Abstract
Background
The cytolethal distending toxin (CDT) of Actinobacillus actinomycetemcomitans is a typical member of this Gram-negative bacterium holotoxin family that targets a wide spectrum of eukarytotic cells, typically causing cell cycle arrest at either the G1 or G2/M phase of the cell cycle. In view of the possible role of the CDT as a prominent A. actinomycetemcomitans virulence factor in periodontal diseases, we have examined the effects of the toxin on primary cultures of human periodontal ligament fibroblasts (HPLF).
Methods
HPLF and an immortalized human gingival epithelial cell line, GMSM-K, were exposed to recombinant A. actinomycetemcomitans CDT. Effects of the toxin on cell proliferation and cell cycle were assessed by a cell viability assay and flow cytometry, respectively. Double-strand DNA damage was detected by pulsed field gel electrophoresis. Binding of the toxin and its individual subunits to HPLF was examined by immunofluorescence microscopy.
Results
Viability of HPLF was not reduced following prolonged exposure to the CDT. There was no indication of cell cycle arrest or double-strand DNA damage. GMSM-K cells exhibited morphological alterations and a rapid decrease in cell viability within 6 and 12 hours, respectively, following exposure to the toxin for 5 minutes. These effects were dependent on toxin dose and age of the cultures and occurred more rapidly compared to CDT-treated HeLa cells. CDT-treated GMSM-K cells displayed cell cycle arrest at the S phase of growth and double-strand DNA damage was observed by 6 hours post-intoxication. Holotoxin and the CdtA subunit were detected on the surface of both HPLF and epithelial cells.
Conclusions
These results demonstrate that HPLF are resistant to the cytotoxic effects of the A. actinomycetemcomitans CDT. The mechanism of resistance is not known but may be related to the inability of the toxin to cause DNA damage. The difference in sensitivities of HPLF and oral epithelial cells to the CDT has important implications for the role of this putative microbial virulence factor in periodontal pathogenesis.
Keywords: Actinobacillus actinomycetemcomitans; cell cycle; cells, epithelial; fibroblasts, periodontal; periodontal diseases/pathogenesis; toxin, cytolethal distending
Cytolethal distending toxin (CDT) is a secreted bacterial protein holotoxin that induces growth arrest in a wide variety of eukaryotic cells. The holotoxin is the product of three genes expressed by a handful of facultative or microaerophilic Gram-negative pathogenic bacterial species. The species identified to date that express a biologically active CDT include select strains of enteropathogenic Escherichia coli,1,2 Campylobacter jejuni,3 Campylobacter upsaliensis,4 Campylobacter coli,5 Shigella dysenteriae,6,7 Haemophilus ducreyi,8 Helicobacter hepaticus,9,10 Helicobacter flexispira,11 Helicobacter bilis,9 Helicobacter canis,9 and Actinobacillus actinomycetemcomitans.12-14 Organization of the genetic locus and the structure and biological activity of the holotoxin are fairly well conserved among the CDT produced by these genera.8,10,12,15,16 The cdt locus is a polycistronic operon containing three essential genes, cdtA, cdtB, and cdtC, which encode for polypeptides having sizes of 27 to 30, 29 to 32, and 20 kDa, respectively.
The various CDTs inhibit the proliferation of immortalized human cell lines, including HeLa,3,17-20 KB,3 HEp-2,3,21-23 Vero,3 HaCat,22,24 Jurkat,25 and Caco-2,20 as well as primary human cells such as lymphocytes4,13,25 and fibroblasts.26-28 The initial step that leads to cytotoxicity appears to be the introduction of double-strand DNA breaks, most likely due to the activity of the CdtB polypeptide.29-31 Subsequent growth arrest results from a block in cell cycle progression. However, the location of the block is cell line or strain dependent. The epithelial cells lines HeLa,10,14,17-20 HEp-2,21 and Caco-220 are arrested at the G2/M phase transition due to inhibition of dephosphorylation of the checkpoint protein kinase Cdc2 (Cdk1) by the protein phosphatase Cdc25. Cdc2 forms a complex with cyclin B which is made in S phase. In order to form this complex, Cdc2 is phosphorylated at residues threonine-161, tyrosine-15, and threonine-14. The Cdc2-cyclin B complexes are inactive and accumulate during S and G2. Cdc25 dephosphorylates threonine-14 and tyrosine-15, which activate the Cdc2-cyclin B complexes, sending the cells into mitosis (summarized in reference 32).
In contrast, it was found that human foreskin and embryonic lung fibroblasts exposed to the H. ducreyi CDT were blocked at both the G1 and G2 phases.33 Toxin-treated fibroblasts exhibited induced expression of the tumor suppressor gene p53 as well as significantly increased expression of p21, an inhibitor of cyclin-dependent kinase activity. Non-proliferating primary human fetal fibroblasts treated with C. jejuni toxin arrested in the G1 phase once they were stimulated to proliferate.28 Belibasakis et al.26 reported that the A. actinomycetemcomitans CDT exhibits a non-lethal inhibition of proliferation of human periodontal ligament (HPLF) and gingival (HGF) fibroblasts. It has also been found that murine NIH 3T3 fibroblasts are resistant to the H. durceyi CDT.22
Actinobacillus actinomycetemcomitans is the only indigenous member of the human oral flora identified to date that expresses the CDT. This bacterium is a facultative Gram-negative pathogen that is strongly associated with the development of localized and generalized forms of aggressive periodontitis. This species produces a variety of products that can be classified as potential virulence factors (see reference 34 for review), but is most notable for the expression of several multi-gene toxins including a leukotoxin and the CDT. A large percentage of strains of A. actinomycetemcomitans isolated from the human oral cavity express biologically active CDT.12,35 However, it has been difficult to demonstrate a clear link between the presence of strains of the bacterium that produce an active CDT and active periodontal lesions.35,36 The greatest potential for involvement of A. actinomycetemcomitans in oral disease is through interactions with the various cellular components of periodontal tissues. Fibroblasts are the predominant cell type within the periodontal ligament (PDL). This heterogeneous population of cells plays an important role in the normal maintenance, repair, and regeneration of not only the PDL but also the cementum and investing alveolar bone.37 To assess the potential role of the A. actinomycetemcomitans CDT in periodontal pathogenesis, it is essential to characterize specific effects on cells making up the human periodontium including PDL (HPLF) fibroblasts.
