Abstract
Estradiol benzoate (EB) has repeatedly been shown to increase hippocampal CA1 spine synapse density in ovariectomized female rats. Although this increase has been assumed to enhance memory, a direct link between increased spine synapse density and memory has not been demonstrated. Furthermore, while androgens, such as testosterone propionate (TP) and dihydrotestosterone (DHT), also increase spine synapse density in females, their effects on memory have yet to be investigated. In the present study, ovariectomized female rats were given two injections, 24 h apart, of sesame oil (control), 10 μg EB, 500 μg TP or 500 μg DHT. Forty-eight hours after the second injection, rats were tested in a 1-day spatial Morris water maze task and then immediately perfused for analysis of CA1 spine synapse density (using electron microscopy and unbiased stereology). In the spatial acquisition phase of testing, EB, but not TP or DHT, significantly impaired memory relative to controls. Hormone treatment did not affect spatial retention or performance in the non-spatial phase of testing. In contrast to previous work, spine synapse density was not increased by EB, TP or DHT. We therefore examined a new set of EB-treated females, only half of which were water maze tested. Consistent with previous work, EB significantly increased spine synapse density among behaviorally naïve females. In contrast, spine synapse densities did not differ among behaviorally tested control and EB females, although they were higher than behaviorally naïve controls. These data indicate that 1-day water maze testing can eliminate the hormone-induced increases in CA 1 spine synapse density typically observed in behaviorally naïve females.
Keywords: dihydrotestosterone, estradiol, spatial memory, testosterone
Abbreviations: DHT, dihydrotestosterone; EB, estradiol benzoate; TP, testosterone proprionate
Introduction
The sex-steroid hormones estrogen and progesterone profoundly alter the dendritic morphology of the hippocampus, a brain region involved in cognition. In naturally cycling female rats, the density of dendritic spines on CA1 pyramidal neurons is approximately 30% higher during proestrus, when estrogen and progesterone levels peak, than during estrus, when these hormones reach their nadir (Woolley et al., 1990; Woolley & McEwen, 1992). Estrogen also reverses the spine synapse density reduction induced by ovariectomy (Woolley & McEwen, 1993). In addition, estrogen enhances hippocampal long-term potentiation (Warren et al., 1995; Foy et al., 1999), increases neurogenesis (Tanapat et al., 1999) and reduces long-term depression (Vouimba et al., 2000), suggesting that estrogen enhances hippocampal plasticity.
It has been assumed that estrogen-induced increases in hippocampal plasticity lead to enhanced memory function. However, studies examining this relationship in the context of the estrous cycle yield no clear consensus regarding effects of cycling estrogen on memory; spatial reference and working memory are reportedly impaired during proestrus (Frye, 1995; Warren & Juraska, 1997), estrus (Healy et al., 1999; Frick & Berger-Sweeney, 2001) or no particular phase of the cycle (Berry et al., 1997; Stackman et al., 1997). Most of these studies utilized the Morris water maze, a spatial reference memory task in which rats swim in water to find a hidden escape platform whose location remains constant throughout testing. This task is widely used to study spatial memory, in part because rats can learn the task quickly. However, submersion in water is stressful (Kim et al., 2001), and elevated glucocorticoid levels induced by training may interfere with estrogen’s effects on the hippocampus. Thus, it is unclear whether estrogen-induced alterations in spine synapse density, as seen in behaviorally naïve females (Woolley & McEwen, 1992; Leranth et al., 2000), occur in females tested in the water maze. One goal of the present experiment was to address this issue.
The effects of testosterone and dihydrotestosterone (DHT) on CA1 spine synapse density and spatial memory in the water maze were also of interest, given recent demonstrations that testosterone propionate (TP), DHT and the adrenal androgen, dehydroepiandrosterone (DHEA) significantly increase CA1 spine synapse density in ovariectomized female rats (Hajszan et al., 2004; Leranth et al., 2004). These data suggest that androgens may modulate spine synapse density and memory in females via both local aromatization to estrogen within the brain and actions at androgen receptors. Although testosterone has been shown to improve spatial memory in young human women (Postma et al., 2000), effects of TP and DHT on spatial memory have not been investigated in female rats.
The present experiment was designed to examine the effects of estrogen, TP and DHT on CA1 spine synapse density and spatial memory in ovariectomized female rats. Spatial memory was tested in a 1-day Morris water maze task, which assesses spatial acquisition, spatial retention and cued acquisition within 3 h (Frick et al., 2000, 2004). This 1-day protocol permitted an examination of memory and spine synapse density within the same day.
