Abstract
The bone morphogenetic proteins (BMPs) play important roles in embryogenesis and normal cell growth. The BMP receptors belong to the family of serine/threonine kinase receptors, whose activation has been investigated intensively for the transforming growth factor-β (TGF-β) receptor subfamily. However, the interactions between the BMP receptors, the composition of the active receptor complex, and the role of the ligand in its formation have not yet been investigated and were usually assumed to follow the same pattern as the TGF-β receptors. Here we demonstrate that the oligomerization pattern of the BMP receptors is different and is more flexible and susceptible to modulation by ligand. Using several complementary approaches, we investigated the formation of homomeric and heteromeric complexes between the two known BMP type I receptors (BR-Ia and BR-Ib) and the BMP type II receptor (BR-II). Coimmunoprecipitation studies detected the formation of heteromeric and homomeric complexes among all the BMP receptor types even in the absence of ligand. These complexes were also detected at the cell surface after BMP-2 binding and cross-linking. Using antibody-mediated immunofluorescence copatching of epitope-tagged receptors, we provide evidence in live cells for preexisting heteromeric (BR-II/BR-Ia and BR-II/BR-Ib) and homomeric (BR-II/BR-II, BR-Ia/ BR-Ia, BR-Ib/ BR-Ib, and also BR-Ia/ BR-Ib) oligomers in the absence of ligand. BMP-2 binding significantly increased hetero- and homo-oligomerization (except for the BR-II homo-oligomer, which binds ligand poorly in the absence of BR-I). In contrast to previous observations on TGF-β receptors, which were found to be fully homodimeric in the absence of ligand, the BMP receptors show a much more flexible oligomerization pattern. This novel feature in the oligomerization mode of the BMP receptors allows higher variety and flexibility in their responses to various ligands as compared with the TGF-β receptors.
INTRODUCTION
Bone morphogenetic proteins (BMPs) are secreted signaling molecules that belong to the transforming growth factor-β (TGF-β) superfamily. BMPs control many temporally distinct and tissue-specific aspects of vertebrate development (Hogan, 1996). Gene-targeting experiments and naturally occurring mutations within the BMPs have shown the substantial effect of BMPs on the regulation of gastrulation, neurogenesis, chondrogenesis, interdigital cell death, and bone morphogenesis (Storm et al., 1994; Storm and Kingsley, 1996; Reddi, 1995; Winnier et al., 1995; Zhang and Bradley, 1996; Dunn et al., 1997).
Signaling by BMP, much like signaling by TGF-β, involves two types of transmembrane serine/threonine kinases, termed type I and type II receptors (Kawabata et al., 1995; Liu et al., 1995; Nohno et al., 1995; Rosenzweig et al., 1995; Hoodless et al., 1996). The human BMP type II receptor (BR-II) is similar to the TGF-β type II receptor (TβR-II) but has in addition a long C-terminal extension of unknown function. There are two known BMP type I receptors (BR-Ia and BR-Ib) that reveal similar signaling characteristics in cell culture (Liu et al., 1995; Rosenzweig et al., 1995; Hoodless et al., 1996; Kretzschmar et al., 1997). Analysis of the expression patterns of BR-Ia and BR-Ib in the developing chicken limb have shown that BR-Ib regulates programmed cell death whereas BR-Ia is essential to the maintenance of the later chondrocyte differentiation program (Zou and Niswander, 1996; Zou et al., 1997). In addition, studies in bone-derived mesenchymal precursor cells have shown that overexpression of BR-Ia induces adipocyte differentiation whereas BR-Ib transmits signals for the osteoblast lineage (Chen et al., 1998). The signaling specificity might be determined at least in part by the particular BR-I complexed with BR-II, as well as by the identity of the specific BMP ligand associated with the active receptor complex. It can also involve the differential usage of several substrates (such as Smads 1 and 5), which might distinguish between activated forms of different type I receptors.
Ligand–cross-linking experiments have shown that BMP ligands, including BMP-2, bind effectively to BR-Ia or BR-Ib but only weakly to singly expressed BR-II; binding to BR-II is enhanced by coexpression with a type I receptor (Liu et al., 1995; Rosenzweig et al., 1995). This situation differs from the one encountered in the case of the TGF-β receptors, in which TβR-II binds TGF-β1 on its own and recruits the TGF-β type I receptor (TβR-I; which by itself does not bind ligand) into a signaling complex (Wrana et al., 1994; Massague, 1998). TGF-β2 and growth and differentiation factor-5, on the other hand, bind very weakly to their respective type II receptors unless the latter are coexpressed with the type I receptors (Lin et al., 1995; Liu et al., 1995; Nohno et al., 1995; Rodriguez et al., 1995; Rosenzweig et al., 1995; Nishitoh et al., 1996; Massague, 1998).
Several lines of evidence suggest both heteromeric and homomeric complex formation among TβR-II and TβR-I, which are closely related to the BMP receptors (Wrana et al., 1992; Moustakas et al., 1993; Chen and Derynck, 1994; Henis et al., 1994; Gilboa et al., 1998; Huse et al., 1999; Wells et al., 1999). Both TβR-II and TβR-I were shown to be present as homodimers in the absence of ligand (Henis et al., 1994; Gilboa et al., 1998), and recent crystallographic data suggest that the cytoplasmic domain of TβR-I in complex with the FK506-binding protein (FKBP12) is a dimer (Huse et al., 1999). Heterocomplexes between the two TGF-β receptor types were detected to a low degree in the absence of ligand and were enhanced significantly by TGF-β1 binding (Wells et al., 1999). Both heteromeric and homomeric complexes appear to be relevant for signaling. Thus, several chimeric receptor systems have established that TβR-II/TβR-I complexes are required for signaling (Okadome et al., 1994; Vivien et al., 1995; Anders and Leof, 1996; Luo and Lodish, 1996; Muramatsu et al., 1997). Studies performed on either chimeric or mutated TβR-I or TβR-II suggested that at least two type I receptors should be present within the signaling complex (Luo and Lodish, 1996; Weis-Garcia and Massague, 1996) and that homo-oligomerization of TβR-II is involved in regulating signal transduction via intermolecular autophosphorylation (Luo and Lodish, 1997).