In this study we examine the effects of recombinant A. actinomycetemcomitans CDT on the proliferation and cell cycle of HPLF in primary cultures. The results are compared to those obtained with an immortalized human oral epithelial cell line.
MATERIALS AND METHODS
Bacterial Strains, Growth Conditions, and Preparation of CDT
Recombinant clones containing all three A. actinomycetemcomitans cdt genes (E. coli DH5α [pCDT1], E. coli BL-21[DE3] [pET15bcdt]), combinations of genes (E. coli BL-21[DE3] [pET15bcdtAB], E. coli BL-21[DE3] [pET15bcdtBC]), and the individual genes (E. coli BL-21[DE3] [pET15bcdtA], E. coli BL-21[DE3] [pET15bcdtC], E. coli BL-21[DE3] [pET15bcdtB]) were previously constructed and characterized.12,38 For preparation of CDT-containing extracts, single colonies of the appropriate clone were inoculated into 10 ml of LB medium containing 50 μg/ml ampicillin and incubated overnight at 37°C with vigorous shaking. Overnight cultures were added to 100 ml of the same medium and grown until late log phase (OD600 = 0.8 to 1.0) was reached. IPTG was added to a final concentration of 1 mM, and the cells grown for an additional 4 to 5 hours. The bacteria were harvested by centrifugation, washed twice with phosphate-buffered saline (PBS), and suspended in PBS. The bacterial suspensions were sonicated‡ in an ice bath using three 30-second pulses separated by 30-second rest. The lysed cells were centrifuged at 12,000 × g for 10 minutes to remove unbroken cells and sterilized by passage through a 0.22 μm pore size filter.§ Total protein concentration was determined with a protein assay kit. The TD50 concentration was determined as described previously.12 Expression of the cdt genes was assessed by analysis of total cell protein on 10% to 20% polyacrylamide Tris-HCl gels.∥ An aliquot of the lysed bacterial suspension was boiled in gel loading buffer (2% SDS, 0.05 M Tris-HCl, pH 6.8, 10% glycerol) for 5 minutes. Apparent molecular size was determined by comparison to prestained molecular weight standards.∥ For some experiments, His6-tagged CdtA, CdtB, and CdtC were purified and the holotoxin reconstituted as previously described.38
Cells and Tissue Culture Conditions
Human epithelial-like (HeLa) cells were used as a standardized cell line because they exhibit a well-characterized response to the CDT. These cells were grown in Eagle's minimal essential medium¶ supplemented with 10% fetal bovine serum (FBS).
Human periodontal ligament fibroblasts (HPLF) were derived from periodontal ligament explants using a technique similar to that described by Somerman et al.39 Clinically healthy third molars were obtained from five patients (three males and two females; age range 23 to 28 years) who presented to the Oral Surgery Clinic of the University of Pennsylvania School of Dental Medicine. Immediately following extraction, the teeth were placed into modified Eagle's medium (DMEM),¶ supplemented with penicillin G (10,000 U/ml), streptomycin (25 μg/ml), and fungizone (0.85%). Within 1 hour of extraction the teeth were washed twice with DMEM and the attached gingival tissue removed. The teeth were then placed in a petri dish containing DMEM and the periodontal ligament dissected away from the mid-third of the root surface by gentle scraping with a disposable surgical scalpel. The collected tissue was centrifuged at 1,000 rpm for 10 minutes at 4°C. The tissue was suspended in 5 ml of growth medium supplemented with 10% fetal calf serum (FCS) and incubated at 37°C in 5% CO2. Monolayers of HPLF were typically observed 2 to 3 weeks after the initial seeding of cultures. Upon reaching confluence, the primary cultures were treated with trypsin (1 mg/ml in PBS) to detach the cells for passaging. Cells were stored in 2 ml of 10% dimethylsulfoxide (DMSO) and 90% DMEM in liquid nitrogen. Cells in the second through seventh passages were used for CDT assays since they typically became senescent in later passages.
Multiple attempts to isolate and maintain pure primary cultures of human gingival epithelial cells were inconsistent. Therefore, an immortalized human oral epithelial cell line was used to compare the effects of the CDT to those obtained with the oral fibroblasts. This cell line, GMSM-K, was grown in keratinocyte serum-free medium¶ as reported.40 Cells in the second through fifth passages were used for CDT assays.
All protocols for obtaining and handling human oral tissues were reviewed and approved by the Institutional Review Board of the University of Pennsylvania.
Microscopy
HeLa cells, HPLF, and GMSM-K cells were examined by bright field microscopy. Cells were seeded in T-75 flasks and incubated for 24 hours. Filter sterilized sonicate from E. coli BL-21(DE3) (pET15bcdt), prepared as described above, was added to cultures at a concentration of 18.4 μg total protein per ml of medium (TD50). The cultures were then incubated up to 96 hours. A second set of cultures received the same concentration of bacterial extract. After incubation for 5 minutes at 37°C, the cells were washed to remove unbound toxin and fresh medium was added. The cultures were then incubated for up to 96 hours post-intoxication. A third set of control cultures did not receive bacterial extract. Cultures were viewed using an inverted microscope.# Images were captured** at 6, 12, 24, 48, 72, and 96 hours after addition of the extracts.
Kinetics of Response to the CDT
HPLF and GMSM-K cell suspensions were seeded in 96-well tissue culture plates (15,000 cells/well) in 0.1 ml/well of appropriate medium. Cultures were incubated overnight at 37°C with 5% CO2 to allow the cells to attach to the plates. For dose response kinetic assays, 1 to 1,000 ng of total soluble protein from E. coli BL-21(DE3) (pET15bcdt) and E. coli BL-21(DE3) (pET15b) were added to triplicate wells. One set of triplicate wells did not receive any bacterial extract. The cultures were incubated for 48 hours after addition of the extracts. Rate kinetics were performed by adding 5 μg of total soluble protein from E. coli BL-21(DE3) (pET15bcdt) and E. coli BL-21(DE3) (pET15b) to 15,000 cells/well in triplicate. The cultures were incubated for 5 minutes at 37°C with 5% CO2. One set of cultures did not receive bacterial extract. All cultures were then washed to remove unbound toxin and fresh medium was added. The cultures were incubated an additional 0.5 to 36 hours.
