of FITC–70K or FITC–FN linear array formation. (A) FN−/− cells were plated on laminin-coated coverslips for 2h. 40nM FITC–70K or 20 nM FITC–FN were incubated with cell monolayers for 5min, 30min, 60min or 24h in DMEM, 0.2% BSA and 400nM LPA. At these time points, monolayers were washed, fixed, mounted and observed by fluorescence microscopy. Note appearance of FITC–70K linear arrays at 5 min time point, and similarity between FITC–70K and FITC–FN linear arrays, except at 24h, when FITC–FN forms a fibrillar network, but FITC–70K remains bound as linear arrays at the cell periphery. (B) FN−/− cells were plated on laminin-coated coverslips for 2h. FITC–70K was added at concentrations ranging from 5nM to 160nM and incubated for 1 h in DMEM, 0.2% BSA and 400nM LPA. Cells were washed, fixed, mounted and observed by fluorescence microscopy. Samples receiving 40–160nM FITC–70K were imaged using equal exposure times; those receiving lesser concentrations of FITC–70K were imaged using progressively longer exposure times. Bar=10μm.
2.4. Bound FITC-labeled FN but not 70K enters the detergent-insoluble pool
FN deposited in the extracellular matrix of cultured fibronectin-secreting fibroblasts is insoluble in 1% deoxycholate and forms complexes that are not dissociated by SDS unless reducing reagent is present, whereas 70K remains in the detergent-soluble pool (Chen and Mosher, 1996; Choi and Hynes, 1979; McKeown-Longo and Mosher, 1983, 1985). We investigated whether linear arrays formed by 70K and FN on FN−/− cells are deoxycholate-insoluble structures and whether linear array formation involves SDS-resistant interactions. FN−/− cells plated on laminin were given FITC–70K or FITC–FN for varying times, and the monolayers were washed and extracted with deoxycholate. The deoxycholate-insoluble and -soluble materials were analyzed by SDS-PAGE under unreducing conditions followed by immunoblotting with anti-FITC antibodies. As shown in Fig. 5, even after 24h, most of the FITC–70K bound to cell monolayers was in the deoxycholate-soluble fraction. No oligomers of 70K were apparent in gels of either the soluble or the insoluble fractions of FN−/− cells. In contrast, 24h after addition of FITC–FN to FN−/− cell monolayers, FN was found in the detergent-insoluble fraction and SDS-non-dissociable multimers were detected at the top of the gel. The multimers of intact FN disappeared when electrophoresis was performed under reducing conditions (not shown). Similar results were found when Triton X-100 was substituted for deoxycholate as the extracting agent (not shown).
Fig. 5.
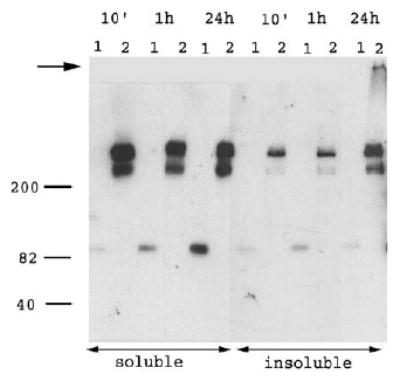
Cell-bound FITC–70K does not form detergent-insoluble multimers. FN –/– cells were plated on laminin-coated tissue culture plastic for 2h. 40nM FITC–70K or 20nM FITC–FN were added for 10min, 1h or 24h in 400nM LPA, DMEM and 0.2% BSA. Monolayers were washed and extracted with 1% deoxycholate in TBS, 2mM EDTA, 2mM iodoacetamide and proteinase inhibitor complex. Samples were centrifuged to separate insoluble from soluble fractions. Samples were run unreduced on 8% acrylamide gels and immunoblotted with rabbit anti-FITC antibodies, followed by peroxidase anti-rabbit conjugates and chemiluminescence development. Lanes: (1) FITC–70K, (2) FITC–FN. Migration of molecular weight standards (kDa) is depicted to the left. The arrow indicates the well level at the top of the 3.5% stacking gel.
2.5. Fluorescence microscopic localization of FITC–r70K or FITC–FN and integrin subunits on FN– /– cells at early time points
As assessed by immunoblotting after SDS-PAGE, bound FITC–r70K became insoluble in deoxycholate or Triton X-100 after cross-linking by a membrane-impermeable reagent, DTSSP (not shown). When the non-extractable fraction was resolved by SDS-PAGE and immunoblotted, cross-linked FITC–r70K migrated largely at the top of the gel without intermediate oligomers (not shown), as in FN-secreting cells (Zhang and Mosher, 1996).
