Abstract
Sympathetic axons embedded in a few arterioles and vasa vasora were recently shown to store tissue plasminogen activator (t-PA) in vesicles. But the extension of such t-PA axons to arteries and arterioles throughout the organism has not been verified. Confirmation of this anatomy would identify a second significant source of vessel wall t-PA. To visualize fine embedded axons independent of endothelium, we created a transgenic mouse whose expressions of the t-PA promoter and enhanced green fluorescent protein are confined to sympathetic neurons and other neural crest derivatives. Confocal images reveal the extension of t-PA axons to arterioles serving heart, brain, kidney, lung, mesentery, and skin; plus aortic, carotid, and mesenteric artery walls. Ganglion neurons and adrenal chromaffin cells also show strong expressions. These new sightings confirm the existence of a system of t-PA axons that is prominent in arterioles, and compatible with the release of neural t-PA into their walls. (Blood. 2006;108:200-202)
Introduction
The presence of tissue plasminogen activator (t-PA) in vascular sympathetic nerves is of interest because of the potential of the released enzyme to activate plasmin proteolysis and its multiple effects within vessel walls.1,2 Sympathetic neurons have recently been shown to synthesize and axonally transport the enzyme in closely packed vesicles, which their axons—in a few small arteries, arterioles, and vasa vasora—have been shown to store.3-5 But the extension of such filaments to small arteries and arterioles throughout the vascular system has not yet been shown. Morphologic confirmation of a t-PA presence within nerve fibers is obviously needed before they can be thought to release the enzyme into vessel walls. Also, demonstrating the existence of a systemic network of t-PA axons would identify an additional, nonendothelial source of t-PA concentration within the vascular system. However, fine (0.5 μm-2.0 μm) axons6 embedded beneath the adventitia of small arteries and arterioles have been especially difficult to differentiate from endothelium in t-PA immunolocalizations,7,8 and endothelial t-PA storage vesicles have been described as possible sources of its acute regulated release.9-11
To better identify t-PA axons in situ, we obtained a new transgenic mouse that specifically targets the t-PA promoter present in sympathetic nerves and other neural crest-derived cells, and crossed with a reporter expressing enhanced green fluorescent protein (EGFP). The prime intent was to verify that axons able to express t-PA are embedded in multiple arterial walls, prior to studies of the release pathway.
Study design
Transgenic mouse model preparation
A mouse line that expresses Cre recombinase under the control of the human tissue plasminogen activator promoter was provided by Professor Sylvie Dufour, Curie Institute, Paris, France.12 Its t-PA Cre transgene is specifically expressed in embryonic neural crest-derived cells, including sympathetic neurons. Crossed with a Z/EG reporter expressing the Lac Z/EGFP transgene, the floxed LacZ is deleted by Cre recombinase, localizing EGFP and the t-PA promoter expressions within separate genes in the same cells. Localization of the promoter, on which tissue-specific t-PA expression depends, with EGFP will therefore identify neural crest-derived axons able to express the t-PA protein. The present model is not designed to show their co-release in response to sympathetic stimulations, as the promoter and EGFP within the same cell lie on different genes. Offspring positive for the t-PA/Cre transgene were selected by genotyping as described elsewhere.13
Tissue recovery and imaging
Tissues were dissected from 27 terminally anesthetized adult male and female offspring at 4 to 6 months, and prepared as sections or whole mounts as previously described.14 Procedures met National Institutes of Health guidelines and were approved by an institutional animal care committee. Preparations were imaged using a Zeiss LSM 510 Meta confocal microscope with a 10 ×/0.3 or 40 ×/1.3 Plan Neofluar objective lens (Carl Zeiss, Jena, Germany) while exciting at 488 nm. Imaging medium was air for the 10 × objective and immersion oil for the 40 × objective. Z axis sections were imaged at 10-μm to 50-μm depths, and sections revealing the nerve fibers with minimal background autofluorescence were printed. LSM 510 version 3.2 software (Carl Zeiss) was used to acquire digital fluorescence images; SP2 software (Zeiss, Thornwood, NY), to acquire digital confocal images; and Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA) was used for color printing. Immunostaining seen in Figure 2D was done as previously described.5
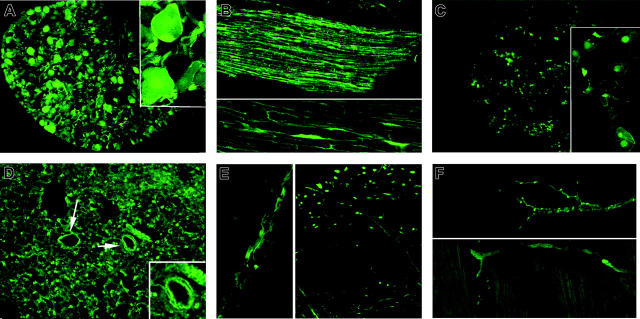
Figure 2.