In spite of the importance of oligomerization for activation and signaling of the TGF-β family of receptors, these issues were not thoroughly investigated for the BMP receptors. In this article, we report studies on the homo- and hetero-oligomerization of BMP receptors (types II, Ia, and Ib). Our studies demonstrate that preformed homomeric and heteromeric complexes of the various BMP receptors exist at the surface of live cells in the absence of ligand. In contrast to the TGF-β receptors, only a fraction of each receptor type resides in homo-oligomers, and BMP-2 binding significantly increases homo-oligomer formation. Ligand-independent heterocomplex formation between BR-II and BR-Ia or BR-Ib occurs at a significant level and is augmented by BMP-2 binding. These findings suggest that the BMP and TGF-β receptor systems differ in their tendency to form oligomeric complexes. The BMP receptor systems exhibit a more flexible oligomerization pattern, reflected not only in the availability of two different type I receptors that can each interact with BR-II but also in the modulation of their homomeric complexes by the ligand.
MATERIALS AND METHODS
Materials
Recombinant human BMP-2 was prepared as described (Ruppert et al., 1996). 9E10 (α-myc, directed against the myc tag [Evan et al., 1985]) mouse ascites was purchased from Babco (Richmond, CA). HA.11 rabbit serum directed against the influenza hemagglutinin (HA) tag (Wilson et al., 1984) and 12CA5 mouse ascites against this tag (α-HA) were from Babco (Richmond, CA). The immunoglobulin G (IgG) fractions were purified from the mouse ascites using standard protocols (Harlow and Lane, 1988). Polyclonal antisera (rabbit) were raised against the following specific peptides from the BMP receptors: LEQDEAFIPVGESLKDLC (human BR-Ia; FB15), KRQEARPRYSIGLEQDET (mouse BR-Ib; FB63), and SMNMMEAAASEPSLDLDN (human BR-II; FB60). Peroxidase-coupled goat anti-mouse (GαM) IgG was obtained from Dianova (Hamburg, Germany). Indocarbocyanine (Cy3)-labeled goat IgG anti-rabbit (GαR) IgG, biotinylated GαM IgG, and FITC-streptavidin were from Jackson ImmunoResearch Laboratories (West Grove, PA). NHS-biotin, protein A-Sepharose, and protein G-Sepharose CL-4B were from Sigma (St. Louis, MO). Disuccinimidyl suberate (DSS) was from Pierce Chemical (Rockford, IL). The cell lines COS7 (CRL 1651), C3H10T1/2 (CCL 226), and C2C12 (CRL 1772) were purchased from American Type Culture Collection (Rockville, MD).
Epitope Tagging of BMP Receptors
The human BR-II construct was supplied by M. Kawabata (Cancer Institute, Tokyo, Japan). The constructs encoding human BR-Ia and mouse BR-Ib were gifts from P. ten Dijke (Ludwig Institute for Cancer Research, Uppsala, Sweden). The cDNAs of the receptors were subcloned into the expression vector pcDNA-I (Invitrogen, San Diego, CA) by double digestion with EcoRI/XbaI for BR-Ib or HindIII/XbaI for BR-Ia and BR-II. The c-myc epitope (Evan et al., 1985) or HA epitope (Wilson et al., 1984) was introduced by recombinant PCR at the N termini of the mature receptors. Each tag was inserted in-frame, immediately downstream of the putative signal sequence. The fragments obtained by recombinant PCR were subcloned into pcDNA-I containing the appropriate BMP receptor cDNA, and the sequences of the tagged constructs were verified by DNA sequencing.
Transfection of COS7 Cells
COS7 cells were grown in DMEM supplemented with 10% FCS and transfected by the DEAE-dextran method (Seed and Aruffo, 1987). For immunoprecipitation or ligand cross-linking, transfections were performed on cells grown in 10-cm plates, using 7–10 μg of DNA per construct. For immunofluorescence copatching studies, COS7 cells were grown on glass coverslips in 30-mm dishes and transfected with 0.25–0.5 μg of DNA of each construct. All experiments were performed 44–48 h after transfection.
Receptor Immunoprecipitation
Transfected COS7 or untransfected C3H10T1/2 cells were washed and incubated with ligand (BMP-2) in KRH buffer (50 mM HEPES, pH 7.5, 128 mM NaCl, 1.3 mM CaCl2, 5 mM MgSO4, and 5 mM KCl) at 4°C as described in Ligand Binding and Cross-linking. Control dishes were incubated with buffer alone. They were washed twice in ice-cold PBS and solubilized in lysis buffer (PBS, pH 7.4, containing 0.5% Triton X-100, 1 mM EDTA, and 10 μg/ml each of leupeptin, aprotinin, soybean trypsin inhibitor, benzamidine-HCl, pepstatin, and antipain) at 4°C for 40 min. Epitope-tagged receptors were immunoprecipitated from extracts of transfected COS7 cells by 12CA5 monoclonal antibodies (α-HA; 20 μg/ml, 4°C, 2 h) followed by incubation with protein A-Sepharose (30 μl of 1:1 suspension in PBS, 1 h, 4°C) or by 9E10 (α-myc; 20 μg/ml) antibodies followed by protein G-Sepharose. Endogenous receptors from C3H10T1/2 cells were precipitated using specific rabbit anti-peptide antisera (FB15 for BR-Ia, FB63 for BR-Ib, and FB60 for BR-II; 1:500 dilution, 4°C, 2 h) followed by protein A-Sepharose as above. The beads were washed three times with PBS. For single immunoprecipitations, the bound protein was eluted by heating the beads in SDS-PAGE sample buffer containing mercaptoethanol (3 min, 95°C). For sequential double immunoprecipitation, the bound protein was eluted from the Sepharose beads in 1% SDS, 50 mM dithiothreitol, and 10% mercaptoethanol (5 min, 95°C). The supernatant was diluted with PBS containing 1 mM EDTA to a final SDS concentration of 0.003%, and the appropriate antibodies were added for the second immunoprecipitation. The proteins finally eluted from the beads were run on 10–12% SDS-PAGE.