Following the various CDT treatments, cell viability was assayed using a cell proliferation assay kit.†† The assay was performed according to the manufacturer's instructions and absorbance of each well was read at 492 nm in a microplate reader. Reduction in cell viability was recorded as a decrease in the A492 relative to untreated cells grown under the same conditions. Cell culture experiments were repeated a minimum of three times and all samples were run in triplicate in each replicate experiment. Standard curves of absorbance versus cell number were prepared to determine appropriate cell concentrations for use in the kinetic experiments. A linear increase in the A492 of the cell cultures over the range of 5,000 to 15,000 cells/well was observed (data not shown).
Cell Cycle Analysis
Cell cycle distribution was determined for untreated and CDT-treated cell populations by flow cytometry. One set of overnight cultures was treated with 18.4 μg of total soluble protein from E. coli BL-21(DE3) (pET15bcdt)/ml of medium. Cultures were incubated 3 to 72 hours post-intoxication. Nuclei from washed cells were isolated and stained by the Vindelov procedure.41 Briefly, cells (1 × 106) were suspended in 1 ml of filter sterilized Tris-buffered saline (pH 7.6) containing (10 μg/ml ribonuclease A,‡‡ 7.5 μg/ml propidium iodide,‡‡ and 0.1% Nonidet P-40‡‡ for 1 to 2 hours. In other experiments HPLF and HGF were fixed in 70% cold ethanol overnight at −20°C, treated with RNase, and stained with propidium iodide. Stained nuclei and cells were analyzed on a flow cytometer§§ at the University of Pennsylvania Cancer Center Flow Cytometry and Cell Sorting Shared Resource facility. The data from 30,000 events were analyzed with a software package.∥∥
Pulse-Field Gel Electrophoresis (PFGE)
HeLa, GMSM-K cells, and HPLF cultures (5 × 106 cells) were incubated overnight. One set of cultures received an LD50 equivalent dose (4.5 μg/ml of medium) of total soluble protein from E. coli BL-21(DE3) (pET15bcdt). A second set of cultures did not receive any extract. Cells were collected after incubation for 3 to 72 hours post-intoxication and suspended in 500 μl of 1% agarose¶¶ made up in F12 medium. Plugs (35 μl) were formed and the cells lysed by suspension in a solution containing 1 mg/ml proteinase K,‡‡ 1% sarcosyl,‡‡ and 0.5 M EDTA (pH 8.0). The suspended plugs were shaken overnight at 50°C. The plugs were washed with 0.5 M EDTA and treated with 0.1 mg/ml DNase-free RNase‡‡ for 30 minutes at 37°C. The plugs were applied to wells cast in a 0.8% agarose gel, containing 0.5 μg/ml ethidium bromide, and electrophoresis was performed at 175 V (starting voltage) for 40 hours at 4°C in a PFGE apparatus. The ratio of DNA in the well and in the gel was estimated from digitized images using a software program.##
Immunofluorescence
Detection of binding of the Cdt polypeptides to the surface of HeLa, HPLF, and GMSM-K cells was performed as described previously.38 Briefly, cells were suspended in the appropriate growth medium and added to each well (1 × 104 cells per well) of an eight-well chamber slide*** and incubated for 48 hours at 37°C in a moist atmosphere containing 5% CO2. Slides used for GMSM-K cells were precoated with 0.2% gelatin to aid cellular attachment. Slides were washed and purified individual His6-tagged proteins or reconstituted holotoxin were added at a concentration of 1 to 5 μg per well. The slides were then incubated on ice for 15 to 30 minutes, washed twice with PBS, and fixed in 10% formalin for 5 minutes at room temperature. The slides were washed, blocked with 3% BSA in PBS, and then incubated with a 1:1,000 dilution of anti-His•Tag monoclonal anti-body††† for 1 hour at room temperature. The slides were then washed and incubated in the dark with a 1:1,000 dilution of goat anti-mouse IgG‡‡‡ (heavy and light chain) conjugate. The slides were thoroughly washed, followed by the addition of mounting solution.§§§ Coverslips were placed on the slides, which were viewed under a fluorescent microscope.∥∥∥ Binding experiments were repeated a minimum of three times.
Statistical Methods
Mean values and standard deviations were plotted. The paired t test (P = 0.05) was used to evaluate the data in some experiments.
RESULTS
Characterization of cdt Gene Products Produced by E. coli BL-21(DE3) (pET15b cdt)
The A. actinomycetemcomitans cdt genes were previously cloned in E. coli to enhance expression, provide a method for isolation of the gene products, and express the CDT free from a background of other reported cytotoxic proteins produced by this oral pathogen (see reference 19 for review). Two recombinant strains E. coli DH5α (pCDT1)12 and E. coli BL-21(DE3) (pET15bcdt)28 contain the cdt locus cloned in two different vectors (pBluescript II SK+ and pET15b). Both constructs yielded active holotoxin based on the inhibition of proliferation of control HeLa cells 48 hours post-intoxication (Fig. 1A). The pET15bcdt construct produces toxin with a His-tag on the amino terminus of the CdtA polypeptide.37 It is possible that the His-tag alters the activity of the toxin. However, both forms of the recombinant toxin had equivalent cytotoxic activities.
Figure 1.

Characterization of cdt gene products produced by E. coli BL-21(DE3) (pET15bcdt). A) No bacterial extract or total soluble protein extract (10 ng of protein/μl of medium) from A. actinomycetemcomitans Y4, E. coli DH5α (pBluescript II SK+), E. coli DH5α (pCDT1), or E. coli BL-21(DE3) (pET15bcdt) was added to cultures of HeLa cells. *Statistically significant differences, P<0.05. B) SDS-PAGE of total soluble protein from recombinant strains of E. coli containing combinations or individual cdt genes cloned in pET15b. A, B, C: native polypeptides CdtB and CdtC; His: His-tagged polypeptides determined by Western blotting; *truncated CdtA.
The relative cytotoxic activity of the recombinant extracts was significantly greater than that of an extract from A. actinomycetemcomitans Y4 (P <0.05). There was no difference between cultures of cells exposed to toxin at the time of plating or after attachment of cells to tissue culture plates overnight (data not shown). Protein extract from bacteria carrying vector plasmid alone (pBluescript II SK+) had no effect on the HeLa cells. CDT-containing extract from E. coli BL-21(DE3) (pET15bcdt), rather than the affinity purified recombinant proteins, were used in the following experiments so that assays could test the effects of holotoxin assembled in situ by the bacterium rather than artificially reconstituted in vitro. The three cdt genes were previously cloned separately in pET15b.38 Expression of the cdt genes by the clones in the pET15b collection is shown in Figure 1B.