Materials and methods
Subjects
Subjects were 40 experimentally naïve female Sprague-Dawley rats (251–275 g) purchased from Charles River (Raleigh, NC, USA). They were group-housed in a climate-controlled vivarium with a 12:12 h light:dark cycle (lights on at 07.00 h). Surgery, injections and behavioral testing took place during the light phase of the cycle. Food (Purina LabDiet 5P00 ProLab RMH 3000) and water were provided ad libitum. Rats were handled by the experimenters daily for 5 days prior to surgery. One rat in the estradiol benzoate (EB) group was excluded prior to testing due to respiratory difficulties. All procedures conformed to the standards set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Yale University.
Ovariectomy
All rats were anesthetized with intramuscular injections of a ketamine cocktail (3 mL/kg), containing ketamine (25 mg/mL), xylazine (1.2mg/mL) and acepromazine (0.03 mg/mL) in 0.9% saline. After a midline incision was made, one ovary, oviduct and uterine horn were isolated, the ovary and oviduct were clamped off and removed, and the uterine horn was placed back into the abdominal cavity. This procedure was repeated for the other ovary. The incision was closed with wound clips, which were removed 7 days later. Rats were given 1 week to recover from surgery. At the completion of behavioral testing, rats that were not perfused (see below) were killed with an overdose of CO2 anesthesia, and their uteri removed and weighed.
Hormone treatment
On the seventh and eighth days after surgery, rats were injected with one of the following in a total volume of 200 μL sesame oil/rat: sesame oil (n = 10), EB (10 μg, n = 9), TP (500 μg, n = 10) or DHT (500 μg, n = 10). Sesame oil and all hormones were obtained from Sigma-Aldrich (St. Louis, MO, USA). Twenty-four hours after the last injection, rats were shaped in the Morris water maze, and 48 h after the last injection they were tested in the Morris water maze and perfused (see below). Although perfusion immediately after training did not enable an examination of long-term effects of hormones and training on spine synapse density, it was of greater interest in this study to examine both spine synapse density and water maze performance on the same day.
Morris water maze
This protocol consists of three phases of testing: spatial acquisition, spatial retention and cued (non-spatial) acquisition. All testing was completed within 3 h. The 1-day water maze protocol was conducted as in our previous work (Frick et al., 2000, 2004), and is described below.
A white circular tank (180cm in diameter) was filled with water (24 ± 2 °C) and was surrounded by a variety of extramaze cues. The tank was divided into four quadrants, and four start positions were located at the intersections of the quadrants. Data were recorded using an HVS 2020 automated tracking system (HVS Image, Hampton, England). Twenty-four hours before water maze testing, all rats were habituated to the water using a four-trial shaping procedure in which a smaller ring (97 cm) was inserted inside the tank to decrease the total swimming area. This procedure habituated the rats to the water and taught them to escape from the water by climbing onto a platform. Each rat was first placed on a visible red lucite platform (10 × 10 cm) for 10s, and then placed at three progressively further distances from the platform where it was allowed 30 s to escape onto the platform. No data were collected during this procedure.
Spatial acquisition
In the spatial acquisition phase, the rats learned to find a submerged platform using extramaze cues. A transparent lucite platform (10 × 10 cm) was submerged 2 cm underneath the water in the north-east quadrant of the tank, where it remained for all spatial trials. Each rat participated in 12 trials, which were organized into three blocks of four trials (1 trial/start position within a block). Each block was considered a separate test session and blocks were separated by 30 min. For each trial, the rat was given a maximum time of 60 s to locate the platform, after which it remained there for 10–15 s. If it did not locate the platform within 60 s, it was guided to it by the experimenter. The next trial started immediately after removal from the platform. After completion of the fourth trial of the block, the rat was removed from the platform and placed in a temporary holding cage. Swim time (s), swim distance (cm) and swim speed (cm/s) were recorded.
Spatial retention
Thirty minutes after completion of spatial acquisition, one 30-s probe trial was conducted to examine how well the rats had learned the exact location of the platform. During this trial, the platform was removed from the tank. The following measures were recorded during the probe trial: quadrant time (percent time spent in the training quadrant) and platform crossings (the number of times the rat crossed the exact location of the platform).
