Abstract
Ovarian steroid production and subsequent local steroid-mediated signaling are critical for normal ovarian processes, including follicle growth, oocyte maturation, and ovulation. In contrast, elevated steroidogenesis and/or increased steroid signaling in the ovary can lead to profound ovarian pathology, such as polycystic ovarian syndrome, the leading cause of infertility in reproductive age women. Through the use of several in vitro and animal models, great strides have been made toward characterizing the mechanisms regulating local steroid production and action in the ovary. Examples of this progress include insights into luteinizing hormone (LH)- and growth factor-mediated signaling, steroidogenic acute regulatory protein (StAR) activation, and both genomic and nongenomic steroid-mediated signaling in somatic and germ cells, respectively. The following review will address these advances, focusing on how this rapidly expanding knowledge base can be used to better understand female reproduction, and to further improve treatments for common diseases of infertility.
Keywords: steroid, oocyte, follicle, receptor, ovary
Abbreviations: StAR. steroidogenic acute regulatory protein; LH, luteinizing hormone; AR, androgen receptor; PR, progesterone receptor; ER, estrogen receptor; PCOS, polycystic ovarian syndrome; EGF, epidermal growth factor
Introduction
Ovarian steroid production is critical for the normal development and function of several distant tissues in women, including the uterus, breast, skeleton, and brain. Importantly, steroids produced from the ovary also have local effects that are essential for normal ovarian physiology. For example, estradiol-mediated signaling through the estrogen receptors ERα and ERβ is critical for follicle development 1–3, as mice with reduced expression of one or both or these receptors have abnormal follicle growth. Likewise, androgen signaling via the androgen receptor (AR) also appears to be important for normal follicle development, as mice lacking the AR have reduced follicle growth and premature ovarian failure 4–6. Finally, local progesterone production and actions at the time of the luteinizing hormone (LH) surge are crucial for ovulation, as mice lacking progesterone receptors (PRs) do not ovulate in response to gonadotropin7.
While normal ovarian steroid production is critical for the maintenance of standard ovarian development and function, excess ovarian steroid secretion and subsequent local steroid signaling can lead to significant ovarian pathology. The most common clinical example of increased steroid signaling in the ovary leading to abnormal function is polycystic ovarian syndrome (PCOS), which is characterized by excess ovarian (and sometimes adrenal) androgen production, abnormal follicle growth, and infertility due in large part to anovulation8. The following review will examine the intracellular signals that regulate ovarian steroid production, as well as the physiologic and pathologic consequences of intra-ovarian actions modulated by these steroid hormones.
Normal steroidogenesis in the ovary
The Two Cell Model
Normal ovarian steroid production is a tightly regulated and complex process that involves several different signaling pathways in multiple kinds of cells (Fig. 1). A typical mammalian ovarian follicle consists of at least four cell types. Each follicle contains an oocyte surrounded by cumulus granulosa cells. These cells are then bounded by outer mural granulosa cells, which in turn are surrounded by theca cells 9, 10. Steroidogenesis in most mammals (including humans and mice) appears to occur via the two cell/two gonadotropin model whereby androgens are synthesized from cholesterol in LH-stimulated theca cells, followed by conversion to estrogens in follicle stimulating hormone (FSH)-exposed granulosa cells. LH receptors and the enzyme CYP17, which converts pregnenolone and progesterone to dehydroepiandrosterone (DHEA) and androstenedione, respectively, are expressed primarily in theca cells, while FSH receptors and aromatase (CYP19), which converts androgens to estrogens, are expressed mainly in granulosa cells. Notably, LH receptors are not exclusively found in theca cells, as their expression levels in mural granulosa cells have been noted to increase in response to FSH just prior to the LH surge 11.
Figure 1.
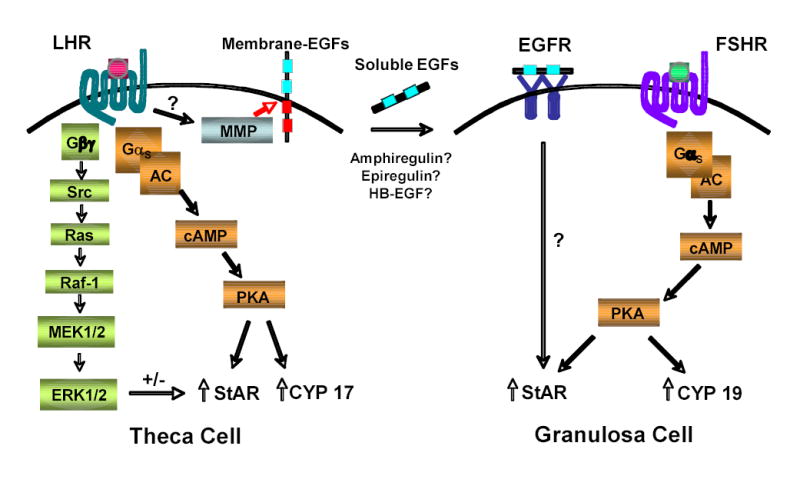
Gonadotropins trigger multiple signals that regulate steroidogenesis. LH-induced activation of the LH receptor on theca, and likely mural granulosa, cells triggers several signaling pathways that can regulate steroid production. Gαs-mediated increases in cAMP lead to elevated PKA activity, which in turn can increase StAR and CYP17 expression and activity via mechanisms that are still not well understood. Concomitant Gβγ-mediated signaling can activate the Src/Ras/MAPK signaling cascade, which in turn has been shown to both increase and decrease StAR and CYP17 activity, depending upon the cell culture system. Of note, LH-induced Gβγ and possibly Gq signaling can also trigger PLC activation, which may affect steroidogenesis (not shown). Finally, through yet unknown mechanisms, LH receptor signaling can trigger activation of MMPs, which in turn may cleave membrane-EGF moieties to release soluble EGFs (possibly amphiregulin, epiregulin, and/or HB-EGF). These soluble EGFs can then activate the EGF receptor on granulosa, and possibly theca, cells to increase StAR activity via mechanisms that have yet to be characterized. Finally, similar to the LH receptor, activation of the FSH receptor on granulosa cells increases intracellular cAMP and activates PKA, which in turn can increase StAR and CYP19 (aromatase) expression and activity.