Microscopic Evaluation of Human Oral Cells Treated With Recombinant A. actinomycetemcomitans CDT
To determine if the A. actinomycetemcomitans CDT has the potential to perturb cell types indigenous to the human periodontium, primary cultures of HPLF were exposed to CDT-containing extract from the E. coli BL-21(DE3) (pET15bcdt). The effect of the toxin on HPLF proliferation was compared to the effect on the GMSM-K and HeLa cell lines. Microscopic inspection revealed that cultures of HPLF did not appear to be altered by exposure to the toxin reaching confluence by 96 hours of growth (Fig. 2). By 96 hours post-intoxication, the appearance of the fibroblast culture was identical to that of the untreated culture with no discernable decrease in cell numbers. In comparison, the proliferation of CDT-treated epithelial cells was significantly reduced. Reduction in proliferation of CDT-treated GMSM-K cells occurred more rapidly than that of the HeLa cells. This did not appear to be a consequence of the absence of serum in the GMSM-K growth medium. Addition of 10% FCS to the growth medium did not alter the response of the GMSM-K cells to the toxin. A change in morphology of the GMSM-K cells and apparent reduction in cell numbers was observed by 12 hours after exposure to the CDT-containing extract. Cultures of HeLa cells did not show the same effects until 48 hours post-exposure. Both the GMSM-K and HeLa cell cultures manifested the toxic effects after exposure to CDT-containing extract for a minimum of 5 minutes. The appearance of these cultures following intoxication was identical to that of cultures which contained the toxin extract over the course of the experiment (data not shown).
Figure 2.

Microscopic examination of time course exposure of HPLF, GMSM-K, and HeLa cell cultures to the CDT.Top row: untreated (–CDT) cell cultures after incubation for 0, 48, or 96 hours. Remaining rows: cell cultures treated (+CDT) with 18 μg protein/ml of CDT-containing extract from E. coli BL-21(DE3) (pET15bcdt).
Kinetics of Response of Human Oral Cells to the CDT
Dose response and rate kinetics were performed to further characterize the differential response of the oral epithelial cells and fibroblasts to the CDT. CDT-containing extract from E. coli BL-21(DE3) (pET15bcdt) exhibited a dose-dependent effect on the viability of GMSM-K cells (Fig. 3A). One nanogram of total soluble protein from the recombinant clone was enough to inhibit cell viability when compared to extract from E. coli carrying the vector plasmid (pET15b) alone (inset). There was a 50% decrease in the A492 of 15,000 cells with 1 ng of total soluble protein from E. coli BL-21(DE3) (pET15bcdt). A maximum decrease in the A492 of 15,000 cells was obtained with 125 ng of total soluble protein. The average A492 of the GMSM-K cell culture that was not exposed to bacterial extract was 0.60. This CDT-induced reduction in A492 was statistically significant when compared to controls (P <0.05).
Figure 3.
Dose-dependent response of GMSM-K cells and HPLF to recombinant CDT -containing extract. A) Increasing concentrations of extract from E. coli BL-21(DE3) (pET15bcdt) (open circles), E. coli BL-21(DE3) (pET15b) (closed squares), and no extract (closed triangles).The absorbances of cultures not receiving bacterial extract were measured only at the 0 concentration point. B) Cultures of HPLF treated as for the GMSM-K cells. Regions of the curves showing the lower concentrations of extract are expanded for clarity (insets).
One thousand times more total soluble protein from E. coli BL-21(DE3) (pET15bcdt) had no effect on primary cultures of HPLF (Fig. 3B). The average A492 of HPLF cultures not exposed to bacterial extract was 1.19. The difference in baseline absorbance values for GMSM-K cells and HPLF was consistent and appeared to be due to differences in the size of the cells. Being the larger of two cell types, the HPLF likely have more mitochondria and are, therefore, more metabolically active on a per cell basis. This would affect the magnitude of the response in the cell viability staining assay.
Rate kinetic experiments showed that exposure of GMSM-K cells to CDT-containing extract in culture for a minimum of 5 minutes was enough to illicit a cytotoxic response. GMSM-K cells exposed to the CDT-containing extract from E. coli BL-21(DE3) (pET15bcdt) for 5 minutes, washed, and then incubated for various lengths of time showed a decrease in A492 by 12 hours post-intoxication, indicating a significant reduction in viability (Fig. 4A, unfilled circles). However, initial effects on cell viability were detected by 3 hours post-intoxication (Fig. 4A, inset). The cultures continued to exhibit a decrease in A492 up to 36 hours post-intoxication. Cultures not exposed to CDT-containing extract (Fig. 4A, filled triangles) and those receiving extract from E. coli BL-21(DE3) (pET15b) (Fig. 4A, unfilled squares) showed an increase in the A492 up to 12 hours, which was indicative of normal cell growth. The A492 of these cultures increased only slightly from 12 to 36 hours, indicating that the cultures were approaching confluence.
Figure 4.

Rate kinetics of the response of GMSM-K and HeLa cells to recombinant CDT containing extract. A) Cultures of GMSM-K cells received 50 ng total protein extract sonic extract from E. coli BL-21(DE3) (pET15bcdt) (open circles) and E. coli BL-21(DE3) (pET15b) (open squares) per ml of medium. B) Cultures of HPLF treated as for the GMSM-K cells except incubation was extended to 50 hours.The region of the GMSM-K curve showing the lower concentrations of extract is expanded for clarity (inset).
A reduction in cell number was not detected in HeLa cell cultures exposed to CDT-containing extract until at least 36 hours post-intoxication (Fig. 4B, unfilled circles). HeLa cell cultures not exposed to CDT-containing extract (Fig. 3B, filled triangles [only at 0 concentration]) and those receiving extract from E. coli BL-21(DE3) (pET15b) (Fig. 4B, unfilled squares) exhibited a steady increase in the number of viable cells up to 26 hours. These values remained constant up to 36 hours. The kinetic data support the visual data (Fig. 2) of the cell-specific effects of the CDT-containing extract on proliferation. The toxin appears to rapidly and irreversibly affect the oral epithelial cell line.