Cued acquisition
In the cued acquisition phase, rats learned to swim to a visible platform. Thus, the spatial cues used to find the hidden platform in the spatial task were not necessary for the cued task. This task examines non-mnemonic aspects of water maze performance, such as swimming ability, motivation and visual ability. The visible platform was the same as that used for shaping. This task was conducted 20 min after completion of the probe trial. Four cued trials were conducted, with start locations randomized and the platform located in a different quadrant during each trial. Swim time, swim distance and swim speed were recorded. The intertrial interval was approximately 15 min.
Tissue processing
Within 4 h of the completion of the last cued trial, three rats from each group were anesthetized with sodium pentobarbital and transcardially perfused with heparinized saline followed by a fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in 0.l m phosphate-buffered saline. The brains were removed and postfixed overnight in the same fixative and then transferred to 0.1 m phosphate-buffered saline the next day. The hippocampi were dissected out and vibratome sections (100 μm) were cut perpendicular to the longitudinal axis of the hippocampus. The sections were osmicated (1% osmium tetroxide in phosphate-buffered saline; 40 min), dehydrated in ethanol (the 70% ethanol contained 1% uranyl acetate; 40 min) and flat embedded in Araldite. After capsule embedding, blocks were trimmed to contain only the stratum pyramidale and stratum radiatum up to its transition to stratum lacunosum moleculare of the CA1 hippocampal subfield.
Synapse counts
Spine synapse density was calculated according to our standard protocol using unbiased stereological methods (Leranth et al., 2000, 2003, 2004). Briefly, to assess possible changes in the volume of the tissue, a correction factor was calculated assuming that the hormonal treatments did not alter the total number of pyramidal cells. Thus, in all hippocampi, six or seven disector pairs (pairs of adjacent 2-μm toluidine blue-stained semithin sections mounted on slides) were analysed using the technique of Braendgaard and Gundersen (Braendgaard & Gundersen, 1986). The pyramidal cell density value (D) was calculated using a formula D = N/sT, where N is the mean disector score across all sampling windows, T is the thickness of the sections (2 μm) and s stands for the length of the window. Based on these values, a dimensionless volume correction factor kv was introduced: kv = D/D1, where D1 is the main density across the groups of hippocampi.
Thereafter, pairs of consecutive serial ultrathin sections (‘reference’ and ‘look-up’) were cut from the vibratome sections taken from all parts of the hippocampus along its longitudinal axis. The section pairs were collected on formvar-coated single-slot grids. Subsequently, digitized images were taken at a magnification of 11,000 × in a Tecnai 12 transmission electron microscope furnished with an AMT Advantage 4.00 HR/HR-B CCD camera system from an area located between the upper and middle third of the CA1 stratum radiatum (300–500 μm from the pyramidal cell layer). Identical regions in reference and look-up sections were identified using landmarks such as myelinated fibers, large dendrites or blood vessels that did not change significantly between neighboring sections due to their size. Areas occupied by potentially interfering structures such as blood vessels, large dendrites or glial cells were subtracted from the measured areas using the NIH Scion image-processing software.
In order to obtain a comparable measure of synaptic numbers, unbiased for possible changes in synaptic size, the disector technique was used (Sterio, 1984). The digitized electron micrographs were printed out using a Brother HL-1450 laser printer. Prior to data analysis, the printed pictures were coded and the code was not broken until the analysis was completed. Only those spine synapses were counted that were present in the reference section, but not in the look-up section (Fig. 1). In order to increase the efficiency of spine synapse counting, the analysis was performed treating each reference section as a look-up section and vice versa (Woolley & McEwen, 1992).
Fig. 1.