Interestingly, both theca and granulosa cells express steroidogenic acute regulatory protein (StAR, see below), CYP11A1 (side chain cleavage enzyme), and 3β-HSD. Both of these cell types are therefore capable of making pregnenolone and/or progesterone from cholesterol. However, in the follicular phase, the relatively avascularized granulosa cells see limited oxygen or cholesterol, thus low amounts of these steroids are produced. In contrast, after exposure to gonadotropins, the granulosa cells become “luteinized,” and are then able to synthesize large amounts of pregnenolone and progesterone from cholesterol 9, 10. Notably, since these granulosa cells lack CYP17, they cannot metabolize progestins to androgens, as would occur in CYP17-expressing theca cells. Thus, most ovarian progesterone production likely comes from granulosa rather than theca cells. Since progesterone is necessary for normal ovulation 12, and appears to be capable of promoting oocyte maturation 13, understanding the mechanisms by which LH-induced signaling in theca cells leads to progesterone production in granulosa cells is essential.
StAR Protein
In order to understand how progesterone production is regulated in the ovary, an appreciation of how StAR protein is expressed and activated is necessary. StAR is the primary transporter of cholesterol from the outer to inner mitochondrial membrane, where most of the steroidogenic enzymes are located. Thus, StAR is a critical, rate-limiting regulator of steroid production in all major steroidogenic tissues. For example, StAR is essential for corticosteroid production in the adrenal gland, as humans or mice lacking StAR activity are born with adrenal insufficiency requiring corticosteroid replacement 14, 15. Similarly, StAR is critical for normal androgen production in the testes, as male humans and mice lacking active StAR have external female genitalia due to insufficient androgen production in utero. However, males still produce enough testosterone to retain their internal Wolffian remnants, suggesting that small amounts of StAR-independent steroidogenesis may still occur in the testes during embryogenesis and perhaps even shortly after birth. Finally, StAR is needed for normal ovarian steroid production, as adult female mice and humans lacking functional StAR have ovarian failure. Similar to the testes in embryonic males lacking StAR, ovarian function is still partially intact in young females; however, unlike the males, females lacking StAR can continue to make sex steroids throughout the pre-pubertal period, with hormone levels usually declining shortly after the onset of puberty 14. In fact, some human females lacking StAR activity still progress through puberty, and even briefly cycle, before amenorrhea ensues 15.
A “two hit” model has been described to explain the variable steroidogenic organ failure in animals and people lacking functional StAR 15. This model proposes that low-level StAR-independent steroidogenesis initially occurs in cells from all steroidogenic tissue. With time, however, these cells accumulate cholesterol that cannot be metabolized, resulting in eventual glandular destruction. With regards to the adrenals, this “second hit” primarily occurs during fetal development, as fetal adrenal tissue normally makes steroids; thus, adrenal cells lacking StAR accumulate significant lipids during embryogenesis. Similarly, the testes make steroids both in utero and just after birth; thus, Leydig cell damage occurs early in males. In contrast, the ovary remains relatively dormant until puberty; thus this gland is often preserved until that time. Once steroidogenesis begins, however, ovaries lacking StAR rapidly accumulate cholesterol and are destroyed like the adrenal gland and testes. Whether early StAR-independent steroid production in the ovary is sufficient to regulate steroid-mediated processes such as follicular growth and oocyte maturation has yet to be determined.
LH-induced signaling molecules that may regulate ovarian steroidogenesis
StAR protein expression and activity is regulated by many factors in steroidogenic tissue, starting with activation of the LH receptor in theca cells and possibly mural granulosa cells. LH-triggered signaling via its G protein-coupled receptor induces several important second messengers that have been implicated as regulators of StAR activity and/or steroidogenesis in general, including the following (Fig. 1):
cAMP and PKA
While many intracellular signaling pathways have been linked to LH-induced ovarian steroid production; gonadotropin-induced elevation of cAMP is one of the few such pathways that has been definitively proven to be important. Both the FSH and LH receptors couple to Gαs when activated by their respective ligands. Gαs in turn stimulates adenylyl cyclase, leading to an elevation in intracellular cAMP levels and subsequent activation of protein kinase A (PKA) 9. Elevation of intracellular cAMP in theca or granulosa cells by gonadotropins, forskolin, or membrane-permeable dibutryl cAMP (dbcAMP) promotes steroidogenesis by increasing expression of StAR as well as by increasing StAR activity via phosphorylation on serine 195 9, 16. Furthermore, cAMP regulates CYP19 (aromatase) and CYP17 expression and activity in granulosa and theca cells, respectively, which contributes to estrogen and androgen production 10.