Vessel-wall t-PA/EGFP expression. (A) Aorta surface: 3D Z-axis composite of adventitial layers shows positve expression by a few discrete axons compatible with sparse innervation. (B) Carotid artery surface: 3D Z-axis composite of adventitial layers reveals positive expression by numerous postitve axons. (C) Renal artery surface: positive expression is confined to a fairly dense nerve net with prominent varicose dilations. (D) Mesenteric artery surface: numerous axons with bright terminal varicosities appear in deep adventitial layer (left). Section immunostained for t-PA shows dense subadventitial plexus on outer smooth-muscle surface (brown color; right). (E) Ear skin surface: positive expression appears confined to walls of branching arteriole, absent from numerous adjacent venules and capillaries. (F) Sciatic nerve section: expression is positive in walls of multiple nutrient vasa nervosa (arrows) and in scattered cross-cut axons. Magnifications are ×40, except as noted.
Results and discussion
Specificity of localizations
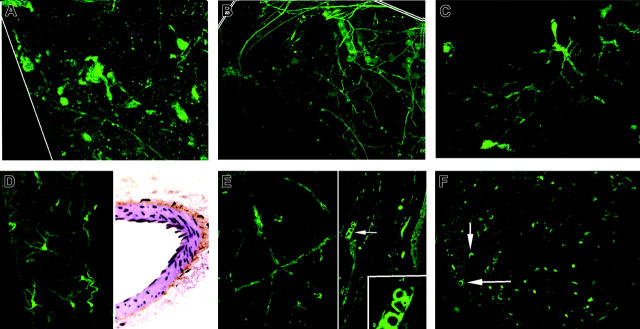
Specificity of the EGFP markings for cells expressing the t-PA promoter was first tested in known neural crest derivatives.15 Neuron cell bodies in the superior cervical sympathetic ganglion showed a distinct bright fluorescence confined to the nucleus, cytoplasm, and cell membrane, as well as the bright specks of adjacent cross-cut axons (Figure 1A). The paravertebral sympathetic chain showed a similar positive fluorescence confined to individual slender axons (Figure 1B). Consistent with past immunolocalizations,16 adrenal medullary chromaffin cells, whose neural crest origin is similar to that of sympathetic neurons, showed a discrete bright fluorescence that was confined to their nuclei but absent in the adjacent cortex (Figure 1C).
Figure 1.
Sympathetic system t-PA/EGFP expressions. (A) Cervical ganglion section: expression confined to neuron cell bodies and axons (inset, ×100). (B) Sympathetic chain longitudinal section: discrete expression confined within individual axons. (C)Adrenal medulla section: chromaffin cell nuclei show prominent expression (inset, ×100); cortex negative. (D) Lung: positve cross-cut fibers, presumed axons, dot all regions of the interstitium. Few appear in bronchial arteriolar walls (arrows and inset). (E) Brain surface: axon showing positive expression is embedded in wall of anterior cerebral artery branch (left). Positive expression is also evident in cross-cut and full-length individual axons (right). (F) Axon terminals heart surface: prominent expression by typical intermittent varicosities in the walls of a branching coronary arteriole (above) and in myocardium (below). Magnifications are ×40, except as noted.
Arterial/arteriolar axon localizations
The presence of embedded t-PA axons in arterial and arteriolar walls was observed in multiple locations. Most of these subadventitial fibers display intermittent, discrete varicosities, a hallmark of sympathetic axon terminals and the storage site of vesicles containing norepinephrine.6 The implied coexistence of t-PA with the neurotransmitter accords with previous reports suggesting their co-release via the regulated pathway.3
Discrete varicose t-PA axon terminals appear in the walls of coronary artery branches on the heart surface and within the myocardium (Figure 1F). Lung sections show uncountable fluorescent cross-cut fibers among the alveoli and interstitium, but scant evidence of a positive t-PA axon fluorescence in small vessels (Figure 1D). This appears compatible with the paucity of autonomic innervation of pulmonary vessels.17
Axons showing positive expression also appear in walls of the anterior cerebral artery. Frontal cortex whole mounts display many cross-cut axons and individual fine, wavy surface axons (Figure 1E).