Biotinylation of Antibodies
Lyophilized IgG (50 μg of 9E10 or 12CA5) was dissolved in 200 μl of 0.1 M sodium citrate buffer, pH 9.1. After addition of 1 μl of NHS-biotin (12 mg/ml in dimethylformamide; Sigma), the reaction was performed for 4 h at 22°C. The reaction was stopped by adding 5 μl of 1 M NH4Cl. The biotinylated antibodies were dialyzed against PBS.
Western Blotting
Western blotting was done according to standard protocols. After electrotransfer and blocking (10 mM Tris, pH 7.9, 150 mM NaCl, 0.5% Tween 20, and 3% BSA; 4°C, 1 h), the blot was incubated with the monoclonal antibodies 9E10 (20 μg/ml) or 12CA5 (10 μg/ml) or with FB60 rabbit antisera (1:425 dilution) for 12 h at 4°C. Detection of adsorbed antibodies was performed by ECL (Amersham, Arlington Heights, IL), using peroxidase-GαM IgG diluted 1:10,000 in blocking buffer. Alternatively, biotinylated 9E10 (20 μg/ml) or 12CA5 (10 μg/ml) was used, followed by detection using peroxidase-streptavidin (0.2 μg/ml) and ECL.
Ligand Binding and Cross-linking
BMP-2 was labeled by 125I using the chloramine T method as described (Cheifetz et al., 1988). Iodination efficiency was 99%.
Confluent 10-cm plates of transfected COS cells or C3H10T1/2 cells were incubated for 2–6 h at 4°C with 5–20 nM 125I-BMP-2 in KRH buffer containing 0.5% fatty acid–free BSA. For C3H10T1/2 cells, 0.01% Tween 20 was added. Cross-linking was performed with DSS as described previously for TGF-β (Moustakas et al., 1993). Cross-linking was stopped by adding sucrose to a final concentration of 7% in KRH. Cell lysis and immunoprecipitation were performed as described above.
Immunofluorescence Copatching
The method used has been described by us previously (Henis et al., 1994; Gilboa et al., 1998; Wells et al., 1999). COS7 cells grown on glass coverslips were transfected (singly or in various combinations) with BMP receptor constructs. Forty-eight hours after transfection, cells were washed twice with serum-free DMEM and incubated 1 h at 37°C to allow endocytosis of ligand-bound receptors. After washing twice with cold Hanks' balanced salt solution with 20 mM HEPES, containing 1% fatty acid–free BSA, the cells were incubated in the same buffer (4°C, 2 h) with normal goat IgG (200 μg/ml) to block nonspecific binding. This was followed by successive incubations (4°C, 1 h each, with three washes between incubations; all performed in the cold to enable exclusive cell-surface labeling by the antibodies and to eliminate internalization) with the following: 1) α-myc mouse IgG (20 μg/ml) together with rabbit HA.11 α-HA serum (1:250), 2) Cy3-GαR IgG (20 μg/ml) together with biotinylated GαM IgG (20 μg/ml), and 3) FITC-streptavidin (2 μg/ml). After washing, the cells were fixed in methanol (5 min, −20°C) and acetone (2 min, −20°C) and mounted with Mowiol (Hoechst, Frankfurt, Germany) containing 29 mM n-propyl gallate (Sigma). Fluorescence digital images were acquired with the Leica DMR microscope (100× oil objective; Nussloch, Germany), coupled to a charge-coupled device camera (SenSys; Photometrics, Tucson, AZ), using the PMIS (Photometrics) software. For each field, FITC and Cy3 images were taken separately using selective filter sets that essentially eliminate leakage. The images were exported in TIFF format to Photoshop (Adobe, Mountain View, CA), superimposed, and printed. The numbers of red, green, and yellow (superimposed red and green) patches were counted on the computer screen on 20 × 20-μm2 flat cell regions (avoiding the nuclear region, which is out-of-focus).
Luciferase Reporter Assay
C2C12 cells (2 × 106 cells in a 6-cm dish) were transfected by electroporation using 18 μg of a luciferase reporter construct containing two inverted repeats of a Smad-binding element from the JunB promoter (pSBE–luc [Jonk et al., 1998]), 8 μg of renilla luciferase (pRL)–Tk (dual luciferase kit; Promega, Madison, WI) as a reference for transfection efficiency, and 10 μg of BMP receptor constructs (in double-receptor transfections, 5 μg of each receptor; in transfections with a single BMP receptor, 5 μg of pcDNA-I–β-Gal replaced the second BMP receptor construct). After 7 h in complete medium, cells were starved in medium with 0.2% FCS (4–6 h). They were incubated with or without 10 nM BMP-2 in low serum for 24 h, and luciferase activity was measured using a dual luciferase assay system (Promega).
RESULTS
BMP Receptors Form Hetero-oligomers in the Absence of Ligand
To study both hetero- and homo-oligomerization of BMP receptors, we have epitope tagged each BMP receptor (BR-Ia, BR-Ib, and BR-II) with either the HA epitope tag or the myc tag at the N terminus of the mature protein (see MATERIALS AND METHODS). The tagged receptors arrived at the cell surface (see Figures 6 and 7), bound ligand (see Figures 3 and 5), and transduced signal as tested by transcriptional activation of a BMP-dependent reporter gene construct (Jonk et al., 1998) in a manner similar to that of the native receptors (our unpublished results).
Figure 6.