Phospholipase C (PLC)
Although primarily coupling with Gαs, evidence suggests that the LH and FSH receptors can also activate Gq and Gαi in response to ligand 17. Both Gq and Gβγ are both known to activate PLC, which then triggers the production of second messengers inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG). These molecules in turn increase intracellular calcium and activate PKC, respectively. Under some conditions, PKC and calcium may inhibit adenylyl cyclase and reduce steroidogenesis in mice 18; however, the precise role of PLC and its downstream signals in regulating ovarian steroidogenesis remains uncertain.
Src and Extracellular-regulated kinases (ERKs)
Activation of Gβγ by G protein-coupled receptors activates Src, which in turn triggers the Ras/Raf/MEK/MAPK/ERK pathway. For example, stimulation of LH and/or FSH receptors can enhance extranuclear receptor signaling kinase (ERK) activity 19, 20, with ERK activation subsequently increasing StAR expression in some steroidogenic cell lines (e.g., Y1 adrenal cells 21). In contrast, Src and MAPK signaling inhibit steroid production in some cultured theca cells by decreasing activity of CYP17 and other steroidogenic enzymes 22, with some studies suggesting that lower MAPK signaling may be contributing to elevated CYP17 activity and subsequent androgen production in PCOS 23. Given these varying observations, more experiments in different models are necessary to clearly elucidate the physiologic role of the LH-induced Src and MAPK signaling in the regulation of ovarian steroidogenesis.
Insulin-like Growth Factors (IGFs)
Several studies have implicated members of the IGF family as promoters of ovarian steroidogenesis 9, 24, 25. These growth factors appear to signal through the IGF1 receptor, and, depending upon the species, have been shown to increase the levels and activities of several steroidogenic enzymes, including CYP17, CYP19 (aromatase), and CYP11A1 (side-chain cleavage enzyme). Furthermore, IGF signaling has been shown to increase LH receptor mRNA transcription, and then in turn to enhance LH-induced upregulation of StAR mRNA transcription 24, 26. Details regarding intermediate IGF receptor-induced signaling pathways that regulate these downstream effects are still unclear; however, cAMP and ERK signaling may be involved 9, 27.
Epidermal Growth Factor (EGF) Receptor
Another potentially important signaling molecule that is activated downstream of the LH receptor is the EGF receptor. G protein-coupled receptors (GPCRs) are known to activate EGF receptor signaling by several mechanisms 28, 29. For example, stimulation of the Gαs-coupled β2-adrenergic receptor activates membrane-bound matrix metalloproteinases (MMPs, see below) that cleave heparin-bound EGF (HB-EGF) from the cell surface to auto-activate the EGF receptor 30.
Interestingly, several studies have demonstrated that EGF receptor signaling can promote steroidogenesis in gonadal cells, including Leydig cell lines and ovarian follicles 31–33. Like LH, EGF appears to be promoting steroidogenesis primarily by activating StAR. In Leydig cells, EGF stimulates StAR production, while in ovarian follicles EGF instead appears to activate StAR post-translationally; perhaps through phosphorylation 13.
Since activation of the EGF and LH receptors similarly increase StAR activity and steroidogenesis, then perhaps the EGF receptor is signaling downstream of the LH receptor. In support of this hypothesis, EGF receptor activation appears necessary for gonadotropin-induced steroid production in pre-ovulatory follicles, as inhibition of the EGF receptor kinase completely abrogates LH-induced progesterone production 13. Intriguingly, inhibition of membrane matrix metalloproteinase (MMP)-mediated cleavage of membrane-bound EGF moieties also abrogates LH-induced steroidogenesis in ovarian follicles, suggesting the following model (Fig. 1): through yet unknown mechanisms, LH triggers activation of one or more MMPs in theca and possibly mural granulosa cells, which in turn triggers the release of membrane-bound EGF molecules, including possibly HB-EGF, amphiregulin, and epiregulin 34. These soluble EGFs in turn function in both an autocrine and paracrine fashion to activate EGF receptors on theca and granulosa cells, respectively, resulting in increased StAR activity and subsequent steroid production.
Notably, as in ovarian follicles, normal LH-induced steroidogenesis in Leydig cells also depends upon activation of the EGF receptor 13, suggesting that EGF receptor signaling is critical for gonadotropin-mediated steroid synthesis in any gonadal tissue. In contrast, however, LH-induced steroid production in Leydig cells does not appear to require activation of MMPs. These differences may reflect the greater need for paracrine signaling in the ovary, where multiple cell types are required for steroidogenesis. Furthermore, these observations suggest that targeted inhibition of ovarian MMP activity might prove to be an effective means of specifically regulating ovarian steroid production in diseases of excess steroidogenesis, such as PCOS (see below).
Physiologic Steroid Signaling in the Ovary (Table 1)
Table 1.
Potential Local Actions of Ovarian Steroids
| Steroid | Effect |
|---|---|
| Progesterone | follicle rupture, ovulation, oocyte maturation |
| Androgen | oocyte maturation?, FSH sensitizer, follicle growth |
| Estradiol | granulosa cell growth, modulation of FSH actions, oocyte maturation?, ovulation |
Steroid concentrations in the ovary
Ovarian steroid levels vary depending upon the day on which they are measured, with androgen, estrogen, and progesterone levels rising during ovulation, and estrogen and progesterone levels reaching even higher levels during the luteal phase. In fact, follicular fluid at the time of ovulation has been noted to contain micromolar amounts of steroid 35. What then prevents these saturating amounts of steroid from constantly signaling via their cognate receptors, which bind ligand in the nanomolar range? One possible explanation for this conundrum may be that, due to steroid metabolism in target cells, the intracellular steroid concentrations are far lower than the extracellular amounts. Alternatively, follicular fluid contains high amounts of sex hormone binding globulin, as well as other proteins that could potentially sequester steroids 36–38; thus, while the total steroid content in follicular fluid may be high, the free, or bioavailable, steroid amounts may be relatively low.