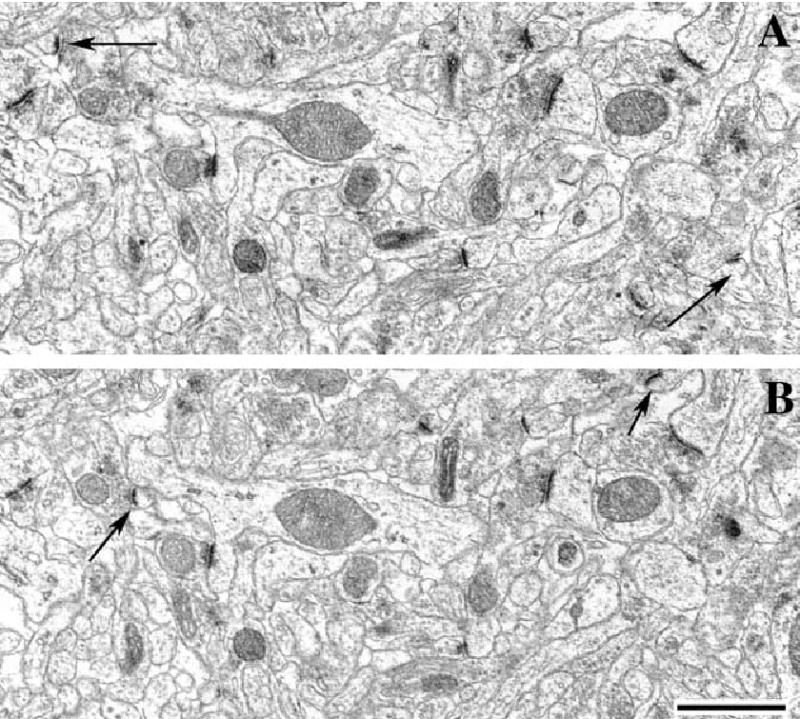
Electron micrographs showing identical areas on two serial sections (reference, A; and look-up section, B). Arrows on both panels point at spine synapses that are present only in one section. Scale bar, 1 μm.
The density of spine synapses of pyramidal cell dendrites was calculated with the help of a reference grid superimposed on the electron microscopic prints. The disector volume (volume of reference) was the unit area of the reference grid multiplied by the distance between the upper faces of the reference and look-up sections (Braendgaard & Gundersen, 1986). Section thickness (average 0.075 μm) was determined using the electron scattering technique. The measured synaptic density values were divided by the volume correction factor kv. This correction provided a synaptic density estimate normalized with respect to the density of pyramidal cells and also accounted for possible changes in hippocampal volume.
At least five neuropil fields (each 80 μm2) were photographed on each electron microscopic grid. With at least three grids (containing a minimum of two pairs of consecutive, serial ultrathin sections) prepared from each vibratome section (cut from the three portions of the hippocampus along its longitudinal axis), each animal provided at least 3 × 3 × 5 × 2 = 90 neuropil fields for evaluation, corresponding to a total section area of 7200 μm2 (a total neuropil volume of 540 μm3) per animal. Individual mean synapse densities for each animal were used to calculate values for overall synapse density in each experimental group.
Data analyses
Hormone treatment was the independent variable for all behavioral analyses. Each spatial acquisition measure was averaged for each block of four trials and analysed using a one-way repeated-measures anova with Block as the repeated measure. To determine whether all groups had learned the spatial task similarly, a separate one-way anova without repeated measures was performed on measures from the last testing block (block 3) only. Scheffe post-hocs were used to determine between-group differences. One-way anovas without repeated measures were performed on spatial retention trial measures. Cued acquisition measures were analysed using one-way repeated measures anovas with Trial as the repeated measure.
In order to avoid bias in the anatomical measurements, the investigator was blind to the experimental treatment. Means determined for each animal were used for the statistical analysis and were compared among groups using a one-way anova and Scheffe post-hoc tests. Uterine weights were also compared using a one-way anova and Scheffe post-hoc tests. P-values < 0.05 were considered to be significant.
Results
Uterine weights
Uterine weights were significantly increased by estrogen treatment only (F3,25 = 11.0, P < 0.0001; post-hoc P < 0.05 EB relative to all other groups). Group means were as follows: control = 0.25 ± 0.02 g; EB = 1.1 ± 0.2 g; TP = 0.32 ± 0.04 g; DHT = 0.29 ± 0.03 g.
Morris water maze
Spatial acquisition was impaired by estrogen treatment during the last block of training (Fig. 2), but spatial retention and cued acquisition were not affected by hormone treatment (Table 1). In the spatial acquisition phase of testing, the main effect of testing Block was significant for both swim time (F2,70 = 70.3, P < 0.0001) and swim distance (F2,70 = 57.1, P < 0.0001), indicating robust learning in the groups as a whole. Although main effects of Treatment and the Treatment-Block interactions were not significant in either measure, one-way anovas conducted on the last testing block only indicated that females receiving EB had longer swim times (F3,35 = 3.9, P < 0.02; Fig. 2A) and distances (F3,35 = 3.5, P < 0.03; Fig. 2B) relative to controls (post-hocs, P < 0.05). No significant differences were observed among control, TP and DHT groups. Despite the adverse effect of EB on swim time and swim distance, there was no effect of any treatment on swim speed either across all blocks (Treatment effect: F3,70=1.8, P > 0.05; Treatment-Block interaction: F6,70 = 0.4, P > 0.05; Fig. 2C) or in block 3 (F3,35 = 0.7, P > 0.05). Swim speeds varied significantly throughout testing in all groups (F2,70 = 4.6, P < 0.02).