Fluorescence microscopy was performed to relate the location of FITC-labeled 70K or FN on cell surfaces after cross-linking and detergent extraction to the location of integrin subunits detected with rhodamine-conjugated IgG. Thus, FN−/−cells were plated on laminin-coated coverslips and incubated for 30min with FITC–r70K or FITC–FN. Coverslips were washed and treated with or without DTSSP for 15min, extracted with 1% Triton X-100, fixed with paraformaldehyde and immunostained. As shown in Fig. 6, most of cell structure apparent by phase contrast was removed by treatment with 1% Triton, leaving only nuclei. Fluorescence microscopy demonstrated FITC–r70K (Fig. 6A) or FITC–FN (Fig. 6B) in arrays after detergent extraction of DTSSP-treated monolayers. In the cross-linked and extracted cells, β 1 was present at the bases of the arrays, partially co-localizing with FITC–r70K or FITC–FN. The tips of the arrays, however, stained only with FITC–r70K (Fig. 6A) or FITC–FN (Fig. 6B). When coverslips not initially treated with 1% Triton were fixed with paraformaldehyde and then permeabilized with 0.2% Triton, we observed the same patterns of FITC–r70K or FITC–FN arrays as was observed after DTSSP and 1% Triton treatment. However, β 1 also had an additional diffuse perinuclear distribution that was not observed after cross-linking with DTSSP and 1% Triton extraction (not shown).
Fig. 6.
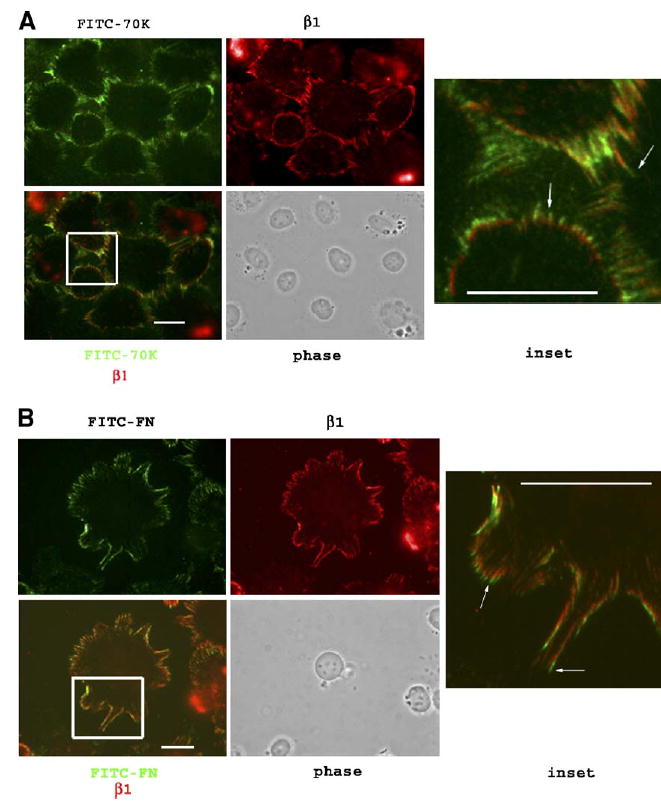
Immunofluorescence microscopy of FITC–r70K or FITC–FN binding to FN−/− cells treated with 1% Triton after DTSSP: partial co-localization with β 1 integrin. FN−/− cells were plated on laminin-coated coverslips for 2h in DMEM and 0.2% BSA. (A) FITC–r70K (40nM) or (B) FITC–FN (20nM) in DMEM, 0.2% BSA and 400nM LPA were added and incubated for 30min. Following two washes with PBS, 1.5mM DTSSP was added to the appropriate coverslips and incubated for 15 min. Coverslips were washed with TBS, 1% Triton, fixed with 3.7% paraformaldehyde and blocked with 5% BSA. β 1 integrin was visualized with rat anti-mouse β 1 antibodies followed by rhodamine-conjugated anti-rat IgG. Lower left panel shows the image obtained from merging the FITC–70K with the β 1 panels above. Yellow tones depict co-localization. Inset from merged image: note FITC–FN and FITC–r70K (arrows) at the outward tip of arrays stained with anti-β 1 at the base of arrays. Bar=10μm.
We carried out similar experiments to determine if α 5 and α 6 localized to the same areas as β 1 and FITC–70K or FITC–FN in cross-linked, detergent-extracted cells. As shown in Fig. 7, after a 1-h incubation with FITC–70K, α 6 localized in the same place as β 1 in relation to FITC–70K, but α 5 was not detected under these conditions. When we incubated FITC–FN with FN−/− cells for 15min, 1h and 4h, β 1 partially co-localized with FITC–FN at all time points (Fig. 8A), whereas co-localization of α 5 was apparent only at 1 and 4h following FITC–FN addition (Fig. 8B). Incubation with 70K, even for 24h, did not promote recruitment of α 5 to the peripheral linear arrays (not shown), defined as areas in the vicinity of cross-linked, detergent-insoluble 70K. The co-localization of α 5 after 1h of incubation of FN−/− cells with FITC–FN was at the central portion of linear arrays (Fig. 8A). β 1 also became localized with FITC–FN in this pattern with increasing time of incubation (Fig. 8B).
Fig. 7.

Localization of FITC–r70K and β 1, α 6 or α 5 integrin subunits on FN−/− cells. FN−/− cells were plated on laminin-coated coverslips for 2h. 40nM FITC–r70K was added for 1h in DMEM and 0.2% BSA in the presence of 400nM LPA. DTSSP (1.5mM) was added for 15min in PBS followed by TBS and 1% Triton washes. Remaining material was fixed with 3.7% paraformaldehyde and immunostained with anti-β 1, anti-α 6 or anti-α 5 monoclonal antibodies as indicated in the text. Primary antibodies were recognized with rhodamine-conjugated secondary IgG. Left and middle panels show the same image photographed using fluorescein filter (FITC–r70K) or rhodamine filters (integrin), respectively. Right panels show merged FITC–r70K and integrin images. Inset from merged images: note α 6 and β 1 integrin staining (red) at the base of arrays, followed by area of co-localization (yellow tones) and FITC–r70K at the outward tip of arrays (green). Note also lack of α 5 staining at FITC–r70K binding sites. Bar=10μm.