Progesterone Actions
Direct measurement of ovarian steroid content during the LH surge in many different mammals reveals a large and rapid rise in progesterone production 13, 35, 39, suggesting that progesterone may play an important role at the time of ovulation. Accordingly, progesterone signaling in the ovary seems to be essential for normal gonadotropin-induced ovulation, as injection of the PR antagonist RU486 blocks ovulation in rodents 40, and knockout mice lacking functional PRs, fail to ovulate in response to gonadotropins 7. Histology in these PR null mice reveals normal development of intraovarian follicles to the tertiary follicle stage, with luteinization of granulosa cells and the production of oocytes that are competent for in vitro fertilization. In fact, the major defect appears to be the complete elimination of LH-induced follicle rupture. Additional studies have revealed the abnormal expression of ADAMTS-1 and cathepsin L, two proteases known to be involved in follicle rupture 41, indicating that trascriptional targets of the PR may be involved in the progesterone-mediated signaling cascade that triggers ovulation.
Estradiol Actions
Estradiol signals primarily via two classical receptors: ERα and ERβ. Granulosa cells mainly express ERβ, suggesting that this receptor may regulate estradiol-mediated stimulation of granulosa cell growth and modulation of FSH action. Accordingly, follicles from ERβ knockout mice have a diminished response to exogenous estradiol, with a reduced cumulus cell mass and a decreased rate of ovulation 2. This defect in granulosa cell growth results in reduced female fertility, with fewer and smaller litters than wild-type mice despite normal sexual behavior.
In contrast to ERβ, ERα is expressed in every location of the hypothalamic/pituitary/gonadal axis, and ERα null mice are completely infertile. Interestingly, early ovarian development is unimpaired in the ERα knockout mice, and primordial and primary follicles are indistinguishable from those of WT females. However, concomitant with elevated serum gonadotropin levels, follicular development is markedly accelerated in the null relative to wild-type mice around day 20 of life. Follicles develop to the late antral stage but fail to progress to final maturation, often containing hemorrhagic and atretic cysts 1. This pathology may in part be explained by a lack of negative feedback by estradiol on the pituitary, leading to excess gonadotropin production and subsequent signaling in the follicular cells.
Similar to the ER knockout mice, elimination of CYP19 aromatase expression results in excess gonadotropin secretion due to the lack of estradiol-mediated negative feedback 42. The hyperstimulation of ovaries by high gonadotropin levels results in the failure to ovulate. In fact, human females with CYP19 deficiency usually have ovarian failure with multicystic ovaries and mild virulization, most likely due the inability to metabolize androgens 43.
Androgen Actions
Theca cells are stimulated by LH to produce androgens, which then act as paracrine regulators within the ovarian cell types. One function of androgens is to modulate FSH signaling by acting through ARs on granulosa cells to amplify cAMP levels 44. Interestingly, AR mRNA and protein levels are appear to be down-regulated during late preovulatory development, which may serve to desensitize granulosa cells until exposure to the LH surge 4. The importance of the AR in ovarian function has been further examined in AR null mice, where females are subfertile with reduced litter size 5, 45. Interestingly, older females lacking the AR develop premature ovarian failure 6, further implicating the AR as an important mediator of normal follicle development.
Nongenomic Steroid-Mediated Oocyte Maturation
One critical steroid-mediated process that is essential for normal female fertility is oocyte maturation, or re-entry into meiosis. Oocytes within the ovary are arrested in prophase I of meiosis until the gonadotropins stimulate follicular growth and development, which then triggers the resumption of meiosis up to metaphase II. Gametes are subsequently again held in meiotic arrest until fertilization, when meiosis is completed.
Oocyte maturation is best studied in Xenopus laevis, where ovarian steroids promote this process in a transcription-independent (nongenomic) fashion. In Xenopus, androgens appear to be the primary physiologic mediators of maturation in vivo, signaling via their classical ARs. In fact, blockade of androgen production significantly reduces gonadotropin induced oocyte maturation and delays ovulation 46. Interestingly, selective androgen receptors modulators, or SARMs, have been characterized that bind to the AR and specifically regulate genomic versus nongenomic AR-mediated signaling in Xenopus laevis 47.
The process of steroid-mediated oocyte maturation appears to be conserved in mammals, as androgens, estrogens, and progestins trigger maturation of both isolated mouse oocytes held in meiotic arrest by phosphodiesterase inhibitors 48 and oocytes maintained in meiotic arrest by surrounding follicular cells 13. As in frogs, steroids appear to promote maturation via their respective classical receptors 13; however, since progesterone is the most abundant steroid during ovulation, it is likely the primary steroid regulating mouse oocyte maturation in vivo. Notably, in contrast to frogs, steroid-production is sufficient but not necessary for oocyte maturation in mice 49, 50, suggesting that higher vertebrates have developed redundant systems to trigger this important process. One hypothesis is that steroids play an important role in selecting which oocyte will mature first in mammals, as the dominant follicles have the highest steroid concentrations 51. Importantly, regardless of their role in regulating normal oocyte maturation, excess ovarian steroid production is potentially problematic, as it could lead to unregulated oocyte maturation and subsequent infertility (see below).