Fig. 2.
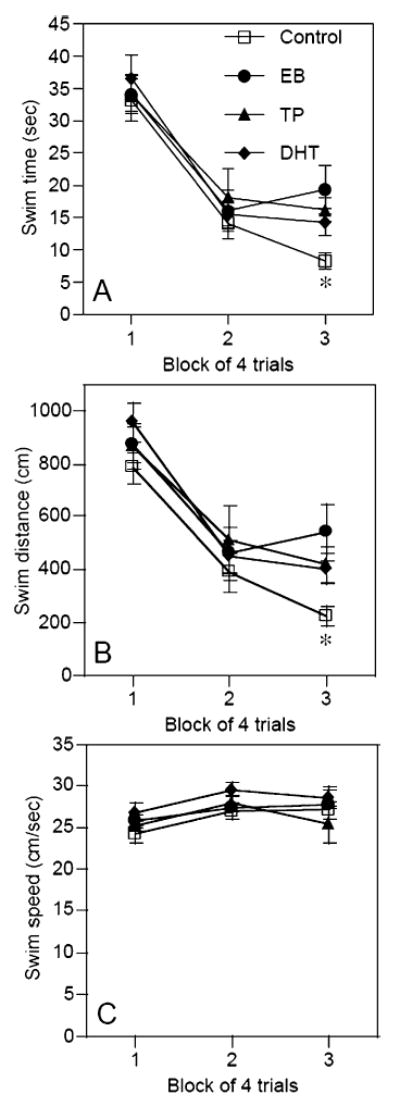
Performance in the spatial acquisition task as illustrated by (A) swim time, (B) swim distance and (C) swim speed. Estradiol benzoate (EB)-treated rats exhibited significantly longer swim times and swim distances than controls during block 3 (*P < 0.05). Hormone treatment did not affect swim speeds. Each symbol represents the mean ± SEM. DHT, dihydrotestosterone; TP, testosterone proprionate.
Table 1.
Group means (± SEM) for the spatial retention trial and cued trials
| Group
|
||||
|---|---|---|---|---|
| Trial and Measure | Control | EB | TP | DHT |
| Probe | ||||
| Quadrant time (%) | 38.2 ± 2.9 | 43.5 ± 6.6 | 35.9 ± 4.8 | 35.7 ± 3.6 |
| Platform crossings (n) | 2.04 ± 0.4 | 2.0 ± 0.5 | 1.2 ± 0.4 | 2.0 ± 0.5 |
| Cued | ||||
| Swim time (s) | 9.1 ± 1.3 | 12.4 ± 1.8 | 8.4 ± 0.8 | 8.6 ± 1.1 |
| Swim distance (cm) | 226.6 ± 32.7 | 282.9 ± 40.9 | 199.4 ± 21.7 | 197.7 ± 29.5 |
| Swim speed (cm/s) | 24.3 ± 1.2 | 23.7 ± 1.1 | 23.7 ± 1.3 | 22.0 ± 1.1 |
DHT, dihydrotestosterone; EB, estradiol benzoate; TP, testosterone proprionate.
In the spatial retention trial, neither quadrant time nor platform crossings were significantly affected by hormone treatment (Table 1). Similarly, the main effects of Treatment were not significant for any of the cued acquisition measures (Table 1). Although Treatment-Trial interactions in the cued task were significant for swim time (F9,105 = 2.3, P < 0.02) and swim distance (F9,105 = 2.8, P < 0.001), these effects were not the result of any systematic effects of the treatments, but were due to the fact that each group performed most poorly in a different trial of testing. Swim speeds in the cued task were not significantly affected by Treatment (Table 1) or Trial.