Fig. 8.

Time course of localization of FITC-FN and β 1 or α 5 integrins. FN−/− cells were plated on laminin-coated coverslips for 2h. FITC–FN (20nM) was incubated with cells for 15min, 1h or 4h in DMEM, 0.2% BSA and 400nM LPA. DTSSP (1.5mM) was added for 20min in PBS followed by TBS and 1% Triton washes. Remaining material was fixed with 3.7% PFA and immunostained with anti-β 1 (A) or anti-α 5 antibodies (B) followed by rhodamine-conjugated IgG as indicated in the text. Left and middle panels show the same image photographed using fluorescein filters (FITC–FN) or rhodamine filters (integrin subunits). Panels to the right show merged enlarged images corresponding to the rectangles depicted in the left panels. Yellow fluorescence represents co-localization. Note that absence of α 5 integrins until 1- or 4-h incubation with FITC–FN brings α 5 into FN assembly sites. Bar=10μm.
3. Discussion
The 70K N-terminal region of FN has been postulated to participate in FN assembly by binding other regions of FN during the progression phase of assembly (Mao and Schwarzbauer, 2005; Wierzbicka-Patynowski and Schwarzbauer, 2003). The 70K N-terminal fragment of FN, however, binds to cells with the same affinity and distribution as FN and is a potent inhibitor of FN assembly (Bultmann et al., 1998; Magnusson and Mosher, 1998; McKeown-Longo and Mosher, 1985). 70K binding to fibroblasts thus mimics the characteristics of initial FN binding. Here, we demonstrate binding of 70K to surfaces of FN−/− cells in the absence of intact FN. The binding was specific and to linear arrays at the periphery of the cells. At early time points, the arrays were indistinguishable from the arrays formed by bound intact FITC–FN. These results are compatible with a role of the 70K region in FN assembly that is independent of binding to other regions of FN.
Except for its restricted distribution at the cell periphery, binding of FITC-labeled FN or 70K to FN−/− cells adherent to laminin were similar to results previously reported for FN-secreting fibroblasts, indicating similarity in binding sites regardless of FN presence. Linear arrays of FITC–70K were detected after brief (5min) incubations with low concentrations (5nM) of FITC–70K and concentrations higher than 40nM did not result in greater array intensity. The saturability of binding suggests that 70K is not binding to itself. These findings are in agreement with the high avidity (Kd of 10nM) binding sites for 70K binding to LPA-treated fibroblast surfaces (Zhang et al., 1994). The linear arrays formed by 70K remained stable for up to 24h and bound 70K did not become deoxycholate-insoluble or form SDS-non-dissociable multimers. In contrast, bound FITC–FN entered the deoxycholate-non-extractable and SDS-insoluble fraction over time, just as FN does when bound to FN-secreting fibroblasts (Choi and Hynes, 1979; McKeown-Longo and Mosher, 1985), and formed an extensive network of fibrils after 24h of incubation with cells. Thus, 70K mimics the high affinity binding of FN that initiates assembly but lacks the interactions that lead to detergent-insoluble fibril formation, i.e., fibril progression.
After cross-linking of FITC–70K bound to FN−/− cells, FITC–70K became detergent-insoluble and migrated on SDS-PAGE as high molecular weight complexes (not shown), just as occurs with 70K bound to FN+/+ cells (Barry and Mosher, 1989; Zhang and Mosher, 1996). One candidate for the high molecular weight protein component of the complexes has been FN that has multimerized and become covalently cross-linked by, e.g., transglutaminase (Akimov and Belkin, 2001; Barry and Mosher, 1988; Barry and Mosher, 1989). However, finding that 70K cross-links to high molecular weight complexes in FN−/− cells indicates that the cross-linked partners responsible for the shift in electrophoretic migration are not FN. This conclusion is compatible with previous studies of FN-expressing cells showing that 125I-70K is cross-linked to molecules that are not immunoprecipitated by anti-FN antibodies recognizing parts of FN outside the 70K region (Zhang and Mosher, 1996).
The rapidity of linear array formation suggests that binding sites are pre-arrayed on the cell surface. α 5β 1 integrin was shown to bind to immobilized 70K in an interaction inhibitable by RGD peptide (Hocking et al., 1998) and 70K localized to focal adhesion sites containing β 1 integrins (Christopher et al., 1997; Dzamba et al., 1994; Wierzbicka-Patynowski and Schwarzbauer, 2002). FITC–70K binding and linear array formation by FN−/− cells adherent to laminin, however, were not blocked by GRGDSP at a concentration that inhibits α 5β 1-mediated cell adhesion to FN. Further, α 5 did not localize to assembly sites on FN−/− cells adherent to laminin. Instead, α 6 and β 1 partially localized with 70K or FN at the bases of the arrays. These findings indicate that α 5β 1 does not bind 70K to form the linear arrays. We presume that β 1 at the base of the arrays is part of the α 6β 1 integrin complex that mediates cell adhesion to laminin. In favor of this interpretation, vinculin also is localized at these sites (not shown). Localization of α 6β 1 at the base of the arrays suggests that β 1 integrins help organize the arrays. Similarly, α 5β 1 interacting with assembling endogenous FN may help organize the additional sites of 70K binding found on FN+/− cells.