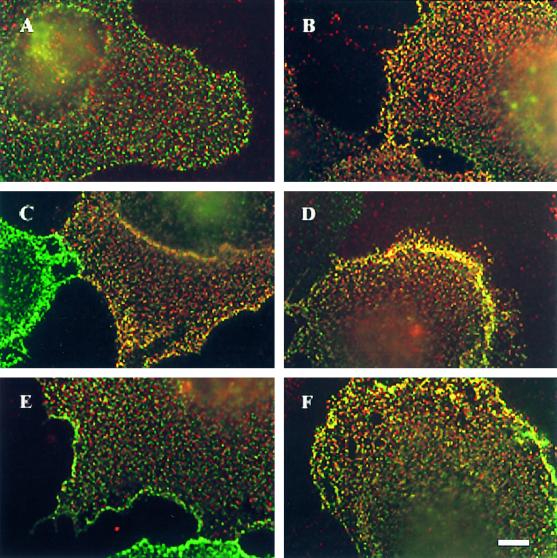
Immunofluorescence copatching reveals a ligand-mediated increase in BMP receptor heterocomplex formation at the cell surface. COS7 cells were cotransfected with myc–BR-Ia and HA–BR-II (A and B), HA–BR-Ib and myc–BR-II (C and D), or myc–BR-Ia and HA–BR-Ib (E and F). Live cells were labeled in the cold consecutively by a series of antibodies to mediate patching and fluorescent labeling, as described in MATERIALS AND METHODS. After this labeling procedure, HA-tagged receptors are labeled by Cy3 (red), myc-tagged receptors are labeled by FITC (green), and patches containing both tags appear yellow when the two fluorescent images are superimposed. The labeling specificity is demonstrated by the existence of separately labeled patches (only red or only green) in the coexpressing cells and by the exclusively green or exclusively red staining of cells singly transfected with either myc–BR-II or HA–BR-II alone and labeled by both antibody sets (see Figure 7E, insets). (A, C, and E) No ligand added. (B, D, and F) Incubation with 10 nM BMP-2. The ligand was added together with the normal goat IgG before copatching (for 1.5 h) and kept in during all successive incubations. Bar, 10 μm.
Figure 7.
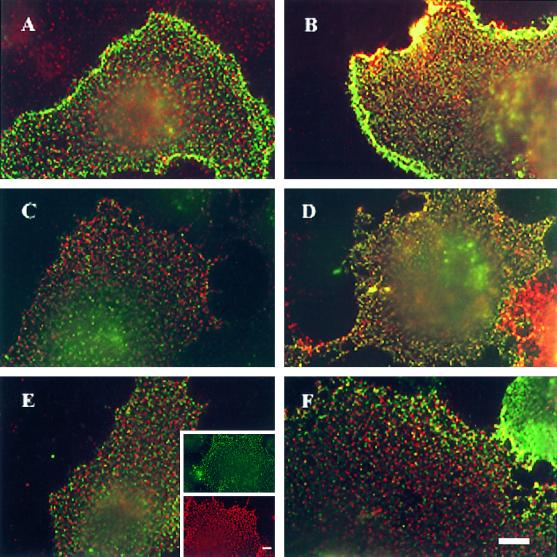
Immunofluorescence copatching demonstrates ligand-enhanced homomeric complexes among all BMP receptor types. COS7 cells were cotransfected with myc–BR-Ia and HA–BR-Ia (A and B), myc–BR-Ib and HA–BR-Ib (C and D), myc–BR-II and HA–BR-II (E and F), myc–BR-II alone (E, green inset), or HA–BR-II alone (E, red inset). Incubation with BMP-2 (10 nM) and labeling with antibodies were as described in Figure 6. The exclusively green or red staining of cells singly expressing myc–BR-II or HA–BR-II after labeling with both antibody sets (E, insets) demonstrates the specificity of the labeling and patching protocol. (A, C, and E) No ligand added. (B, D, and F) Incubation with 10 nM BMP-2. Bars, 10 μm.
Figure 3.
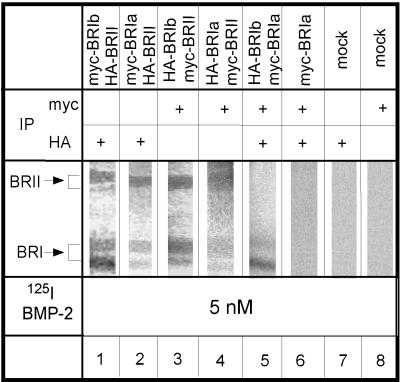
Detection of cell-surface BMP receptor heterocomplex formation by ligand binding and cross-linking. COS7 cells were cotransfected with pairs of epitope-tagged receptors, each carrying a different tag, as indicated above each lane. After binding and cross-linking of 125I-BMP-2 (5 nM), one tagged receptor was immunoprecipitated by 12CA5 (α-HA) or 9E10 (α-myc). In the case of coexpressed myc–BR-Ia and HA–BR-Ib (lane 5), whose bands colocalize, as well as in the relevant control (lane 6), sequential immunoprecipitation with α-myc and α-HA was used. Immunoprecipitates derived from the same amount of cell lysates (1 10-cm dish) were loaded on each lane of the gel and analyzed by SDS-PAGE and autoradiography. Lanes 1–4 depict complexes of BR-II with BR-Ia or BR-Ib, and lane 5 shows BR-Ia/BR-Ib complex formation. Lanes 7 and 8 depict controls of mock-transfected cells (using empty vector), whereas lane 6 indicates the specificity of the double immunoprecipitation protocol (cells singly transfected with myc–BR-Ia and immunoprecipitated first by α-myc and then by α-HA). Single transfections with HA–BR-Ia or epitope-tagged BR-Ib gave similar results (our unpublished results).
Figure 5.