Effect of the CDT on Cell Cycle of HPLF and Oral Epithelial Cells
The differential effects of the recombinant A. actinomycetemcomitans CDT on the proliferation of HPLF and oral epithelial cells prompted an assessment of the ability of the toxin to perturb the cell cycle of these various cell types. The effect of recombinant CDT on the cell cycle was examined by flow cytometry (Fig. 5). In untreated cultures, the number of HeLa cells with a 4n DNA content represented less than 1% of the total cell population (Table 1). HeLa cells exposed to CDT-containing extract for 12 and 24 hours were arrested at the G2/M phase transition. By 12 hours and 24 hours post-intoxication the percentage of cells exhibiting a 4n DNA content increased to 16% and 81%, respectively. There was no significant difference in the percentage of arrested HeLa cells whether cultured in the continuous presence of CDT for 24 hours or for 24 hours following exposure for 5 minutes (Fig. 5, inset and Table 1).
Figure 5.

Effects of CDT on the cell cycle of HeLa, GMSM-K cells, HPLF, and HGF. Approximately 1 × 106 cells from cultures untreated (top row) or treated with 18.4 μg/ml protein extract from E. coli BL-21(DE3) (pET15bcdt) per ml of medium were incubated for 12 to 72 hours post-exposure. Cell cycle profiles obtained by flow cytometry are shown.The solid line (PI) is the DNA profile determined by propidium iodide staining. G1, G2/M (solid filled) and S (diagonal line filled) peaks were determined by computer analysis.
Table 1.
Cell Cycle Analysis of HPLF and Oral Epithelial Cells Treated With CDT
| Cell Type | CDT Exposure (hours) | Diploid G1 (2n) (%) | Diploid G2 (4n) (%) | Diploid S (%) | Coefficient of Variance (%) |
|---|---|---|---|---|---|
| HeLa | 0 | 72.86 | 0.91 | 26.24 | 5.00 |
| 12 | 31.81 | 15.42 | 52.77 | 3.37 | |
| 24 | 0.60 | 80.54 | 18.86 | 8.37 | |
| 24 (5 minutes) | 0.46 | 95.19 | 4.35 | 6.13 | |
| GMSM-K | 0 | 39.22 | 8.00 | 52.78 | 3.94 |
| 3 | 32.02 | 8.00 | 59.98 | 4.29 | |
| 6 | 25.40 | 4.16 | 70.44 | 3.31 | |
| 12 | 12.84 | 0.18 | 86.98 | 3.87 | |
| 24 | 9.58 | 0.15 | 90.27 | 4.05 | |
| HPLF | 0 | 85.84 | 1.97 | 12.19 | 7.53 |
| 24 | 63.49 | 0.31 | 36.20 | 6.78 | |
| 48 | 77.36 | 1.16 | 21.49 | 7.07 | |
| 72 | 90.15 | 1.23 | 8.62 | 9.01 |
The DNA content of a population of CDT-treated GMSM-K cells was very different than that of HeLa cells. A small percentage (8%) of GMSM-K cells in untreated cultures consistently had a DNA content of 4n. There was little difference between the DNA content of untreated cells and those treated with CDT-containing extract for 3 hours. By 6 hours post-intoxication the percentages of cells with both 2n and 4n DNA contents decreased. By 12 hours, less than 1% of the cells in a population exposed to CDT extract had a DNA content of 4n and those with a 2n content were reduced to 13%. This trend continued up to 24 hours post-intoxication. Most of the cells in the CDT-treated population had a DNA content between 2n and 4n represented by the large S peak. Unlike HeLa cells, the proliferation of GMSM-K cells may be arrested in the S phase (Fig. 4). These data indicate that the CDT may block DNA replication in the GMSM-K cell line.
Primary cultures of HPLF treated with CDT extract failed to show evidence of cell cycle arrest. There was no significant accumulation of cells having a DNA content of 4n nor was there a decrease in the percentage of cells with a 2n DNA content associated with increased time of exposure (72 hours maximum) to the CDT (Fig. 5 and Table 1). Unlike the GMSM-K cells, there was no indication that CDT-treated fibroblasts were arresting in the S phase. The cell cycle analysis data support the observation that there is no inhibition of proliferation of CDT-treated HPLF as determined by microscopic examination and by evaluation of growth kinetics.
CDT-Induced DNA Damage
An early step in the intoxication process is the uptake of the CdtB polypeptide and its targeting to the cell nucleus resulting in DNA double-strand breaks.30 Pulsed field gel electrophoresis (PFGE) was used to determine if the recombinant A. actinomycetemcomitans CDT induced DNA damage in HPLF. DNA extracted from HeLa cell cultures contained an extensive size range of DNA fragments only when the cells were treated with CDT-containing extract from E. coli BL-21(DE3) (pET15bcdt) (Fig. 6). DNA fragmentation was observed by 15 hours of growth following exposure to the toxin. Approximately 60% of the ethidium bromide-stained DNA migrated into the gel. Double-strand DNA damage was not observed in cells treated with extract from E. coli that does not express the CDT. We previously showed that E. coli BL-21(DE3) does not contain DNA nicking activity.38 GMSM-K cells were very sensitive to the DNA-damaging effects of the CDT. Approximately 75% of the ethidium bromide stained DNA from cells treated for 6 hours entered the gel following electrophoresis. The small amount of damaged DNA (16%) observed in untreated GMSM-K cells may have resulted from cell lysis during the preparation of the agarose plugs. No fragmentation of DNA from CDT-treated HPLF was observed, up to 72 hours post-exposure, following analysis by PFGE. The absence of evidence of CDT-induced DNase activity further supports our observation that HPLF are resistant to the CDT.
Figure 6.
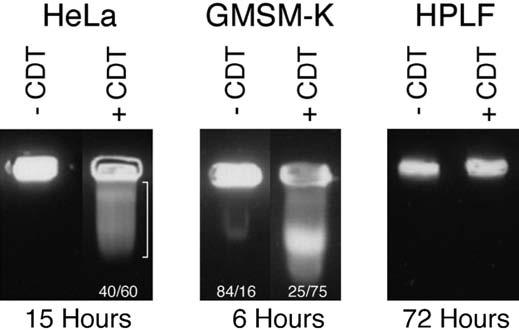
Pulse-field gel electrophoresis of CDT-treated oral cells. The numbers represent the relative percent of DNA in the well versus the gel as determined by scanning densitometry of the ethidium bromide stained gel.