Spine synapse density
Spine synapse density in the CA1 region of the hippocampus was significantly affected by hormone treatment (F3,8 = 8.3, P < 0.01; Fig. 3A), such that density was reduced in the TP group relative to all other groups (P < 0.05). Neither the EB nor DHT groups differed from controls. The fact that none of the hormones increased spine synapse density sharply contrasts with previous data from our lab and others which indicate that two injections of EB, TP or DHT 24 h apart in behaviorally naïve ovariectomized rats significantly increase spine synapse density (Woolley & McEwen, 1992, 1993; Leranth et al., 2000, 2004). Because the primary difference between this study and previous studies was Morris water maze testing, we decided to examine spine synapse density in a new set of six EB-treated ovariectomized female Sprague-Dawley rats (all treated identically to the rats in the primary experiment). We decided to focus on EB because of the extensive literature pertaining to effects of this hormone on spine density and memory. Three rats were injected and behaviorally tested as described above, whereas three rats were injected and perfused 4 days later, but NOT behaviorally tested 24 and 48 h after the last injection. All six rats were perfused on the second day after the last injection, after behavioral testing was completed. An analysis of these brains, plus the water maze-tested controls from this study and non-water maze-tested controls from a previous study, demonstrated that water maze testing had a significant effect on spine synapse density. A two-way anova with Behavior (water maze or no water maze) and Treatment (oil or EB) as independent variables demonstrated a significant Behavior effect (F1,8=12.8, P < 0.01), Treatment effect (F1,8= 144.4, P < 0.0001) and Behavior-Treatment interaction (F1,8= 134.4, P < 0.0001); spine synapse density did not differ between control and EB-treated rats tested in the water maze, but was increased in EB-treated rats vs. control rats not tested in the water maze (Fig. 3B). Post-hoc tests conducted comparing the four groups confirmed that there was no difference in spine density between the two water maze-tested groups, but that control females not tested in the water maze had significantly fewer spine synapses than EB females not tested in the water maze (P < 0.05). Compared with both groups tested in the water maze, EB-treated rats not tested in the water maze had significantly more spine synapses, whereas controls not tested in the water maze had significantly fewer spine synapses (P < 0.05).
Fig. 3.
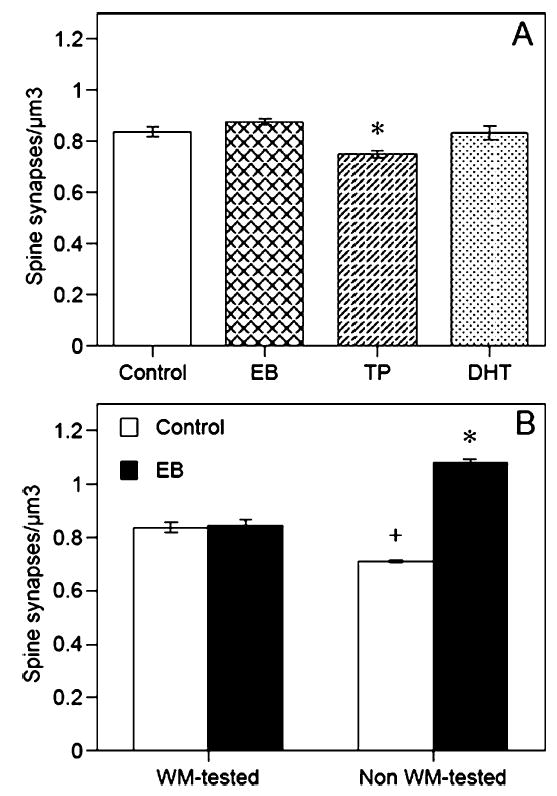
Among rats tested in (A) the water maze, testosterone proprionate (TP) significantly reduced spine synapse density (*P < 0.05), but estradiol benzoate (EB) and dihydrotestosterone (DHT) had no effect. As shown in (B), spine synapse density did not differ between Control and EB-treated rats tested in the water maze (WM). In contrast, EB-treated rats not tested in the water maze exhibited a significantly higher density of spine synapses than non-water maze-tested Controls and both water maze-tested groups (*P < 0.05). Controls not tested in the water maze also had fewer spines than both water maze-tested groups (+P < 0.05 relative to all other groups). Each bar represents the mean ± SEM.
Discussion
The present study is the first to show that EB, TP and DHT given to ovariectomized rats tested in the Morris water maze do not increase CA1 dendritic spine synapse density. Indeed, TP significantly reduced spine synapse density relative to controls tested in the water maze. Furthermore, EB-treated females were impaired in learning the spatial version of the task, as illustrated by impaired performance relative to controls during the last block of spatial acquisition testing. However, neither spatial retention nor cued acquisition were significantly affected by hormone treatment, which is not surprising given that hormones such as EB do not affect performance in the cued task and that the spatial retention trial is not as sensitive to EB as other retention trials administered after multiple days of testing (Frick et al., 2002). Interestingly, EB-treated females not tested in the water maze exhibited increased spine synapse density similar to previous studies (Leranth et al., 2000). These data suggest that the 1-day water maze testing protocol eliminates hormone-induced changes in hippocampal synaptic plasticity.