Surfaces of the aorta and carotid arteries display multiple curving axon filaments not previously visualized in our past immunolocalizations5 (Figure 2A-B). More numerous axon terminals appear in the outer walls of the renal artery (Figure 2C). Surface views of the mesenteric artery also confirm the presence of still more varicose t-PA axons. Sections immunostained for neuropeptide Y confirm the adrenergic identity and subadventitial location of a dense axon plexus layer (Figure 2D). Sciatic nerve microvessels, presumably vasa nervosa, display a positive fluorescence within their thin walls (Figure 2F). Similar to prior immunostainings of vasa vasoram,5 their presence appears consistent with the peripheral extension of t-PA axons to small nutrient microvessels. Positive immunostaining of these vessel walls for neuropeptide Y confirm the adrenergic identity of embedded t-PA axons (not shown). The dense micromesh of vessels normally seen in ear skin18 here displays only intermittent fluorescent varicosities confined to its branching arterioles (Figure 2E), but not visible in larger veins or capillaries. Sections of the skin arterioles reveal a prominent positive t-PA axon fluorescence throughout the wall thickness, consistent with their typically dense adrenergic innervation.19,20
Collectively, the images offer a first glimpse of the extension of t-PA axons to all sizes of arteries and arterioles, with the greatest concentration in arterioles. The greater number now identified also suggests the storage of a sizeable reservoir of neural t-PA within the vast microvasculature, in addition to that in its endothelium. Beyond showing the extent and patterns of a t-PA axon network, there remains the need to determine whether sympathetic excitation induces release of the whole enzyme from vascular axon terminals per se, or only from endothelium. Indirect evidence for an axonal source includes (1) large decreases of blood fibrinolytic activity following guanethidine sympathetomy, which selectively destroys the axon terminals21; (2) agonist-stimulated t-PA release from isolated arterial segments in vitro is proportional to the sympatheic innervation density, and inversely related to the endothelial surface area22; (3) abrupt large increases in regional blood t-PA activity during in vivo electrical stimulations of sympathetic nerves and norepinephrine infusions23,24; (4) more modest and gradual (1.5×, 30 minutes) increases in t-PA release from resting cultured endothelial cells following epinephrine infusion; and (5) confirmed prominent arteriolar t-PA immunostaining in striated muscle arterioles, but not in venules.25 The possible co-release of t-PA and EGFP from stimulated axon terminals is under study.
We conclude that the extension of sympathetic axons able to express t-PA to multiple artery walls is revealed in a new transgenic mouse. Expressions of the t-PA promoter and EGFP appear most prominent in axons embedded in arteriolar walls.
Acknowledgments
We thank Ms Donna Fiorente for editorial assistance.
Prepublished online as Blood First Edition Paper, March 2, 2006; DOI 10.1182/blood-2005-12-4884.
Supported by National Institutes of Health (NIH) grant EY 13 243 and the Lions Clubs International Foundation.
Z.H. performed research and assisted with tissue sample preparations; C.G. performed transgenic breeding; X.J. assisted in tissue preparations and immunostaining; S.K. performed confocal tissue sample analysis; S.D. and T.P. provided and oversaw use of screened promoter line; R.E.C. oversaw immunolocalizations; and J.O'R. designed and supervised the project and wrote the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Syrovets T, Simmet T. Novel aspects and new roles for the serine protease plasmin. Cell Mol Life Sci. 2004;61: 873-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myohanen H, Vaheri A. Regulations and interactions in the activation of cell-associated plasminogen. Cell Mol Life Sci. 2004;61: 2840-2858. [DOI] [PubMed] [Google Scholar]
- 3.Parmer RJ, Mahata S, Sebald MT, O'Connor DT, Miles LA. Tissue plasminogen activator (t-PA) is targeted to the regulatory secretary pathway; catecholamine storage vesicles as a reservoir for the rapid release of t-PA. J Biol Chem. 1997;292: 1976-1982. [DOI] [PubMed] [Google Scholar]
- 4.Lochner JE, Kingma M, Kuhn S, Meliza CD, Cutler B, Scalettar BA. Real time imaging of the axonal transport of granules containing a tissue plasminogen activator/green fluorescent protein hybrid. Mol Biol Chem. 1998;9: 2463-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang X, Wang Y, Hand AR, Gillies C, Cone RE, O'Rourke J. Presence of tissue plasminogen activator (t-PA) in the adventitial nerves that innervate small arteries: morphologic evidence for a neural fibrinolysis. Fibrinolys and Proteolys. 2000:14: 35-46. [Google Scholar]
- 6.Burnstock G, Costa M. Adrenergic Neurons: Their Organization, Function and Development in the Peripheral Nervous System. London, England: Chapman and Hall; 1975:19-37.