Binding of Specific CDT Polypeptides to Human Oral Cells
It was previously shown that reconstituted CDT holotoxin and CdtA could be detected on the surface of Chinese hamster ovary cells by immunofluorescence.38 Resistance of human oral fibroblasts to the CDT could be due to the lack or alteration of a CDT receptor on the cell surface. To test this possibility, reconstituted recombinant holotoxin and the individual recombinant Cdt polypeptides were examined for the their ability to bind to HeLa, HPLF, and GMSM-K cells. Both reconstituted toxin and CdtA were detected on the surface of all three cell types (Fig. 7). The GMSM-K cells attach poorly to the glass slides typically used for immunofluorescence. Consequently, gelatin coated slides had to be used. The gelatin coating reduced the clarity of the immunofluorescence and most likely is the reason for the apparent weak detection of CdtC on the GMSM-K cells. However, these data indicate that the apparent resistance of HPLF to the CDT is not due to the inability of the cells to bind the toxin.
Figure 7.

Immunofluorescent detection of recombinant holotoxin and Cdt polypeptides on the surface of HeLa, HPLF, and GMSM-K cells. Individual His6-tagged polypeptides and reconstituted toxin were incubated with each cell type on chamber slides for 15 minutes at 4°C. Bound protein was detected with His•Tag monoclonal antibody (1:1,000 dilution) and fluorescein-conjugated second antibody (1:1,000 dilution).
DISCUSSION
The CDT is an unusual cytotoxin because it is expressed by a group of bacterial species that are pathogens in disparate diseases and colonize dissimilar environments. Strains of E. coli,2 Campylobacter spp.,3,42 and S. dysenteriae1,43 that express the CDT participate in diarrheal diseases. Haemophilus ducreyi causes chancroid ulcers,8 while Helicobacter spp.10 are involved in the formation of stomach ulcers. Actinobacillus actinomycetemcomitans is thought to be a prominent pathogen in multiple forms of periodontal disease.34,44 It is not clear at the present time if the CDT is a virulence factor in all of these diseases. Since A. actinomycetemcomitans is the only member of the human oral flora to carry cdt genes and express active toxin, we are interested in its potential role as a virulence factor in periodontal disease. It is assumed that the CDT has an important role in disease-associated activities of the bacterium since a proportionately high number of fresh clinical isolates express cdt genes.12,35,36 This is a good indication that there is selective pressure to maintain the cdt operon in A. actinomycetemcomitans.
Periodontal ligament fibroblasts have a key role in maintaining the integrity of the ligament that is responsible for supporting the teeth within the adjacent alveolar bone. Epithelial cells represent another abundant cell type in the oral cavity that are morphologically and functionally distinct from HPLF. Thus, our aims were primarily to examine the effects of the CDT on HPLF and secondarily to compare and contrast these with the effects of the CDT on oral epithelial cells. Difficulties in the routine culturing of primary oral epithelial cells persuaded us to use a recently immortalized epithelial cell line (GMSM-K) for our studies. The comparative data should be viewed with caution since the epithelial cells are immortalized and this may influence the response of the cells to the CDT. However, the most intriguing finding was that primary cultures of HPLF failed to exhibit typical cellular responses to the CDT. There was no effect on proliferation or cell cycle. This is in contrast to results obtained by others28,33 who worked with fibroblasts derived from non-oral tissue compartments. Several studies examining the activities of the H. ducreyi CDT have used fibroblasts from non-human or non-oral sites. It was found that Chinese hamster lung (Don) fibroblasts were irreversibly sensitive to the CDT but required a relatively long exposure time to achieve intoxication.22 The Don fibroblasts became distended following 24 hours of continuous treatment and this was accompanied by a decrease in their rate of proliferation. In contrast, neither the morphology nor proliferation of mouse 3T3 fibroblasts were altered by the H. ducreyi CDT. The growth of human foreskin fibroblasts appeared to be inhibited by H. ducreyi CDT but the cells were not killed.24 We did not see an effect of the A. actinomycetemcomitans CDT on proliferation of HPLF up to 96 hours of exposure with relatively high levels of toxin. It has been reported that both human foreskin and human embryonic lung fibroblasts had a CDT-induced pattern of cell cycle arrest different from that of epithelial cells and keratinocytes which arrested in the G2 phase.33 The CDT-treated fibroblasts had an incomplete block in G2 phase up to 48 hours post-intoxication and were arrested in the S and G1 phases. Cdc2 was down-regulated with a corresponding accumulation of phospho-Cdc2. The human lung fibroblasts also exhibited increased expression of p53 and upregulation of p21 upon exposure to the toxin. Collectively, these studies established that the response to CDT was cell specific and not necessarily associated with a G2 arrest. We failed to detect cell cycle arrest at either G1 or G2/M phase transition of HPLF. These opposing results could be attributed to differences between HPLF and fibroblasts from other tissue compartments or differences between the CDT from A. actinomycetemcomitans and H. ducreyi. However, the deduced amino acid sequences of the Cdt polypeptides from these two species are more than 90% identical.8,12
Several studies have reported a growth inhibitory effect of A. actinomycetemcomitans on HPLF and human gingival fibroblasts (HGF).26,45 One of these studies directly implicated the CDT.26 However, the conditions under which these studies were conducted were not particularly stringent. Helgeland and Nordby45 reported a cell cycle inhibition in HGF with uncharacterized secreted material from A. actinomycetemcomitans. The fibroblasts accumulated in G2/M phase between 28 to 40 hours post-exposure to the A. actinomycetemcomitans material. The cytotoxic activity in the inhibitory fraction was not identified and this crude fraction was most likely heavily contaminated with lipopolysaccharide (LPS) and other cytotoxic proteins. This work was published prior to the identification of the CDT in A. actinomycetemcomitans. The strain used by these investigators was A. actinomycetemcomitans ATCC 33384,18 which is the type strain (NCTC 9710) of this species. We previously showed that strain NCTC 9710 produces biologically active CDT.12 It is not clear if the reported effects on HGF were due to other factors produced by the bacterium. This is one reason why recombinant A. actinomycetemcomitans CDT was used in our study.
In another study, a crude extract of whole cells of A. actinomycetemcomitans was used to treat cultures of HPLF and HGF.26 There was a rapid inhibition of DNA synthesis. The fibroblasts became distended by 72 hours but remained viable. It was reported that these effects were abolished when extract from a CDT knockout mutant was used. However, it is extremely difficult to evaluate these results because: 1) these investigators did not attempt to identify either cdt genes or Cdt polypeptides in their test strain A. actinomycetemcomitans HK 1519; 2) their bacterial extract was a crude preparation contaminated with significant levels of LPS (6.5 μg/ml); 3) the concentration of extract used in their assays was not reported; and 4) effects on cell cycle and evaluation of DNA damage, standard measures of CDT activity, were not determined.