Based on the above considerations, we postulate that FN fibrillogenesis is initiated by the display of linearly arrayed molecules that bind the N-terminal region of FN (Fig. 9). After this initial binding, a conformational change in FN follows that exposes the FN regions involved in FN–FN and FN–integrin interactions (Fig. 9). These latter interactions drive fibril progression and formation of detergent-insoluble FN–FN complexes. The FN–FN interactions may involve the 70K region in FN binding to type III domains (Aguirre et al., 1994; Bultmann et al., 1998; Hocking et al., 1994, 1996). Thus, this model assigns roles to the 70K region in both initiation and progression of FN assembly.
Fig. 9.
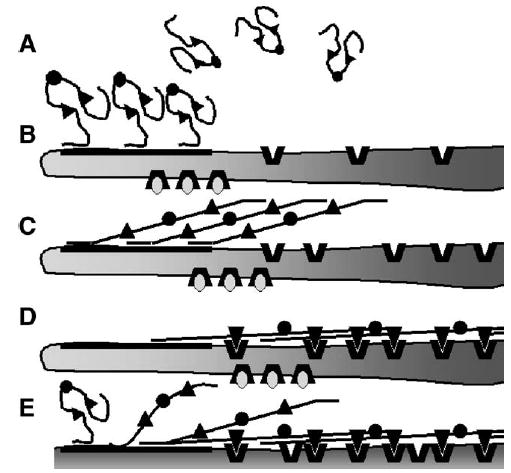
Model of fibronectin assembly on cells adhered to an assembly supportive substratum. (A) Soluble FN dimer with disulfides (circles) connecting monomers and RGD-containing regions (triangles). (B) N-termini of FN bind to linearly arrayed assembly sites. Display of these sites is controlled by cell adhesion mediated by integrins engaging permissive ligands (ovals). (C) Binding of the N-termini of FN to assembly sites promotes unfolding of FN exposing the RGD-containing regions. (D) The RGD-containing regions bind FN-binding integrins (thick V’s). The tension generated from integrins engaging the cytoskeleton stretches the FN molecule exposing FN–FN interacting sites with ensuant fibril formation. (E) Translocation of integrins towards the cell center removes the nascent FN fibrils from the assembly sites, thus opening up the sites for further fibrillogenesis.
Numerous studies employing various approaches implicate integrins as key players in FN assembly (reviewed in Mao and Schwarzbauer, 2005; Pankov and Yamada, 2002; Wierzbicka-Patynowski and Schwarzbauer, 2003). Our results suggest that integrins may (1) mediate cell adhesion in such a way as to control display of FN assembly sites, (2) interact with conformationally changed FN to enable fibril progression and (3) transduce force to the elongated and assembled FN to stretch the molecules and open individual type III modules, thus allowing FN–FN interactions that result in the SDS-non-dissociable complexes (Baneyx et al., 2002; Ohashi et al., 2002; Zhong et al., 1998). In agreement with these ideas, a FN construct lacking the RGD sequence was found to bind in “short stitches” on FN−/− cells cultured on collagen in 72-h assays (Sottile et al., 2000), indicating that binding to integrins via the RGD region is not essential for binding of full length FN to cell surfaces but is important for fibril progression. The co-localization of α 5 and β 1 with FITC–FN during the progression phase of assembly by FN−/− cells is in accord with demonstration of α 5β 1 in matrix contacts co-localizing with elongating FN fibrils in FN-secreting cells (Clark et al., 2005; Pankov et al., 2000). It should be emphasized, however, that the postulated model allows a non-FN binding integrin such as α 6β 1 to support display of the FN assembly sites that recognize the N-terminal region, whereas progression of FN fibril formation would require engagement of the type III module region by a FN-binding integrin.
4. Experimental procedures
4.1. Materials
The following were purchased: bovine serum albumin (BSA), Sigma (St. Louis, MO); fluorescein isothiocyanate (FITC) and rabbit anti-FITC IgG, Molecular Probes (Eugene, OR); mouse laminin-1, Invitrogen (Carlsbad, CA); rat anti-mouse β 1 (MAB 1997), Chemicon (Temecula, CA); rat anti-mouse α 5 (MFR 5) and rat anti-mouse/human α 6 (GoH3), Pharmingen (San Diego, CA); peroxidase-conjugated goat anti-rabbit IgG and Rhodamine Red-X-conjugated goat anti rat IgG, Jackson Immunoresearch (West Grove, PA); chemiluminescence kit, Perkin Elmer Life Sciences (Boston, MA); lysophosphatidic acid (LPA), Avanti Polar Lipids (Birmingham, AL); DTSSP [3,3′ dithiobis(sulfosuccinimidyl-propionate)], a reducible, membrane-impermeable, amine-reactive cross-linker, Pierce (Rockford, IL); GRGDSP and GRGESP, Bachem (King of Prussia, PA). FN-free fetal calf serum was obtained by passing fetal calf serum (Intergen, Purchase, NY) twice over a gelatin-Sepharose (Sigma) column. Polyclonal antibodies to human FN or the N-terminal FN were raised in rabbits (Williams et al., 1983; Zhang and Mosher, 1996).