The fact that EB affected performance during spatial acquisition, but not cued acquisition, indicates that the adverse effect of EB in the 1-day water maze is specific to spatial learning and memory. This spatial acquisition impairment was subtle, given that it was evident only during the last block of testing. However, the fact that spatial memory continued to improve in controls, but not in the EB group, suggests that hormone treatment interfered with normal learning of this task. This effect is somewhat surprising given that many studies of exogenous estrogen replacement find that estrogen given to ovariectomized female rats can improve spatial memory (e.g. O’Neal et al., 1996; Packard & Teather, 1997; Gibbs et al., 1998; Luine et al., 1998; Bimonte & Denenberg, 1999; Gibbs, 1999). Furthermore, EB given to female rats in a similar manner as the present study significantly improved spatial memory retention (Sandstrom & Williams, 2001). However, the study by Sandstrom & Williams (2001) used a different version of the Morris water maze testing a different type of memory (working memory). They also tested rats for several days under varying hormone conditions, and each test day only included six trials, as opposed to the 17 trials employed in the 1-day task used here. These methodological differences may contribute to the divergent findings of these studies. Nevertheless, others have found that chronic exogenous EB can impair spatial memory in the water maze in both rats (Holmes et al., 2002) and mice (Fugger et al., 1998). Given the effect of water maze testing on EB-induced spine synapse modulation, it will be necessary to determine whether an EB-induced impairment would be observed in other non-water maze spatial memory tasks and other memory tasks (e.g. object memory, Luine et al., 2003).
Interestingly, neither TP nor DHT significantly affected spatial memory in this study, despite the fact that TP reduced spine synapse density relative to tested controls. However, the performance of the TP and DHT groups during block 3 is closer to that of the EB group than the controls, suggesting a tendency for these hormones to impair spatial memory. Because our follow-up study of behaviorally naïve and experienced rats did not include TP- and DHT-treated groups, we cannot say conclusively whether the lack of effect of these hormones on spines is due to water maze testing. However, recent studies from this laboratory have clearly demonstrated that hippocampal spine synapse density is androgen sensitive in ovariectomized female rats (Hajszan et al., 2004; Leranth et al., 2004), suggesting that water maze testing interfered with the response. As such, more work will be needed to elucidate the effects of these hormones on memory in female rats.
The lack of a hormone-induced increase in CA1 spine synapse density in water maze-tested females is interesting and challenges the assumption that EB-induced changes in hippocampal synaptic plasticity lead to spatial memory improvements in the water maze. A likely explanation for an interaction between water maze testing and EB treatment is the stress produced by water maze treatment. A single day of training in a Morris water maze task has been associated with levels of corticosterone similar to those seen after acute restraint stress (Kim et al., 2001; J.J. Kim, personal communication), and in the hippocampus, stress severely reduces synaptic plasticity, alters dendritic morphology and inhibits neurogenesis (see Kim & Diamond, 2002 for a recent review). Furthermore, classical conditioning in intact female rats is impaired by stress (Wood & Shors, 1998; Wood et al., 2001). Although it is difficult to equate the stress induced by water maze shaping and testing with the chronic stress induced in most stress experiments, these data support the hypothesis that elevated corticosterone levels produced by water maze testing interfere with EB-induced effects on spatial memory and hippocampal morphology. Of course, measurement of corticosteroid levels will be necessary to support this hypothesis. Although the design of our experiment cannot exclude the possibility that water maze testing on days 3 and 4 after the first injection prevented EB from increasing spine synapse density, it seems more likely that the testing decreased spine synapses after the EB-induced increase had already occurred; anatomical plasticity at synapses can occur extremely rapidly (Fischer et al., 1998; Engert & Bonhoeffer, 1999; Maletic-Savatic et al., 1999), as illustrated by the demonstration that progesterone-induced reductions in dendritic spines can occur within 18 h (Woolley & McEwen, 1993).