Our data clearly show that HPLF do not undergo the classic double-strand DNA damage and cell cycle arrest associated with the CDT. It appears that a combination of factors may contribute to this resistance. Results of the PFGE indicate that either the CDT does not get into the HPLF nucleus, that the chromatin is protected from the DNase I-like nicking activity of the toxin, or that the DNase I-like activity is neutralized in the cells.4,29,30 Immunofluorescence detection of both whole toxin and CdtA on the surface of HPLF cells suggest that failure of toxin binding to the cells is not a likely reason for resistance. If this is the case then resistance of HPLF to the toxin is most likely related to a direct or indirect inhibition of the DNase I-like activity of the CdtB polypeptide. Preliminary data from our studies also indicate that HGF exhibit the same pattern of resistance to the CDT (unpublished observations). This suggests that human oral fibroblasts, in general, may be resistant to the A. actinomycetemcomitans toxin.
In addition to the finding that HPLF are resistant to the CDT, it was also found that at least one type of oral epithelial cell is exceptionally sensitive to the toxin. Furthermore, the growth inhibitory effect of the CDT on these epithelial cells is atypical since arrest at G1 or G2/M was not observed. Results of FACS (fluorescence-activated cell sorter) analyses indicate that a block in DNA replication may lead to the inhibition of proliferation of these cells. The arrest of cells in the S phase has not been previously reported for the CDT.
These findings have important implications for the potential role of the CDT and A. actinomycetemcomitans in the pathogenesis of periodontal disease. Epithelial cells, by virtue of their forming a physical barrier, provide an innate form of host defense against microbial infection. The epithelial cells of the gingiva thereby protect the underlying supporting structures of the periodontium from the deleterious effects of bacterial colonization. The ability of the CDT to inhibit the growth of oral epithelial cells may lead to disruption of this protective barrier allowing A. actinomycetemcomitans, along with other putative periodontal pathogens, access to the underlying gingival connective tissues. In this tissue compartment other virulence factors as well as the concomitant activation of a host inflammatory response are likely to play more direct roles in destruction of the tooth-supporting structures, including the periodontal ligament. Thus, the A. actinomycetemcomitans CDT may have a role in the initial stages of developing periodontal lesions.
ACKNOWLEDGMENTS
This study was supported by USPHS grant R01-DE-012593 from the National Institutes of Health. We thank Valerie Murrah, University of North Carolina, Chapel Hill, North Carolina, for the generous gift of GMSM-K cells; Eric Gilchrist, University of North Carolina at Chapel Hill, for helpful advice for culturing these cells; Gary Cohen, University of Pennsylvania, for supplying HeLa cells and the use of an enzyme-linked immunosorbent assay plate reader; Charles Pletcher, University of Pennsylvania, for help with the flow cytometry; Thomas Stamato, Lankenau Research Institute, Wynnwood, Pennsylvania, for use of the PFGE apparatus; and Edward Macarak, University of Pennsylvania, for use of the fluorescent microscope.
Footnotes
Braun-Sonic 2000, B. Braun Biotech, Inc., Allentown, PA.
Millipore Corporation, Billerica, MA.
Ready-Gels, Bio-Rad Laboratories, Hercules, CA.
Invitrogen, Carlsbad, CA.
Nikon TMS-F inverted microscope, Nikon Instrument, Inc., Melville, NY.
Nikon N70 camera, Nikon Corporation, Melville, NY.
CellTiter96, AQueous Non-Radioactive Cell Proliferation Assay Kit, Promega Corporation, Madison, WI.
Sigma Chemical, St. Louis, MO.
FACSCalibur, Becton Dickinson, Franklin Lakes, NJ.
ModFit 3.0, Verity Software House, Topsham, ME.
InCert agarose, FMC Corporation, Philadelphia, PA.
ImageJ Version 1.32a, available at http://rsbweb.nih.gov/nih-image/index.html.
Nalgene, Nunc International, Rochester, NY.
Novagen, EMD Biosciences, San Diego, CA.
Alexa Fluor 488, Molecular Probes, Inc., Eugene, OR.
Prolong Anti-Fade, Promega Corporation.
Nikon Eclipse E600, Nikon Instruments, Inc.
REFERENCES
- 1.Johnson WM, Lior H. Response of Chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol Lett. 1987;43:19–23. [Google Scholar]
- 2.Johnson WM, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microbial Pathol. 1988;4:103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 3.Johnson WM, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microbial Pathol. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 4.Mooney A, Clyne M, Curran T, Doherty D, Kilmartin B, Bourke B. Campylobacter upsaliensis exerts a cytolethal distending toxin effect on HeLa cells and T lymphocytes. Microbiology. 2001;147:735–743. doi: 10.1099/00221287-147-7-1815. [DOI] [PubMed] [Google Scholar]
- 5.Hickey TE, McVeigh AL, Scott DA, et al. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect Immun. 2000;68:6535–6541. doi: 10.1128/iai.68.12.6535-6541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson WM, Lior H. Production of Shiga toxin and a cytolethal distending toxin (CLDT) by serogroups of Shigella spp. FEMS Microbiol Lett. 1987;48:235–238. [Google Scholar]
- 7.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathol. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 8.Cope LD, Lumbley S, Latimer JL, et al. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci (USA) 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien CC, Taylor NS, Ge Z, Schauer DB, Young VB, Fox JG. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J Med Microbiol. 2000;49:525–534. doi: 10.1099/0022-1317-49-6-525. [DOI] [PubMed] [Google Scholar]
- 10.Young VB, Knox KA, Schauer DB. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun. 2000;68:184–191. doi: 10.1128/iai.68.1.184-191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kostia S, Veijalainen P, Hirvi U, Hanninen ML. Cytolethal distending toxin B gene (cdtB) homologues in taxa 2, 3 and 8 and in six canine isolates of Helicobacter sp. flexispira. J Med Microbiol. 2003;52:103–108. doi: 10.1099/jmm.0.04920-0. [DOI] [PubMed] [Google Scholar]