Human plasma FN and 70K derived from a cathepsin digest of FN were isolated and FITC-labeled as described previously (McKeown-Longo and Mosher, 1983, 1985; Peters et al., 1990). 70K was radiolabeled with 125I as described previously (McKeown-Longo and Mosher, 1985). Vitronectin was isolated from human plasma (Bittorf et al., 1993). Recombinant FUD (functional upstream domain, clone pUR4) encompassing the non-repeated domain plus 6 amino acids from the first repeat region of F1, a FN-binding adhesin from S. pyogenes was prepared as a recombinant His-tagged protein as described previously (Tomasini-Johansson et al., 2001). The plasmid for expression of repeats III7–10 of FN was a generous gift from Harold Erickson (Duke University) and used to produce purified III7–10 as described previously (Leahy et al., 1994; Zhang et al., 1999).
Human recombinant 70K (r70K) was prepared from FN cDNA (generous gift from Dr. Alberto Kornblihtt, University of Buenos Aires). PCR was used to amplify a fragment encompassing residues 32 to 608 of full length FN, lacking the pre–pro region. This product was cloned into the Xma1 and Pst1 sites of the baculovirus transfer vector pAcGP67.coco. The construct has an addition of 4 residues (ADPG) encoded by vector sequence N-terminal to the start of the FN gene and a short linker (SSAG) between the end of the 70K sequence (QTYP) and the six histidine residue tag (Mosher et al., 2002). r70K protein was expressed and purified using the His-tag as previously described (Mosher et al., 2002). r70K was labeled with FITC as described above for proteolytically derived 70K.
4.2. Cell culture
FN−/− and FN+/− mouse fibroblasts had been derived from FN−/− and FN+/− embryonic stem cells obtained from homozygous and heterozygote FN knock-out mice (George et al., 1993; Saoncella et al., 1999). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies, Inc.) with 10% fetal bovine serum (Intergen, Purchase, NY) and 100 IU/ml penicillin and 100 μg/ml streptomycin. FN −/− cells were also cultured in serum-free defined medium, a 1:1 mixture of Cellgro (Mediatech, Herndon, VA) and Aim V (Life Technologies, Gaithersburg, MD) as described (Sottile et al., 1998).
4.3. Fluorescence microscopy
Cell-bound FITC-labeled 70K or FN were visualized as follows. Glass coverslips were coated with laminin (15μg/ml), vitronectin (5μg/ml) or FN (5μg/ml) in phosphate-buffered saline (20mM phosphate, 150mM NaCl, pH 7.4, PBS) overnight at 4°C. Cells in culture plates were washed twice with PBS containing 0.3mM EDTA, followed by treatment with 4mM trypsin for 3–5min. Trypsin was inactivated by addition of 10% FN–free fetal calf serum in DMEM. Cells were collected by centrifugation, resuspended in DMEM containing 0.2% bovine serum albumin (DMEM-BSA) and plated at 1×105 cells/well. After 2-h incubation, FITC–70K or FITC–FN were added in the absence or presence of potential inhibitors of binding and incubated at various concentrations and periods of time, as indicated. FITC–70K or FITC–FN were added in DMEM-BSA containing 400nM LPA, which enhances binding of 70K and assembly of FN (Zhang et al., 1994). Monolayers on coverslips were washed twice with DMEM-BSA, fixed with 4% paraformaldehyde for 10 min and mounted using Vectashield (Vector, Burlingame, CA). Cells were viewed on an Olympus epifluorescence microscope and digitally photographed using an RT slider digital camera (Spot Diagnostic Instruments, Inc., Sterling Heights, MI) and processed with Spot RT Software and Adobe Photoshop 5.0. In Figs. 6–8, the images in panels showing the localization of individual molecules were photographed with the appropriate filter in grayscale mode; these images were then colorized to their respective original color to allow merging for visualization of co-localization.
4.4. Immunofluorescence of bound 70K
Cell monolayers were incubated with unlabeled proteolytically derived or recombinant 70K (40nM) and treated as described above up to the fixation step, after which 5% BSA in PBS was added to coverslips. Coverslips were incubated for 1h with 1:100 dilution of rabbit antiserum to 70K and, after washing three times with PBS, incubated with FITC or rhodamine-labeled secondary antibodies to rabbit IgG. Cover-slips were washed three times with PBS, mounted and visualized as described above.
4.5. Binding of radiolabeled 70K to FN– /– cell monolayers
125I-70K binding to cells was carried out in the absence of serum utilizing previously described methods (McKeown-Longo and Mosher, 1985; Tomasini-Johansson et al., 2001). Tissue culture plastic wells in 24-well plates were coated with laminin at 10–20μg/ml in PBS overnight at 4°C. FN−/− cells (3×105 per well) were plated in DMEM-BSA and incubated overnight before addition of 42nM 125I-70K in DMEM-BSA containing 400 nM LPA. Unlabeled 70K (600nM) was included in replicate wells for each condition and competed for 125I-70K binding to cells by approximately 60%. The amount of binding not competed for by 70K was denoted non-specific binding and was subtracted from total binding measurements to calculate specific binding. The effects of potential inhibitors were determined by adding 1μM FUD, 1.5mM GRGDSP or 1.5mM GRGESP to appropriate wells. Monolayers were incubated with 125I-70K with or without additions for 60min followed by three washes with Tris-buffered saline (10mM Tris, 150mM NaCl, pH 7.4, TBS) and recovery of bound label by the addition of 1N NaOH. Bound radiolabel in extracts was measured in a gamma radiation counter. The assay was performed in duplicate and repeated twice.