Pathologic steroid signaling in the ovary – Polycystic Ovarian Syndrome
Polycystic ovarian syndrome (PCOS) is a major cause of infertility, with approximately 5–10% of all reproductive age women being affected. It is associated with high ovarian androgen production and anovulation; however, many patients with PCOS also have features of the metabolic syndrome, including obesity, hyperlipidemia, and insulin resistance 52–54. The architecture of the ovaries in PCOS patients is abnormal, consisting of multiple irregular-sized follicles, many of which have degenerated and contain nonfunctioning oocytes. This ovarian anatomy is consistent with unregulated follicular growth resulting in the absence of dominant follicle formation that is necessary for normal ovulation.
The pathophysiology of PCOS is not well understood, and debate exists over whether insulin or androgen actions are the primary cause of the ovarian abnormalities 54–57. In favor of insulin signaling being the primary defect is: 1) Insulin promotes follicle growth and steroidogenesis in vitro 58. 2) Fertility in patients with PCOS and insulin resistance is often restored by insulin sensitizers that lower plasma insulin levels 59–64.
Alternatively, other evidence points to excess androgens being responsible for the ovarian pathology in PCOS patients. First, polycystic ovaries and infertility are found in women with other forms of androgen excess that often are not accompanied by insulin resistance, including exogenous androgen use (e.g., female transgender patients and women on anabolic steroids 65, 66) or excess adrenal androgen production (e.g., congenital adrenal hyperplasia 67). Second, androgen receptor (AR) antagonists such as flutamide restore ovulation in certain subsets of patients 68–71. Finally, physiologic levels of androgens and androgen receptors are important for normal follicle development 6, 72, and therefore might enhance growth when in excess.
The following model can been used to reconcile some of these complex issues regarding PCOS and insulin resistance: normal insulin levels may stimulate baseline ovarian androgen production, which in turn participates in regulating follicle development. In contrast, excess insulin may promote increased ovarian androgen production 58, resulting in unregulated follicle growth with an absence of dominant follicle formation and subsequent ovulation.
If elevated androgen signaling in the ovary contributes to the ovarian pathology in PCOS, then AR antagonists should be helpful in treating this disease. As mentioned, both flutamide and spironolactone have indeed been used successfully to improve fertility in PCOS 69–71; however, the efficacy of these drugs is limited due to their low potency. As an alternate approach, rather than use weak AR antagonists to block androgen effects, targeted reduction of ovarian androgen production might attenuate excess androgen signaling and restore fertility in women with PCOS. One possible means of decreasing ovarian androgen production might be to block MMP or EGF receptor activity, both of which are important for ovarian steroidogenesis. Alternatively, specific inhibition of CYP17 lyase might reduce androgen production without affecting adrenal cortisol synthesis in the adrenal gland 73. Further translational studies linking laboratory research to the infertility clinic will allow these types of studies to be performed, and may lead to new and exciting advances in the field of female reproductive biology.
Acknowledgments
We thank Keith Parker and Jean Wilson (University of Texas Southwestern Medical Center) for their valuable advice regarding steroidogenesis in the ovary. S.R.H. is a W. W. Caruth, Jr. Endowed Scholar in Biomedical Research. M.J. is funded in part by the NIH training grant T32 GM07062-29. Work from our laboratory was supported by the NIH (DK59913) and the March of Dimes (FY05-78).
References
- 1.Schomberg DW, Couse JF, Mukherjee A, Lubahn DB, Sar M, Mayo KE, Korach KS. Targeted disruption of the estrogen receptor-alpha gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology. 1999;140:2733–44. doi: 10.1210/endo.140.6.6823. [DOI] [PubMed] [Google Scholar]
- 2.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–82. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couse JF, Korach KS. Reproductive phenotypes in the estrogen receptor-alpha knockout mouse. Ann Endocrinol (Paris) 1999;60:143–8. [PubMed] [Google Scholar]
- 4.Hillier SG, Tetsuka M. Role of androgens in follicle maturation and atresia. Baillieres Clin Obstet Gynaecol. 1997;11:249–60. doi: 10.1016/s0950-3552(97)80036-3. [DOI] [PubMed] [Google Scholar]
- 5.Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci U S A. 2004;101:11209–14. doi: 10.1073/pnas.0404372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. From the Cover: Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci U S A. 2006;103:224–9. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–78. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 8.Lobo RA. What are the key features of importance in polycystic ovary syndrome? Fertil Steril. 2003;80:259–61. doi: 10.1016/s0015-0282(03)00733-7. [DOI] [PubMed] [Google Scholar]