- 7.Levin E, Del Zoppo GJ. Localization of tissue plasminogen activator in the endothelium of a limited number of vessels. Am J Pathol. 1994;144: 855-861. [PMC free article] [PubMed] [Google Scholar]
- 8.Levin EG, Santell L, Osborn KG. The expression of endothelial tissue plasminogen activator in vivo: a function defined by vessel size and anatomic location. J Cell Sci. 1997;110: 139-148. [DOI] [PubMed] [Google Scholar]
- 9.Van den Eijnden-Schrauwen, Kooistra T, deVries REM, Emeis JJ. Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood. 1995;85: 3510-3517. [PubMed] [Google Scholar]
- 10.Emeis JJ, van den Eijnden-Schrauwen Y, van den Hoogen W, de Priester W. An endothelial storage granule for tissue-type plasminogen activator. J Cell Biol. 1997;39: 245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fosnoblet C, Visccher UM, Gerard RD, Irminger J-C, Halban PA, Kruithof EKO. Storage of tissue-type plasminogen activator in Weibel-Palade bodies of human endothelial cells. Arterioscler Thromb Vasc Biol. 199l;19: 1976-1803. [DOI] [PubMed] [Google Scholar]
- 12.Pietri T, Eder O, Blanche M, Thiery JP, Dufour S. The human plasminogen activator-Cre mouse: a new tool for targeting specifically neural crest cells and their derivatives in vivo. Dev Biol. 2003;259: 176-187. [DOI] [PubMed] [Google Scholar]
- 13.Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28: 147-155. [PubMed] [Google Scholar]
- 14.JiangX, WangY, Hand AR, et al. Storage and release of tissue plasminogen activator by sympathetic axons in resistance vessel walls. Microvasc Res. 2002;64: 438-447. [DOI] [PubMed] [Google Scholar]
- 15.Hall B, Houstadius S. The Neural Crest. London: Oxford University Press; 1988: 3-19.
- 16.Kristensen P, Hougaard DM, Nielsen LS, Dano KO. Tissue-type plasminogen activator in rat adrenal medulla. Histochemistry. 1986;85: 431-436. [DOI] [PubMed] [Google Scholar]
- 17.Guyton AC, Hall JE. Pulmonary circulation; pulmonary edema; pleural fluid. In: Textbook of Medical Physiology. 9th ed. Philadelphia, PA: W.B. Saunders, 1996; 491-499.
- 18.Saunders RL de CH. X-ray microscopy. In: Winters WL, Brest AN, eds. The Microcirculation. Springfield, IL: Charles C Thomas; 1969: 50-63.
- 19.Burnstock G. Innervation of vascular smooth muscle: histochemistry and electron microscopy. Clin Exper Pharm Physiol. l975;2: 7-20. [PubMed] [Google Scholar]
- 20.Luff SE. The ultrastructure of arterioles. In: Bevan JA, ed. The Resistance Vasculature. Totowa, NJ: Humana Press; l998: 93-113.
- 21.Wang Y, Jiang X, Hand AR, et al. Additional evidence that the sympathetic nervous system regulates the vessel wall release of tissue plasminogen activator. Blood Coagul Fibrinolysis. 2002;13: 471-481. [DOI] [PubMed] [Google Scholar]
- 22.Hao Z, Jiang X, Sharafeih R, Cone RE, Hand AR. Stimulated release of tissue plasminogen activator from artery wall sympathetic nerves: implications for stress-associated wall damage. Stress. 2005;8: 141-149. [DOI] [PubMed] [Google Scholar]
- 23.Jern C, Selin L, Jern S. In vivo release of tissue-type plasminogen activator across the human forearm during mental stress. Thromb Haemost. 1994;72: 285-291. [PubMed] [Google Scholar]
- 24.Bjorkman JA, Jern S, Jern C. Cardiac sympathetic nerve stimulation triggers coronary t-PA release. Arterioscler Thromb Vasc Biol. 2003;23: 1091-1097. [DOI] [PubMed] [Google Scholar]
- 25.Huber D, Cramer EM, Kaufinann JE, et al. Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endotheial cells both in vitro and in vivo. Blood. 2002;99: 3637-3645. [DOI] [PubMed] [Google Scholar]