4.6. Deoxycholate extraction, SDS-PAGE and immunoblotting
FN−/− cells (1×105 per well) were plated on laminin-coated wells for 2h in DMEM-BSA. Cells were then incubated with 40 nM FITC–70K or 20nM FITC–FN in 400nM LPA, DMEM-BSA for 10min, 1h or 24h. Monolayers were washed twice with DMEM-BSA and scraped into 1% deoxycholate in 20mM Tris buffer (pH 8.3), 2mM EDTA, 2 mM iodoacetamide, and serine and protease inhibitors in Complete-EDTA-free cocktail (Roche, Penzberg, Germany). Extracts were centrifuged at 14,000rpm for 30min. Deoxycholate-insoluble material was resuspended in SDS-PAGE sample buffer (60mM Tris pH 6.8, 4% SDS, 5% glycerol, 4M urea). Samples were analyzed under reduced or unreduced conditions on 8/3% discontinuous acrylamide slab gels, transferred to PDVF membranes, and followed by immunoblotting with 0.2μg/ml rabbit anti-FITC IgG and 40ng/ml peroxidase-conjugated goat anti-rabbit IgG in 4% fat-free dry milk, 0.1% Tween-20 in TBS (Tween-TBS). Blots were developed using a chemiluminescence kit from Perkin Elmer Life Sciences, according to the manufacturer’s instructions.
4.7. Cross-linking of FITC – 70K to cell monolayers
FN−/− cells (7×105 cells/well) were plated for 2h on 12-well plates pre-coated with 15μg/ml mouse laminin. Cell monolayers were washed once with DMEM-BSA and incubated for 45min with 40nM FITC–70K in DMEM-BSA containing 400nM LPA. Monolayers were washed twice with PBS and incubated for 15min with 1.5mM DTSSP. Cells were washed three times with TBS, once with 1% Triton X-100, 2mM EDTA in TBS and twice with PBS. 1% deoxycholate, 2mM EDTA in TBS was used alternatively with similar results. Material left behind was extracted with 200μl sample buffer, as described above. Samples were boiled for 10min and 30μl was loaded per well onto 8% acrylamide gels without stackers. SDS-PAGE was followed by blotting unto PDVF membranes utilizing a transfer buffer containing 0.05% SDS and 20% ethanol. Electrophoretic transfer was carried overnight at 30V, followed by blocking with Tween-TBS containing 8% non-fat dry milk and 1% BSA. FITC–70K was detected by incubating the membranes with rabbit anti-FITC IgG (0.2μg/ml) for 1h, followed by washing with Tween-TBS and incubation with peroxidase-conjugated goat and rabbit Ig (40ng/ml). After washing with Tween-TBS, blots were developed using chemiluminescence according to manufacturer’s instructions.
4.8. Cross-linking of FITC – 70K or FITC – FN to FN– /– cell surfaces and microscopic localization with integrins
FN null cells were plated on laminin-coated coverslips in DMEM-BSA for 2h before the addition of 40nM FITC–70K or 20nM FITC–FN in DMEM, 0.2% BSA containing 400nM LPA and incubated for various time points. Cells on coverslips were washed twice with PBS and treated with 1.5mM DTSSP for 15min. Cells were washed twice with TBS, once with 1% Triton X-100 for 5min and twice with PBS before fixing coverslips with 3.7% paraformaldehyde for 10min. After washing three times with PBS, coverslips were blocked with 5% BSA overnight at 4°C. Coverslips were incubated for 1 h with antibodies to integrin subunits: 5 μg/ml rat anti-mouse β 1, rat anti-mouse α 5 or rat anti-mouse α 6 diluted in 2% BSA in PBS. Following three PBS washes, coverslips were incubated with rhodamine-conjugated goat anti-rat IgG at 5 μg/ml for 1 h, washed three times with PBS, mounted and observed as described above.
Acknowledgments
We thank Drs. Donna Peters and Mats Johansson for helpful discussions and review of the manuscript. This work was supported by grants from the National Institutes of Health (HL21644).