- 9.Wood JR, Strauss JF., 3rd Multiple signal transduction pathways regulate ovarian steroidogenesis. Rev Endocr Metab Disord. 2002;3:33–46. doi: 10.1023/a:1012748718150. [DOI] [PubMed] [Google Scholar]
- 10.Havelock JC, Rainey WE, Carr BR. Ovarian granulosa cell lines. Mol Cell Endocrinol. 2004;228:67–78. doi: 10.1016/j.mce.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Piquette GN, LaPolt PS, Oikawa M, Hsueh AJ. Regulation of luteinizing hormone receptor messenger ribonucleic acid levels by gonadotropins, growth factors, and gonadotropin-releasing hormone in cultured rat granulosa cells. Endocrinology. 1991;128:2449–56. doi: 10.1210/endo-128-5-2449. [DOI] [PubMed] [Google Scholar]
- 12.Conneely OM, Lydon JP, De Mayo F, O’Malley BW. Reproductive functions of the progesterone receptor. J Soc Gynecol Investig. 2000;7:S25–32. doi: 10.1016/s1071-5576(99)00062-3. [DOI] [PubMed] [Google Scholar]
- 13.Jamnongjit M, Gill A, Hammes SR. Epidermal Growth Factor Receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci U S A. 2005;102:16257–61. doi: 10.1073/pnas.0508521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasegawa T, Zhao L, Caron KM, Majdic G, Suzuki T, Shizawa S, Sasano H, Parker KL. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol. 2000;14:1462–71. doi: 10.1210/mend.14.9.0515. [DOI] [PubMed] [Google Scholar]
- 15.Bose HS, Sato S, Aisenberg J, Shalev SA, Matsuo N, Miller WL. Mutations in the steroidogenic acute regulatory protein (StAR) in six patients with congenital lipoid adrenal hyperplasia. J Clin Endocrinol Metab. 2000;85:3636–9. doi: 10.1210/jcem.85.10.6896. [DOI] [PubMed] [Google Scholar]
- 16.Strauss JF, 3rd, Kallen CB, Christenson LK, Watari H, Devoto L, Arakane F, Kiriakidou M, Sugawara T. The steroidogenic acute regulatory protein (StAR): a window into the complexities of intracellular cholesterol trafficking. Recent Prog Horm Res. 1999;54:369–94. discussion 94–5. [PubMed] [Google Scholar]
- 17.Rajagopalan-Gupta RM, Lamm ML, Mukherjee S, Rasenick MM, Hunzicker-Dunn M. Luteinizing hormone/choriogonadotropin receptor-mediated activation of heterotrimeric guanine nucleotide binding proteins in ovarian follicular membranes. Endocrinology. 1998;139:4547–55. doi: 10.1210/endo.139.11.6302. [DOI] [PubMed] [Google Scholar]
- 18.Leung PC, Steele GL. Intracellular signaling in the gonads. Endocr Rev. 1992;13:476–98. doi: 10.1210/edrv-13-3-476. [DOI] [PubMed] [Google Scholar]
- 19.Seger R, Hanoch T, Rosenberg R, Dantes A, Merz WE, Strauss JF, 3rd, Amsterdam A. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J Biol Chem. 2001;276:13957–64. doi: 10.1074/jbc.M006852200. [DOI] [PubMed] [Google Scholar]
- 20.Cameron MR, Foster JS, Bukovsky A, Wimalasena J. Activation of mitogen-activated protein kinases by gonadotropins and cyclic adenosine 5′-monophosphates in porcine granulosa cells. Biol Reprod. 1996;55:111–9. doi: 10.1095/biolreprod55.1.111. [DOI] [PubMed] [Google Scholar]
- 21.Gyles SL, Burns CJ, Whitehouse BJ, Sugden D, Marsh PJ, Persaud SJ, Jones PM. ERKs regulate cyclic AMP-induced steroid synthesis through transcription of the steroidogenic acute regulatory (StAR) gene. J Biol Chem. 2001;276:34888–95. doi: 10.1074/jbc.M102063200. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi G, Arai K, Limback D, Roby KF, Terranova PF. Src tyrosine kinase regulates CYP17 expression and androstenedione secretion in theca-enriched mouse ovarian cells. Endocrine. 2004;25:147–54. doi: 10.1385/ENDO:25:2:147. [DOI] [PubMed] [Google Scholar]
- 23.Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, Kimball SR, Strauss JF, 3rd, McAllister JM. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19:379–90. doi: 10.1210/me.2004-0178. [DOI] [PubMed] [Google Scholar]
- 24.Sekar N, Lavoie HA, Veldhuis JD. Concerted regulation of steroidogenic acute regulatory gene expression by luteinizing hormone and insulin (or insulin-like growth factor I) in primary cultures of porcine granulosa-luteal cells. Endocrinology. 2000;141:3983–92. doi: 10.1210/endo.141.11.7763. [DOI] [PubMed] [Google Scholar]
- 25.Demeestere I, Gervy C, Centner J, Devreker F, Englert Y, Delbaere A. Effect of insulin-like growth factor-I during preantral follicular culture on steroidogenesis, in vitro oocyte maturation, and embryo development in mice. Biol Reprod. 2004;70:1664–9. doi: 10.1095/biolreprod.103.023317. [DOI] [PubMed] [Google Scholar]
- 26.Hirakawa T, Minegishi T, Abe K, Kishi H, Ibuki Y, Miyamoto K. A role of insulin-like growth factor I in luteinizing hormone receptor expression in granulosa cells. Endocrinology. 1999;140:4965–71. doi: 10.1210/endo.140.11.7112. [DOI] [PubMed] [Google Scholar]
- 27.LaVoie HA, Garmey JC, Veldhuis JD. Mechanisms of insulin-like growth factor I augmentation of follicle-stimulating hormone-induced porcine steroidogenic acute regulatory protein gene promoter activity in granulosa cells. Endocrinology. 1999;140:146–53. doi: 10.1210/endo.140.1.6407. [DOI] [PubMed] [Google Scholar]