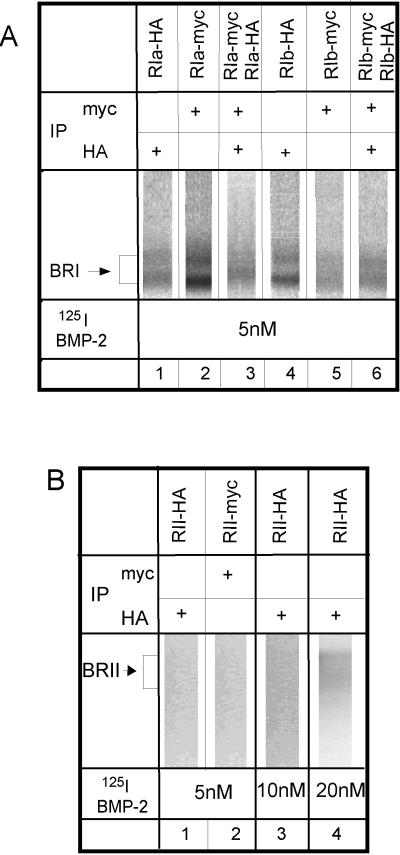
Homo-oligomerization of ligand–cross-linked BMP receptors. (A) BR-Ia and BR-Ib are shown. COS7 cells were singly transfected with HA- or myc-tagged type I receptors or cotransfected with two differently tagged forms of either BR-Ia or BR-Ib as indicated above each lane. After binding and cross-linking with 5 nM 125I-BMP-2, single (lanes 1, 2, 4, and 5) or double (lanes 3 and 6) immunoprecipitation was performed as indicated for each lane, using 9E10 (α-myc) and/or 12CA5 (α-HA) monoclonal antibodies. The immunoprecipitation, SDS-PAGE, and autoradiography were performed as described in Figure 3, which also shows a negative control (lane 6) for the sequential immunoprecipitation. Lanes 1, 2, 4, and 5 depict positive controls of the ligand–cross-linked tagged receptors after single immunoprecipitation with the appropriate antibodies. Lanes 3 and 6 show the coprecipitation of coexpressed BR-Ia and BR-Ib receptors after sequential immunoprecipitation with the two antibodies. (B) Singly expressed BR-II binds the BMP-2 only weakly. COS7 cells were singly transfected with HA- or myc-tagged BR-II, cross-linked with 125I-BMP-2 at the indicated concentration, and subjected to single immunoprecipitation with the relevant anti-tag antibody followed by SDS-PAGE and autoradiography. Although the exposure time was two times longer than that in A, weak labeling could be detected only if the 125I-BMP-2 concentration was very high (20 nM).
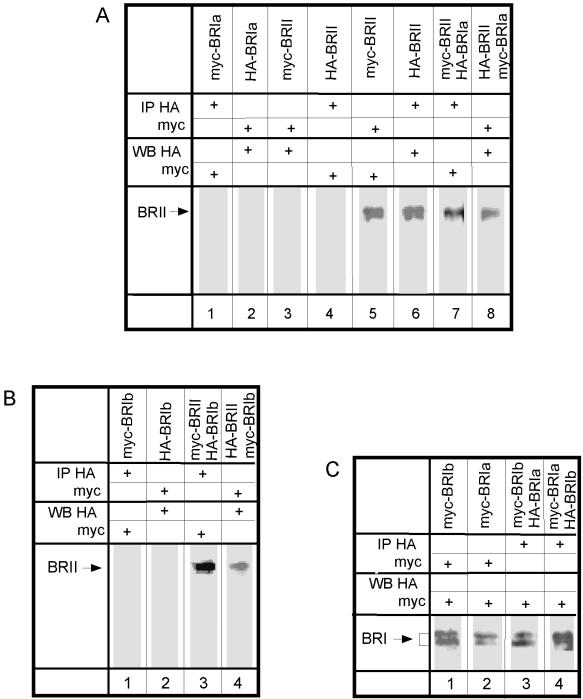
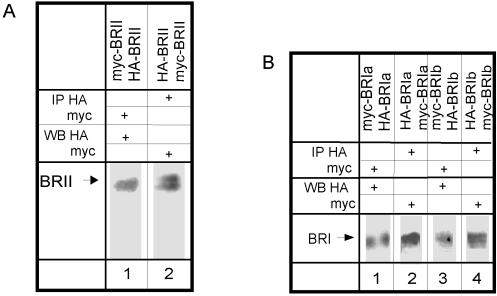
To study heterocomplex formation of BR-II with BR-Ia or BR-Ib, we performed coimmunoprecipitation studies on COS7 cells cotransfected with BR-II together with BR-Ia or BR-Ib carrying different epitope tags (e.g., HA–BR-II together with myc–BR-Ia). After lysis in a mild lysis buffer (0.5% Triton X-100), lysates were immunoprecipitated with antibodies directed against the HA tag (α-HA) or against the c-myc epitope tag (α-myc). The immunoprecipitates were subjected to SDS-PAGE and Western blotting, and the blots were analyzed with α-myc or α-HA mouse monoclonal IgG followed by peroxidase-coupled GαM IgG. These experiments showed that BR-II is coprecipitated with BR-Ia (Figure 1A, lanes 7 and 8). The identity of BR-II was established in control cells transfected with either myc–BR-II or HA–BR-II alone and immunoprecipitated and blotted with the same antibody (α-myc or α-HA, respectively; Figure 1A, lanes 5 and 6). Blots from control cells transfected with myc–BR-Ia alone and precipitated by α-HA were not stained by α-myc, and HA–BR-Ia could not be detected by α-HA after immunoprecipitation using α-myc (Figure 1A, lanes 1 and 2), showing the specificity of the antibodies used for immunoprecipitation. The specificity of the blotting step is demonstrated in lanes 3 and 4 (Figure 1A), where singly expressed myc–BR-II precipitated by α-myc was not detected by α-HA and HA–BR-II precipitated by α-HA was not recognized by α-myc.
Figure 1.
Coimmunoprecipitation of different BMP receptors without ligand. (A) Complex formation between BR-II and BR-Ia. COS7 cells were transiently transfected with plasmids encoding epitope-tagged BR-Ia or BR-II or cotransfected with both (each carrying a different tag) as indicated above each lane. After immunoprecipitation (IP) with 12CA5 (α-HA) or 9E10 (α-myc) antibodies, Western blotting (WB) was performed using α-myc or α-HA, and detection was by ECL. All lanes were loaded with immunoprecipitates derived from the same amount of cell lysates (1 10-cm dish). BR-II migrates as a doublet of ∼150 kDa. (B) Complex formation between BR-II and BR-Ib. Conditions were as described in A, except that epitope-tagged BR-Ib replaced BR-Ia. Lanes 3 and 4 show hetero-oligomers of BR-II with BR-Ib; lanes 1 and 2 are controls. (C) Complex formation between BR-Ia and BR-Ib. The experiment was performed as described in A, except that epitope-tagged BR-Ib replaced BR-II and the blotting used biotinylated α-myc or α-HA followed by peroxidase-streptavidin. Controls identical to those shown in B (lanes 1 and 2) were similarly negative (our unpublished results). BR-I migrates as a doublet of ∼55 kDa. Lanes 3 and 4 show hetero-oligomerization of BR-Ia with BR-Ib.