References
- Aguirre KM, McCormick RJ, Schwarzbauer JE. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J Biol Chem. 1994;269:27863–27868. [PubMed] [Google Scholar]
- Akimov SS, Belkin AM. Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin: a role in TGFbeta-dependent matrix deposition. J Cell Sci. 2001;114:2989–3000. doi: 10.1242/jcs.114.16.2989. [DOI] [PubMed] [Google Scholar]
- Bae E, Sakai T, Mosher DF. Assembly of exogenous fibronectin by fibronectin-null cells is dependent on the adhesive substrate. J Biol Chem. 2004;279:35749–35759. doi: 10.1074/jbc.M406283200. [DOI] [PubMed] [Google Scholar]
- Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci U S A. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry EL, Mosher DF. Factor XIII cross-linking of fibronectin at cellular matrix assembly sites. J Biol Chem. 1988;263:10464–10469. [PubMed] [Google Scholar]
- Barry ELR, Mosher DF. Factor XIIIa-mediated cross-linking of fibronectin in fibroblast cell layers. Cross-linking of cellular and plasma fibronectin and of amino-terminal fibronectin fragments. J Biol Chem. 1989;264:4179–4185. [PubMed] [Google Scholar]
- Bittorf SV, Williams EC, Mosher DF. Alteration of vitronectin: characterization of changes induced by treatment with urea. J Biol Chem. 1993;268:24838–24846. [PubMed] [Google Scholar]
- Bultmann H, Santas AJ, Pesciotta Peters DM. Fibronectin fibrillogenesis involves the heparin II binding domain of fibronectin. J Biol Chem. 1998;273:2601–2609. doi: 10.1074/jbc.273.5.2601. [DOI] [PubMed] [Google Scholar]
- Chen H, Mosher DF. Formation of sodium dodecyl sulfate-stable fibronectin multimers occurs without detectable thioldisulfide exchange. J Biol Chem. 1996;271:2084–2089. doi: 10.1074/jbc.271.15.9084. [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Metsis ML, Koteliansky VE. Studies of extracellular fibronectin matrix formation with fluoresceinated fibronectin and fibronectin fragments. FEBS. 1985;183:365–369. doi: 10.1016/0014-5793(85)80811-5. [DOI] [PubMed] [Google Scholar]
- Choi MG, Hynes RO. Biosynthesis and processing of fibronectin in NIL. 8 hamster cells. J Biol Chem. 1979;254:12050–12055. [PubMed] [Google Scholar]
- Christopher RA, Kowalczyk AP, McKeown-Longo PJ. Localization of fibronectin matrix assembly sites on fibroblasts and endothelial cells. J Cell Sci. 1997;110:569–581. doi: 10.1242/jcs.110.5.569. [DOI] [PubMed] [Google Scholar]
- Clark K, Pankov R, Travis MA, Askari JA, Mould AP, Craig SE, Newham P, Yamada KM, Humphries MJ. A specific alpha5-beta1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation. J Cell Sci. 2005;118:291–300. doi: 10.1242/jcs.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussen F, Choquet D, Sheetz MP, Erickson HP. Trimers of the fibronectin cell adhesion domain localize to actin filament bundles and undergo rearward translocation. J Cell Sci. 2002;115:2581–2590. doi: 10.1242/jcs.115.12.2581. [DOI] [PubMed] [Google Scholar]
- Cue D, Southern SO, Southern PJ, Prabhakar J, Lorelli W, Smallheer JM, Mousa SA, Cleary PP. A nonpeptide integrin antagonist can inhibit epithelial cell ingestion of Streptococcus pyogenes by blocking formation of integrin alpha 5beta 1-fibronectin-M1 protein complexes. Proc Natl Acad Sci U S A. 2000;97:2858–2863. doi: 10.1073/pnas.050587897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamba BJ, Bultmann H, Akiyama SK, Peters DM. Substrate-specific binding of the amino terminus of fibronectin to an integrin complex in focal adhesions. J Biol Chem. 1994;269:19646–19652. [PubMed] [Google Scholar]
- Ensenberger MG, Tomasini-Johansson BR, Sottile J, Ozeri V, Hanski E, Mosher DF. Specific interactions between F1 adhesin of Streptococcus pyogenes and N-terminal modules of fibronectin. J Biol Chem. 2001;276:35606–35613. doi: 10.1074/jbc.M105417200. [DOI] [PubMed] [Google Scholar]
- Ensenberger MG, Annis DS, Mosher DF. Actions of the functional upstream domain of protein F1 of Streptococcus pyogenes on the conformation of fibronectin. Biophys Chemist. 2004;112:201–207. doi: 10.1016/j.bpc.2004.07.020. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, McKeown-Longo PJ. Fibronectin’s III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J Biol Chem. 1994;269:19183–19191. [PubMed] [Google Scholar]
- Hocking DC, Smith RK, McKeown-Longo PJ. A novel role for the integrin-binding III-10 module in fibronectin matrix assembly. J Cell Biol. 1996;133:431–444. doi: 10.1083/jcb.133.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, McKeown-Longo PJ. Activation of distinct alpha5beta1-mediated signaling pathways by fibronectin’s cell adhesion and matrix assembly domains. J Cell Biol. 1998;141:241–253. doi: 10.1083/jcb.141.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham KC, Brew SA, Isaacs BS. Interaction of fibronectin and its gelatin-binding domains with fluorescent-labeled chains of type I collagen. J Biol Chem. 1988;263:4624–4628. [PubMed] [Google Scholar]
- Isaacs BS, Brew SA, Ingham KC. Reversible unfolding of the gelatin-binding domain of fibronectin: structural stability in relation to function. Biochemistry. 1989;28:842–850. doi: 10.1021/bi00428a065. [DOI] [PubMed] [Google Scholar]