An alternative explanation for the effect of water maze testing on hormone-induced spine changes is that the learning associated with water maze training increased spine synapse density in all groups, thereby occluding hormone effects normally seen in behaviorally naïve rats. This idea is supported by the fact that controls tested in the water maze had significantly more spine synapses than those not tested in the water maze (Fig. 3B). Previous studies have found that motor skill learning, such as learning to navigate an obstacle course or learning to reach for food pellets, can increase synaptogenesis in the cerebellum (Black et al., 1990; Kleim et al., 1998) and motor cortex (Kleim et al., 2002), respectively. It is possible that similar changes occur in the hippocampus as a result of hippocampal-dependent learning. Although the data in Fig. 3A may be interpreted to suggest that both learning and hormones increased spine synapse density, density in all of the water maze-tested groups was lower than that of the non-tested EB-treated rats (Fig. 3B), suggesting that estrogen alone can increase spine synapse density to a greater extent than testing alone. If learning and hormone treatment acted synergistically to increase spine synapse density, then density should be highest in rats with both testing and hormone treatment. In contrast, hormone treatment did not increase spine synapse density beyond that induced by learning, supporting the conclusion that water maze testing interfered with the effects of EB on spine synapses. Alternatively, in light of the behavioral findings, the possibility exists that because EB-treated rats learned more poorly than controls, they may have experienced less of a learning-induced increase in spine synapse density than controls, which may then have yielded an overall spine synapse density similar to that of controls. Although we find this explanation somewhat less likely, given the subtle nature of the learning deficit in EB-treated rats, this interesting possibility warrants further study.
In conclusion, these results suggest that 1-day Morris water maze training interferes with the ability of EB, TP and DHT to increase CA1 dendritic spine synapse density. Despite the fact that other studies have shown that chronic or acute estrogen treatment can improve spatial memory in this task, the present findings indicate that the water maze should be used with caution as a means of correlating hormone-induced changes in hippocampal morphology and spatial memory.
Acknowledgments
This work was supported by NIH grants (MH60858 and NS42644 to C.L. and MH65460 to K.M.F.), an American Federation for Ageing Research/Pfizer grant in Hormones and Ageing to K.M.F., and by a grant from the Deutsche Forschungsgemeinschaft (PR 703/1–1) to J.P.-K. We would like to thank Dr. Tibor Hajszan for assistance with neuroanatomical measurements.
References
- Berry B, McMahan R, Gallagher M. Spatial learning and memory at defined points of the estrous cycle: effects of performance on a hippocampal-dependent task. Behav Neurosci. 1997;111:267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braendgaard H, Gundersen HJ. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Meth. 1986;18:39–78. doi: 10.1016/0165-0270(86)90112-3. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim JJ, Baxter MG. Effects of complete cholinergic basal forebrain removal on fear conditioning and spatial learning. Hippocampus. 2004;14:244–254. doi: 10.1002/hipo.10169. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stillner ES, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11:3461–3465. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Cunningham SG, Rissman EF, Foster TC. Sex differences in the activational effect of ERα on spatial learning. Horm Behav. 1998;34:163–170. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Burke AM, Johnson DA. Estrogen replacement attenuates effects of scopolamine and lorazepam on memory acquisition and retention. Horm Behav. 1998;34:112–125. doi: 10.1006/hbeh.1998.1452. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Dehydroepiandrosterone increases hippocampal spine synapse density in ovariectomized female rats. Endocrinology. 2004;145:1042–1045. doi: 10.1210/en.2003-1252. [DOI] [PubMed] [Google Scholar]
- Healy SD, Braham SR, Braithwaite VA. Spatial working memory in rats: no differences between the sexes. Proc Royal Soc London B. 1999;266:2303–2308. doi: 10.1098/rspb.1999.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LAM. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Swain RA, Armstrong KA, Napper RMA, Jones TA, Greenough WT. Selective synaptic plasticity within the cerebellar cortex following complex motor skill learning. Neurobiol Learn Mem. 1998;69:274–289. doi: 10.1006/nlme.1998.3827. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–499. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Horvath TL. Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience. 2000;101:349–356. doi: 10.1016/s0306-4522(00)00369-9. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of mono-aminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- O’Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology. 1996;21:51–65. doi: 10.1016/0306-4530(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Postma A, Meyer G, Tuiten A, van Honk J, Kessels RP, Thijssen J. Effects of testosterone administration on selective aspects of object-location memory in healthy young women. Psychoneuroendocrinology. 2000;25:563–575. doi: 10.1016/s0306-4530(00)00010-x. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiol Learn Mem. 1997;67:167–171. doi: 10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microscopy. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouimba RM, Foy MR, Foy JG, Thompson RF. 17β-estradiol suppresses expression of long-term depression in aged rats. Brain Res Bull. 2000;53:783–787. doi: 10.1016/s0361-9230(00)00377-4. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and non-spatial learning across the rat estrous cycle. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]