Analogous experiments using pairs of coexpressed epitope-tagged receptors were conducted to explore the formation of hetero-oligomers of BR-II and BR-Ib and of BR-Ia with BR-Ib. Figure 1B, lanes 3 and 4, depicts the coprecipitation of BR-II with BR-Ib, which was very similar to that observed for BR-II with BR-Ia. The immunoprecipitation of tagged BR-Ib receptors was as specific as that of the BR-Ia receptors (Figure 1B, lanes 1 and 2). Interestingly, the two type I receptors BR-Ia and BR-Ib also associated into heterocomplexes, as shown in Figure 1C. Thus, the coimmunoprecipitation experiments reveal the existence of heterocomplexes between all the BMP receptors in the absence of ligand.
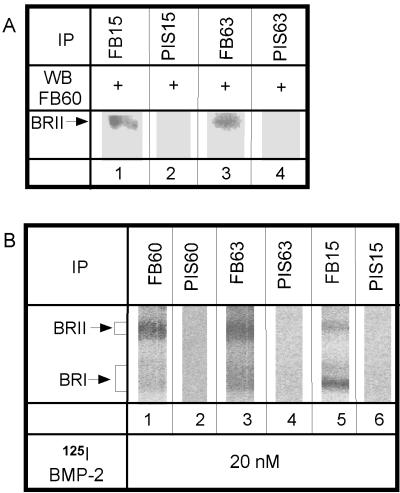
To confirm that hetero-oligomeric complexes are also formed in a cell line that naturally expresses the BMP receptors, we used the pluripotent mouse fibroblast cell line C3H10T1/2. These cells were shown to differentiate into three cell types upon addition of BMP-2 or BMP-7 (Ahrens et al., 1993; Wang et al., 1993; Asahina et al., 1996). Lysates of C3H10T1/2 cells were immunoprecipitated with specific anti-peptide antisera against BR-Ia or BR-Ib (Figure 2A, lanes 1 and 3) and blotted using anti-BR-II antiserum. Both lanes depict a clear band, demonstrating that BR-II was coprecipitated with either type I receptor. No precipitation of BR-II was observed with preimmune serum against either BR-Ia or BR-Ib (Figure 2A, lanes 2 and 4). These experiments, which use a naturally expressing cell line, substantiate the existence of BMP receptor heterocomplexes in the absence of ligand and indicate that this observation is not the result of receptor overexpression.
Figure 2.
Hetero-oligomeric BMP receptor complexes in naturally expressing cells. (A) Coimmunoprecipitation of BR-II with BR-Ia and BR-Ib in the absence of ligand is shown. Lysates from C3H10T1/2 cells were immunoprecipitated with anti-peptide antisera against either BR-Ia (FB15; lane 1) or BR-Ib (FB63; lane 3), followed by Western blotting using an antibody (FB60) against BR-II. Lanes 2 and 4 show controls using each corresponding preimmune serum (PIS) for immunoprecipitation. Immunoprecipitates derived from the same amount of cell lysates were loaded on each lane. (B) Immunoprecipitation after ligand cross-linking detects receptor heterocomplexes at the cell surface. After ligand binding and cross-linking using 20 nM 125I-BMP-2, one receptor type was precipitated using specific anti-peptide antibodies. Immunoprecipitates derived from one 14-cm dish were loaded on each lane. Lane 1, 3, and 5 depict complexes of BR-II with BR-Ia or BR-Ib, whereas the controls for the preimmune sera (lanes 2, 4, and 6) are negative.
Hetero-oligomerization among Ligand–cross-linked BMP Receptors at the Cell Surface
The above experiments detected heterocomplex formation among BMP receptors in whole-cell extracts. These include receptors that might form nonspecific aggregates within the endoplasmic reticulum. To follow exclusively the cell-surface population, we used two independent methods: ligand binding and cross-linking followed by immunoprecipitation (described in this section) and immunofluorescence-copatching studies to explore the state of the receptors in the intact membrane on live cells (see Figures 6 and 7). For ligand–cross-linking experiments, COS7 cells were transiently transfected with different combinations of epitope-tagged BMP receptors (as indicated in Figure 3). Forty-eight hours after transfection, they were incubated with 5 nM 125I-BMP-2 at 4°C to eliminate internalization, cross-linked by DSS, and lysed. Lysates were immunoprecipitated with α-myc or α-HA and analyzed by SDS-PAGE and autoradiography. As shown in Figure 3, ligand-bound hetero-oligomers of all possible combinations of the BMP receptors were detected: BR-II with BR-Ia (lanes 2 and 4), BR-II with BR-Ib (lanes 1 and 3), and BR-Ia with BR-Ib (lane 5, where sequential immunoprecipitation was used). No signal was detected in lysates from mock-transfected cells precipitated by either α-myc or α-HA (Figure 3, lanes 7 and 8). The specificity of the sequential immunoprecipitation protocol used in Figure 3, lane 5, is shown by the lack of signal in cells singly expressing one epitope-tagged receptor form and subjected to sequential double immunoprecipitation (lane 6). Because under the labeling conditions used only cell-surface receptors are exposed to cross-linking by the ligand, these experiments demonstrate that the BMP receptors at the cell surface can form heterocomplexes, at least when bound to BMP-2. These findings hold also for endogenous BMP receptors in untransfected cells (Figure 2B). By the use of specific anti-peptide antibodies, analogous binding and cross-linking studies conducted on naturally expressing C3H10T1/2 cells detected the existence of ligand-bound BR-II/BR-Ia and BR-II/BR-Ib complexes at the cell surface (Figure 2B, lanes 1, 3, and 5).