- Khan MY, Medow MS, Newman SA. Unfolding transitions of fibronectin and its domains. Stabilization and structural alteration of the N-terminal domain by heparin. Biochem J. 1990;270:33–38. doi: 10.1042/bj2700033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy DJ, Erickson HP, Aukhil I, Joshi P, Hendrickson WA. Crystallization of a fragment of human fibronectin: introduction of methionine by site-directed mutagenesis to allow phasing via selenomethionine. Proteins. 1994;19:48–54. doi: 10.1002/prot.340190107. [DOI] [PubMed] [Google Scholar]
- Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol. 1998;18:1363–1370. doi: 10.1161/01.atv.18.9.1363. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Quade BJ, Broekelmann TJ, LaChance R, Forsman K, Hasegawa E, Akiyama S. Fibronectin’s cell adhesive domain and an amino-terminal matrix assembly domain participate in its assembly in fibroblast pericellular matrix. J Biol Chem. 1987;262:2957–2967. [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983;97:466–472. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF, Huwiler KG, Misenheimer TM, Annis DS. Expression of recombinant matrix components using baculoviruses. Methods Cell Biol. 2002;69:69–81. doi: 10.1016/s0091-679x(02)69008-9. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Sakai T, Cho KH, Caparon MG, Fassler R, Bjorck L. Interactions with fibronectin attenuate the virulence of Streptococcus pyogenes. EMBO J. 2004;23:2166–2174. doi: 10.1038/sj.emboj.7600214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi T, Kiehart DP, Erickson HP. Dual labeling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. J Cell Sci. 2002;115:1221–1229. doi: 10.1242/jcs.115.6.1221. [DOI] [PubMed] [Google Scholar]
- Ozeri V, Tovi A, Burstein I, Natanson-Yaron S, Caparon MG, Yamada KM, Akiyama SK, Vlodavsky I, Hanski E. A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J. 1996;15:989–998. [PMC free article] [PubMed] [Google Scholar]
- Ozeri V, Rosenshine I, Mosher DF, Fässler R, Hanski E. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol Microbiol. 1998;30:625–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DM, Mosher DF. Localization of cell surface sites involved in fibronectin fibrillogenesis. J Cell Biol. 1987;104:121–130. doi: 10.1083/jcb.104.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DM, Portz LM, Fullenwider J, Mosher DF. Co-assembly of plasma and cellular fibronectins into fibrils in human fibroblast cultures. J Cell Biol. 1990;111:249–256. doi: 10.1083/jcb.111.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci U S A. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer JE. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J Cell Biol. 1991;113:1463–1473. doi: 10.1083/jcb.113.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J, Schwarzbauer J, Selegue J, Mosher DF. Five type I modules of fibronectin form a functional unit that binds to fibroblasts and Staphylococcus aureus. J Biol Chem. 1991;266:12840–12843. [PubMed] [Google Scholar]
- Sottile J, Hocking DC, Swiatek PJ. Fibronectin matrix assembly enhances adhesion-dependent cell growth. J Cell Sci. 1998;111:2933–2943. doi: 10.1242/jcs.111.19.2933. [DOI] [PubMed] [Google Scholar]
- Sottile J, Hocking DC, Langenbach KJ. Fibronectin polymerization stimulates cell growth by RGD-dependent and -independent mechanisms. J Cell Sci. 2000;113 (Pt 23):4287–4299. doi: 10.1242/jcs.113.23.4287. [DOI] [PubMed] [Google Scholar]
- Tomasini-Johansson BR, Kaufman NR, Ensenberger MG, Ozeri V, Hanski E, Mosher DF. A 49-residue peptide from adhesin F1 of Streptococcus pyogenes inhibits fibronectin assembly. J Biol Chem. 2001;276:23430–23439. doi: 10.1074/jbc.M103467200. [DOI] [PubMed] [Google Scholar]
- Ugarova TP, Zamarron C, Veklich Y, Bowditch RD, Ginsberg MH, Weisel JW, Plow EF. Conformational transitions in the cell binding domain of fibronectin. Biochemistry. 1995;34:4457–4466. doi: 10.1021/bi00013a039. [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Schwarzbauer JE. Regulatory role for SRC and phosphatidylinositol 3-kinase in initiation of fibronectin matrix assembly. J Biol Chem. 2002;277:19703–19708. doi: 10.1074/jbc.M200270200. [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Schwarzbauer JE. The ins and outs of fibronectin matrix assembly. J Cell Sci. 2003;116:3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- Williams EC, Janmey PA, Johnson RB, Mosher DF. Fibronectin. Effect of disulfide bond reduction on its physical and functional properties. J Biol Chem. 1983;258:5911–5914. [PubMed] [Google Scholar]
- Zhang Q, Mosher DF. Crosslinking of the N-terminal region of fibronectin to molecules of large apparent molecular mass (LAMMs): characterization of fibronectin assembly sites induced by the treatment of fibroblasts with lysophosphatidic acid. J Biol Chem. 1996;271:33284–33292. doi: 10.1074/jbc.271.52.33284. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Checovich WJ, Peters DM, Albrecht RM, Mosher DF. Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J Cell Biol. 1994;127:1447–1459. doi: 10.1083/jcb.127.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Sakai T, Nowlen J, Hayashi I, Fässler R, Mosher DF. Functional beta 1 integrins release the suppression of fibronectin matrix assembly by vitronectin. J Biol Chem. 1999;274:368–375. doi: 10.1074/jbc.274.1.368. [DOI] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]