- 28.Pierce KL, Luttrell LM, Lefkowitz RJ. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene. 2001;20:1532–9. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- 29.Herrlich A, Daub H, Knebel A, Herrlich P, Ullrich A, Schultz G, Gudermann T. Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic, acid-stimulated mitogenic activity in L cells. Proc Natl Acad Sci U S A. 1998;95:8985–90. doi: 10.1073/pnas.95.15.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maudsley S, Pierce KL, Zamah AM, Miller WE, Ahn S, Daaka Y, Lefkowitz RJ, Luttrell LM. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem. 2000;275:9572–80. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 31.Manna PR, Huhtaniemi IT, Wang XJ, Eubank DW, Stocco DM. Mechanisms of epidermal growth factor signaling: regulation of steroid biosynthesis and the steroidogenic acute regulatory protein in mouse Leydig tumor cells. Biol Reprod. 2002;67:1393–404. doi: 10.1095/biolreprod.102.007179. [DOI] [PubMed] [Google Scholar]
- 32.Ascoli M, Segaloff DL. Regulation of the differentiated functions of Leydig tumor cells by epidermal growth factor. Ann N Y Acad Sci. 1989;564:99–115. doi: 10.1111/j.1749-6632.1989.tb25891.x. [DOI] [PubMed] [Google Scholar]
- 33.Jezova M, Scsukova S, Nagyova E, Vranova J, Prochazka R, Kolena J. Effect of intraovarian factors on porcine follicular cells: cumulus expansion, granulosa and cumulus cell progesterone production. Anim Reprod Sci. 2001;65:115–26. doi: 10.1016/s0378-4320(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 34.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–4. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 35.Enien WM, Chantler E, Seif MW, Elstein M. Human ovarian granulosa cells and follicular fluid indices: the relationship to oocyte maturity and fertilization in vitro. Hum Reprod. 1998;13:1303–6. doi: 10.1093/humrep/13.5.1303. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Rafael Z, Mastroianni L, Jr, Meloni F, Lee MS, Flickinger GL. Total estradiol, free estradiol, sex hormone-binding globulin, and the fraction of estradiol bound to sex hormone-binding globulin in human follicular fluid. J Clin Endocrinol Metab. 1986;63:1106–11. doi: 10.1210/jcem-63-5-1106. [DOI] [PubMed] [Google Scholar]
- 37.Martin B, Rotten D, Jolivet A, Gautray JP. Binding of steroids by proteins in follicular fluid of the human ovary. J Clin Endocrinol Metab. 1981;53:443–7. doi: 10.1210/jcem-53-2-443. [DOI] [PubMed] [Google Scholar]
- 38.Phocas I, Mantzavinos T, Sarandakou A, Dimitriadou F, Zourlas PA. Serum and follicular fluid sex hormone-binding globulin in stimulated and unstimulated cycles. J Assist Reprod Genet. 1995;12:348–53. doi: 10.1007/BF02215725. [DOI] [PubMed] [Google Scholar]
- 39.Stouffer RL. Progesterone as a mediator of gonadotrophin action in the corpus luteum: beyond steroidogenesis. Hum Reprod Update. 2003;9:99–117. doi: 10.1093/humupd/dmg016. [DOI] [PubMed] [Google Scholar]
- 40.Loutradis D, Bletsa R, Aravantinos L, Kallianidis K, Michalas S, Psychoyos A. Preovulatory effects of the progesterone antagonist mifepristone (RU486) in mice. Hum Reprod. 1991;6:1238–40. doi: 10.1093/oxfordjournals.humrep.a137519. [DOI] [PubMed] [Google Scholar]
- 41.Robker RL, Russell DL, Espey LL, Lydon JP, O’Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A. 2000;97:4689–94. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huynh K, Jones G, Thouas G, Britt KL, Simpson ER, Jones ME. Estrogen is not directly required for oocyte developmental competence. Biol Reprod. 2004;70:1263–9. doi: 10.1095/biolreprod.103.022111. [DOI] [PubMed] [Google Scholar]
- 43.Conte FA, Grumbach MM, Ito Y, Fisher CR, Simpson ER. A syndrome of female pseudohermaphrodism, hypergonadotropic hypogonadism, and multicystic ovaries associated with missense mutations in the gene encoding aromatase (P450arom) J Clin Endocrinol Metab. 1994;78:1287–92. doi: 10.1210/jcem.78.6.8200927. [DOI] [PubMed] [Google Scholar]
- 44.Hillier SG, de Zwart FA. Androgen/antiandrogen modulation of cyclic AMP-induced steroidogenesis during granulosa cell differentiation in tissue culture. Mol Cell Endocrinol. 1982;28:347–61. doi: 10.1016/0303-7207(82)90132-0. [DOI] [PubMed] [Google Scholar]
- 45.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99:13498–503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White SN, Jamnongjit M, Gill A, Lutz LB, Hammes SR. Specific modulation of nongenomic androgen signaling in the ovary. Steroids. 2005;70:352–60. doi: 10.1016/j.steroids.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Lutz LB, Jamnongjit M, Yang WH, Jahani D, Gill A, Hammes SR. Selective modulation of genomic and nongenomic androgen responses by androgen receptor ligands. Mol Endocrinol. 2003;17:1106–16. doi: 10.1210/me.2003-0032. [DOI] [PubMed] [Google Scholar]