BMP Receptor Homo-oligomers Detected by Coimmunoprecipitation and by Ligand Binding and Cross-linking
The type I and type II TGF-β receptors were shown to form ligand-independent homo-dimers (Chen and Derynck, 1994; Henis et al., 1994; Gilboa et al., 1998; Huse et al., 1999), and there is evidence of the functional importance of the homomeric complexes (Luo and Lodish, 1996, 1997; Weis-Garcia and Massague, 1996). However, the homo-oligomeric structure of the BMP receptors has not been investigated. We therefore explored the existence of homo-oligomers of each BMP receptor in the absence of ligand by coimmunoprecipitation. The experiments were performed on COS7 cells cotransfected with pairs of two differently tagged versions of each receptor (e.g., HA–BR-II together with myc–BR-II). The experimental design was as described in Figure 1. Figure 4A demonstrates coprecipitation of HA–BR-II with myc–BR-II and vice versa, whereas Figure 4B depicts the coprecipitation of myc- and HA-tagged BR-Ia pairs and BR-Ib pairs. Because only pairs of the tagged receptors are overexpressed in the transfected cells, these results indicate that at least part of each BMP receptor population resides in homo-oligomers.
Figure 4.
Coimmunoprecipitation of two differently tagged forms of the same BMP receptor. The experiments were performed as described in Figure 1. The controls are identical to those shown in Figure 1. The antibodies used for the immunoprecipitation and Western blotting are indicated for each lane. (A) Coimmunoprecipitation of epitope-tagged BR-II receptors. COS7 cells were cotransfected with HA- and myc-tagged BR-II. Immunoblotting was performed with 12CA5 (α-HA) or 9E10 (α-myc). ECL detection used peroxidase-GαM IgG as in Figure 1, A and B. (B) Coimmunoprecipitation of epitope-tagged BR-Ia or BR-Ib. Cells were cotransfected with HA- and myc-tagged BR-Ia (lanes 1 and 2) or BR-Ib (lanes 3 and 4). Immunoblotting was done with biotinylated α-myc or α-HA, and ECL detection was with peroxidase-streptavidin as in Figure 1C.
To ascertain that homo-oligomers of the BMP receptors also exist at the cell surface, we performed ligand–cross-linking experiments followed by sequential immunoprecipitation (Figure 5), as well as immunofluorescence-copatching studies (see the following section). The cross-linking of 125I-BMP-2 to COS7 cells cotransfected with pairs of differently tagged versions of each receptor followed the protocol described in Figure 3. Because the HA- and myc-tagged versions of the same receptor cannot be distinguished according to size, the cell lysates were subjected to double immunoprecipitation before SDS-PAGE and autoradiography. As shown in Figure 5A, homo-oligomers of both BR-Ia and BR-Ib can be detected (lanes 3 and 6). These bands match the ones obtained with a positive control of cells singly transfected with HA- or myc-tagged type I receptor and immunoprecipitated with the matching antibody (Figure 5A, lanes 1, 2, 4, and 5). Similar experiments could not be applied to measure homo-oligomerization of BR-II, because in the absence of coexpressed type I receptors, BR-II binds ligand very weakly and cannot be detected by autoradiography after cross-linking with 125I-BMP-2 (Figure 5B, lanes 1–3). Weak binding can be observed only with high concentrations of 125I-BMP-2 (Figure 5B, lane 4). Similar results were obtained using untagged BR-II (our unpublished results).
Immunofluorescence-copatching Studies Demonstrate a Ligand-induced Increase in BMP Receptor Oligomerization at the Surface of Live Cells
The experiments described above suggest that some fraction of the BMP receptor population can associate into heteromeric and homomeric complexes in the absence of ligand and that such complexes can also be detected among ligand–cross-linked receptors at the cell surface. However, both methods involve detergent solubilization that might alter receptor interactions, and their results cannot be compared directly, thus not enabling a direct estimation of the effect of ligand binding on receptor complex formation. To overcome these limitations we used immunofluorescence copatching, a method that we developed and have described previously (Henis et al., 1994; Gilboa et al., 1998; Wells et al., 1999). These studies have several advantages: 1) they are performed on receptors embedded at the live cell membrane, without any potential detergent interference; 2) only coexpressing cells are selected for analysis under the microscope, eliminating the contribution of singly expressing cells that prevents quantification of immunoprecipitation-based experiments; and 3) they enable direct comparison of the same parameters in the presence and absence of ligand, providing a semiquantitative measure of complex formation (direct or indirect). In this method, two receptors carrying different tags at their extracellular regions are coexpressed at the surface of live cells. Each receptor is forced into micropatches at the surface by labeling with a specific bivalent IgG directed against it, followed by secondary antibodies coupled to different fluorophores (FITC and Cy3). The labeling/copatching step is performed in the cold, to avoid any possible internalization and allow only surface labeling by the antibodies. The cells are then fixed and examined by fluorescence microscopy to determine whether the two receptors are swept by the cross-linking antibodies into mutual (yellow) or separate (red or green) micropatches; copatching (yellow patches) will occur only if the two receptors form mutual complexes. This approach was used successfully by us to demonstrate homo- and hetero-oligomerization among TGF-β receptors (Henis et al., 1994; Gilboa et al., 1998; Wells et al., 1999).
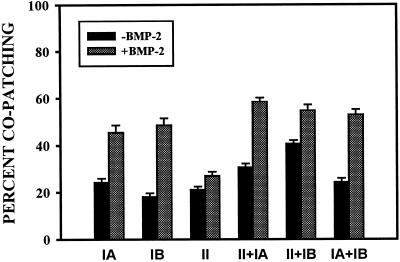
Figure 6 shows results of copatching experiments aimed at analyzing hetero-oligomer formation among BMP receptors. COS7 cells were cotransfected with different pairs of receptors (Figure 6, A and B [BR-II and BR-Ia], C and D [BR-II and BR-Ib], and E and F [BR-Ia and BR-Ib]), each carrying a different epitope tag. The images reveal a significant amount of copatching (yellow patches) in the absence of ligand (Figure 6, A, C, and E). These results are in accord with the coimmunoprecipitation data showing heterocomplexes of the BMP receptors both in COS7 cells and in naturally expressing cells (Figures 1 and 2). Importantly, a substantial increase in the percentage of mutual (yellow) patches was induced by BMP-2 (Figure 6, B, D, and F). The calculated percentages of each receptor type in mutual patches is depicted (see Figure 8). The percentage of a receptor carrying one tag (e.g., the tag labeled with FITC-coupled antibodies) in heterocomplexes is proportional to the number of yellow patches (resulting from overlapped green and red labeling, because of the presence of receptors containing both tags) divided by the sum of yellow and green patches. Similarly, the number of yellow over yellow plus red patches is proportional to the percentage of the red-labeled receptors in heterocomplexes. As can be seen (see Figure 8), this percentage for the pair BR-II and BR-Ia was ∼30%, whereas the equivalent number for BR-II and BR-Ib was 40%. Approximately 25% of coexpressed BR-Ia and BR-Ib appeared in mutual patches. Exposure to ligand increased the percentage of copatching in all cases to 50–60% (see Figure 8).