- 12.Mayer MPA, Bueno LC, Hansen EJ, DiRienzo JM. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shenker BJ, McKay TL, Datar S, Miller M, Chowhan R, Demuth D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- 14.Sugai M, Kawamoto T, Pérès SY, et al. Cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickett CL, Cottle DL, Pesci EC, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott DA, Kaper JB. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comayras C, Tasca C, Pérès SY, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escalas N, Davezac N, De Rycke J, Baldin V, Mazars R, Ducommun B. Study of the cytolethal distending toxin-induced cell cycle arrest in HeLa cells: Involvement of the CDC25 phosphatase. Exper Cell Res. 2000;257:206–212. doi: 10.1006/excr.2000.4878. [DOI] [PubMed] [Google Scholar]
- 19.Pérès SY, Marchès O, Daigle F, et al. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 20.Whitehouse CA, Balbo PB, Pesci EC, Cottle DL, Mirabito PM, Pickett CL. Camphylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akifusa S, Poole S, Lewthwaite J, Henderson B, Nair SP. Recombinant Actinobacillus actinomycetemcomitans cytolethal distending toxin proteins are required to interact to inhibit human cell cycle progression and to stimulate human leukocyte cytokine synthesis. Infect Immun. 2001;69:5925–5930. doi: 10.1128/IAI.69.9.5925-5930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J Clin Invest. 1999;103:107–115. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiRienzo JM, Song M, Wan LSY, Ellen RP. Kinetics of KB and HEp-2 cell responses to an invasive, cytolethal distending toxin producing strain of Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 2002;17:245–251. doi: 10.1034/j.1399-302x.2002.170407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens MK, Latimer JL, Lumbley SR, et al. Characterization of a Haemophilus ducreyi mutant deficient in expression of cytolethal distending toxin. Infect Immun. 1999;67:3900–3908. doi: 10.1128/iai.67.8.3900-3908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelfanova V, Hansen EJ, Spinola SM. Cytolethal distending toxin of Haemophilus ducreyi apoptotic death of Jurkat T cells. Infect Immun. 1999;67:6394–6402. doi: 10.1128/iai.67.12.6394-6402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belibasakis G, Johansson A, Wang Y, et al. Inhibited proliferation of human periodontal ligament cells and gingival fibroblasts by Actinobacillus actinomycetemcomitans: Involvement of the cytolethal distending toxin. Eur J Oral Sci. 2002;110:366–373. doi: 10.1034/j.1600-0722.2002.21350.x. [DOI] [PubMed] [Google Scholar]
- 27.Deng K, Hansen EJ. A CdtA-CdtC complex can block killing of HeLa cells by Haemophilus ducreyi cytolethal distending toxin. Infect Immun. 2003;71:6633–6640. doi: 10.1128/IAI.71.11.6633-6640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassane DC, Lee RB, Pickett CL. Campylobacter jejuni cytolethal distending toxin promotes DNA repair responses in normal human cells. Infect Immun. 2003;71:541–545. doi: 10.1128/IAI.71.1.541-545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elwell CA, Dreyfus LA. Dnase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol Microbiol. 2000;37:952–963. doi: 10.1046/j.1365-2958.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 30.Frisan T, Cortes-Bratti X, Chaves-Olarte E, Stenerlow B, Thelestam M. The Haemophilus ducreyi cytolethal dis-tending toxin induces DNA double-strand breaks and promotes ATM-dependent activation of RhoA. Cell Microbiol. 2003;5:695–707. doi: 10.1046/j.1462-5822.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 31.Lara-Tejero M, Galán JE. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290:354–357. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- 32.Pickett CL, Whitehouse CA. The cytolethal distending toxin family. Trends Microbiol. 1999;7:292–297. doi: 10.1016/s0966-842x(99)01537-1. [DOI] [PubMed] [Google Scholar]
- 33.Cortes-Bratti X, Karlsson C, Lagergard T, Thelestam M, Frisan T. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J Biol Chem. 2001;276:5296–5302. doi: 10.1074/jbc.M008527200. [DOI] [PubMed] [Google Scholar]
- 34.Henderson B, Nair SP, Ward JM, Wilson M. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu Rev Microbiol. 2003;57:29–55. doi: 10.1146/annurev.micro.57.030502.090908. [DOI] [PubMed] [Google Scholar]
- 35.Fabris AS, DiRienzo JM, Wïkstrom M, Mayer MPA. Detection of cytolethal distending toxin activity and cdt genes in Actinobacillus actinomycetemcomitans isolates from geographically diverse populations. Oral Microbiol Immunol. 2002;17:231–238. doi: 10.1034/j.1399-302x.2002.170405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan K-S, Song K-P, Ong G. Cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Occurrence and association with periodontal disease. J Periodontal Res. 2002;37:268–272. doi: 10.1034/j.1600-0765.2002.01618.x. [DOI] [PubMed] [Google Scholar]
- 37.Lekic PC, Pender N, McCulloch CA. Is fibroblast heterogeneity relevant to the health, diseases, and treatments of periodontal tissues? Crit Rev Oral Biol Med. 1997;8:253–268. doi: 10.1177/10454411970080030201. [DOI] [PubMed] [Google Scholar]
- 38.Mao X, DiRienzo JM. Functional studies of the recombinant subunits of a cytolethal distending holotoxin. Cell Microbiol. 2002;4:245–255. doi: 10.1046/j.1462-5822.2002.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Somerman MJ, Archer SY, Imm GR, Foster RA. A comparative study of human periodontal ligament cells and gingival fibroblasts in vitro. J Dent Res. 1988;67:66–70. doi: 10.1177/00220345880670011301. [DOI] [PubMed] [Google Scholar]
- 40.Gilchrist EP, Moyer MP, Shillitoe EJ, Clare N, Murrah VA. Establishment of a human polyclonal oral epithelial cell line. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:340–347. doi: 10.1067/moe.2000.107360. [DOI] [PubMed] [Google Scholar]
- 41.Vindelov LL. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions: A new method for rapid isolation and staining of nuclei. Virchows Arch B Cell Pathol. 1977;24:227–242. [PubMed] [Google Scholar]
- 42.Pickett CL, Pesci EC, Cottle DL, Russell G, Erdem AN, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: Suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bueno LC, Mayer MPA, DiRienzo JM. Relationship between conversion of localized juvenile periodontitis-susceptible children from health to disease and Actinobacillus actinomycetemcomitans leukotoxin promoter structure. J Periodontol. 1998;69:998–1007. doi: 10.1902/jop.1998.69.9.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helgeland K, Nordby Ø. Cell cycle-specific growth inhibitory effect on human gingival fibroblasts of a toxin isolated from the culture medium of Actinobacillus actinomycetemcomitans. J Periodontal Res. 1993;28:161–165. doi: 10.1111/j.1600-0765.1993.tb01064.x. [DOI] [PubMed] [Google Scholar]