- 48.Gill A, Jamnongjit M, Hammes SR. Androgens promote maturation and signaling in mouse oocytes independent of transcription: a release of inhibition model for mammalian oocyte meiosis. Mol Endocrinol. 2004;18:97–104. doi: 10.1210/me.2003-0326. [DOI] [PubMed] [Google Scholar]
- 49.Lieberman ME, Tsafriri A, Bauminger S, Collins WP, Ahren K, Lindner HR. Oocytic meiosis in cultured rat follicles during inhibition of steroidogenesis. Acta Endocrinol (Copenh) 1976;83:151–7. doi: 10.1530/acta.0.0830151. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita Y, Shimada M, Okazaki T, Maeda T, Terada T. Production of progesterone from de novo-synthesized cholesterol in cumulus cells and its physiological role during meiotic resumption of porcine oocytes. Biol Reprod. 2003;68:1193–8. doi: 10.1095/biolreprod.102.010934. [DOI] [PubMed] [Google Scholar]
- 51.Teissier MP, Chable H, Paulhac S, Aubard Y. Comparison of follicle steroidogenesis from normal and polycystic ovaries in women undergoing IVF: relationship between steroid concentrations, follicle size, oocyte quality and fecundability. Hum Reprod. 2000;15:2471–7. doi: 10.1093/humrep/15.12.2471. [DOI] [PubMed] [Google Scholar]
- 52.Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;96:520–7. doi: 10.1172/JCI118064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–74. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 54.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 55.Goodarzi MO, Korenman SG. The importance of insulin resistance in polycystic ovary syndrome. Fertil Steril. 2003;80:255–8. doi: 10.1016/s0015-0282(03)00734-9. [DOI] [PubMed] [Google Scholar]
- 56.Azziz R. Androgen excess is the key element in polycystic ovary syndrome. Fertil Steril. 2003;80:252–4. doi: 10.1016/s0015-0282(03)00735-0. [DOI] [PubMed] [Google Scholar]
- 57.Dunaif A. Hyperandrogenemia is necessary but not sufficient for polycystic ovary syndrome. Fertil Steril. 2003;80:262–3. doi: 10.1016/s0015-0282(03)00732-5. [DOI] [PubMed] [Google Scholar]
- 58.Franks S, Gilling-Smith C, Watson H, Willis D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin North Am. 1999;28:361–78. doi: 10.1016/s0889-8529(05)70074-8. [DOI] [PubMed] [Google Scholar]
- 59.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–23. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 60.Nestler JE, Jakubowicz DJ. Lean women with polycystic ovary syndrome respond to insulin reduction with decreases in ovarian P450c17 alpha activity and serum androgens. J Clin Endocrinol Metab. 1997;82:4075–9. doi: 10.1210/jcem.82.12.4431. [DOI] [PubMed] [Google Scholar]
- 61.Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med. 1998;338:1876–80. doi: 10.1056/NEJM199806253382603. [DOI] [PubMed] [Google Scholar]
- 62.Seli E, Duleba AJ. Treatment of PCOS with metformin and other insulin-sensitizing agents. Curr Diab Rep. 2004;4:69–75. doi: 10.1007/s11892-004-0014-8. [DOI] [PubMed] [Google Scholar]
- 63.Romualdi D, Guido M, Ciampelli M, Giuliani M, Leoni F, Perri C, Lanzone A. Selective effects of pioglitazone on insulin and androgen abnormalities in normo- and hyperinsulinaemic obese patients with polycystic ovary syndrome. Hum Reprod. 2003;18:1210–8. doi: 10.1093/humrep/deg264. [DOI] [PubMed] [Google Scholar]
- 64.De Leo V, la Marca A, Petraglia F. Insulin-lowering agents in the management of polycystic ovary syndrome. Endocr Rev. 2003;24:633–67. doi: 10.1210/er.2002-0015. [DOI] [PubMed] [Google Scholar]
- 65.Pache TD, Chadha S, Gooren LJ, Hop WC, Jaarsma KW, Dommerholt HB, Fauser BC. Ovarian morphology in long-term androgen-treated female to male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopathology. 1991;19:445–52. doi: 10.1111/j.1365-2559.1991.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 66.Franke WW, Berendonk B. Hormonal doping and androgenization of athletes: a secret program of the German Democratic Republic government. Clin Chem. 1997;43:1262–79. [PubMed] [Google Scholar]
- 67.Speiser PW. Congenital adrenal hyperplasia: transition from childhood to adulthood. J Endocrinol Invest. 2001;24:681–91. doi: 10.1007/BF03343913. [DOI] [PubMed] [Google Scholar]
- 68.Gambineri A, Pelusi C, Genghini S, Morselli-Labate AM, Cacciari M, Pagotto U, Pasquali R. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60:241–9. doi: 10.1111/j.1365-2265.2004.01973.x. [DOI] [PubMed] [Google Scholar]
- 69.Rittmaster RS. Antiandrogen treatment of polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:409–21. doi: 10.1016/s0889-8529(05)70077-3. [DOI] [PubMed] [Google Scholar]
- 70.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–52. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 71.De Leo V, Lanzetta D, D’Antona D, la Marca A, Morgante G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:99–102. doi: 10.1210/jcem.83.1.4500. [DOI] [PubMed] [Google Scholar]
- 72.Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39–46. doi: 10.1016/s0303-7207(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 73.Qin KN, Rosenfield RL. Role of cytochrome P450c17 in polycystic ovary syndrome. Mol Cell Endocrinol. 1998;145:111–21. doi: 10.1016/s0303-7207(98)00177-4. [DOI] [PubMed] [Google Scholar]