Figure 8.
Quantification of copatching among various pairs of BMP receptors. Immunofluorescence-copatching experiments were performed on COS7 cells expressing various combinations of differently tagged BMP receptors, as described in Figures 6 and 7. Superimposed red and green images were analyzed by counting the numbers of green, red, and yellow (resulting from overlapped green and red labeling) patches. On each cell, the patches were counted on a flat region of 20 × 20 μm2. The percentage of copatching (percentage of a given tagged receptor in mutual patches with the other receptor) of the FITC-labeled receptor was calculated as 100 × Y/(Y + G), where Y and G are the numbers of yellow and green patches, respectively. The percentage of copatching of the Cy3-labeled receptor was similarly calculated as 100 × Y/(Y + R), where R is the number of red patches. Because these values were similar for all the receptor pairs, only one value is shown for each pair. The results are the mean ± SE of measurements performed on ≥30 cells in each case.
To investigate the tendency of each BMP receptor type to form homomeric complexes, we conducted analogous studies on COS7 cells expressing two differently tagged versions of the same receptor. Typical results are depicted in Figure 7, A and B (BR-Ia), C and D (BR-Ib), and E and F (BR-II). For all of the BMP receptor types, a significant but relatively low amount appeared in mutual patches in the absence of ligand (Figure 7, A, C, and E). Patch-counting analysis of many such experiments indicated that ∼20–25% of each receptor type reside in mutual patches (Figure 8). Ligand binding mediated a significant increase in the formation of mutual patches for either BR-Ia or BR-Ib (Figure 7, B and D), whose percentage in yellow patches increased to 45–50% (Figure 8). It should be noted that for homo-oligomers, the percentage of copatching underestimates the actual percentage of receptors in homomeric complexes, because oligomers containing identically tagged receptors may also form but would not be swept into mutual patches (Henis et al., 1994; Gilboa et al., 1998). This underestimate is the highest in the case of dimer formation (approximately one-third of the percentage of copatching); thus, if all the receptors (100%) are in homodimers, the maximal percentage of copatching that will be obtained is 66.6%.
Notably, BR-II, which binds ligand very poorly on its own, was shown clearly in these experiments to form homomeric complexes at the surface of live cells (Figure 7E). Incubation with BMP-2 failed to increase significantly the percentage of BR-II in yellow patches (Figures 7F and 8), in accord with its ineffective binding to this receptor type.
Signaling via BMP Receptor Complexes
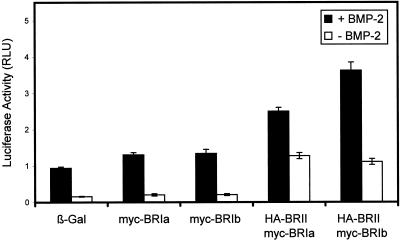
To investigate signaling via BMP receptor complexes, we studied the transcriptional activation of a luciferase reporter construct containing a BMP-2–responsive promoter (pSBE–luc), which is a measure for BMP signaling via the Smad pathway (Jonk et al., 1998). These studies used the murine mesenchymal C2C12 cells, which express endogenously all three BMP receptor types at low levels and respond to BMP-2 by differentiating into osteoblasts (Katagiri et al., 1994). Figure 9 depicts the results of such experiments conducted with various combinations of BMP receptor constructs. Comparison of the basal levels (in the absence of BMP-2; Figure 9, open bars) of pSBE–luc transcriptional activation reveals that single transfection with one receptor type had only a marginal effect on the basal activity, whereas cotransfection of BR-II with a BR-I subtype significantly elevated the luciferase activity. As shown in Figure 9, transfection with BR-Ia or BR-Ib alone increased the basal luciferase activity very slightly, suggesting that the homomeric complexes that can form under the overexpression conditions are not active in this assay. Similar results (our unpublished results) were obtained for BR-II. On the other hand, cotransfection of BR-II with BR-Ia or BR-Ib raised the basal transcriptional activation level by a factor of 5–6 (Figure 9, compare open bars), demonstrating that the preformed type II/type I receptor complexes do have signaling capability. The ligand-mediated induction of the reporter gene activity was similar (6- to 7-fold) in the mock-infected cells (expressing only endogenous receptors) and in cells transfected with a single BMP receptor type and was ∼2-fold weaker in cells cotransfected with type II and type I receptors. This is most likely caused by the higher basal activity in the cotransfected cells (as a result of higher heterocomplex formation before ligand binding), which would reduce the scale for ligand-mediated association into heterocomplexes.
Figure 9.
Signaling of BMP receptors via the Smad pathway measured by transcriptional activation of a luciferase reporter construct. C2C12 cells were transfected with the reporter construct pSBE–luc together with the reference construct pRL–Tk (Promega) and the indicated BMP receptor constructs. Cells transfected with a β-Gal construct served as a control representing cells expressing only the endogenous BMP receptors. Cells were incubated with (filled bars) or without (open bars) 10 nM BMP-2 for 24 h. Luciferase activity was measured as described in MATERIALS AND METHODS. Data were normalized to pRL–Tk luminescence activity to control for transfection efficiency and represent the mean ± SE of three independent experiments.
DISCUSSION
BMPs are a family of related molecules within the TGF-β superfamily. They regulate a broad spectrum of processes ranging from cell proliferation, lineage determination, differentiation, to cell death (Hogan, 1996). As in the related TGF-β receptor system, BMP signaling requires both type I and type II BMP receptors (Kawabata et al., 1995; Liu et al., 1995; Nohno et al., 1995; Rosenzweig et al., 1995; Hoodless et al., 1996), suggesting the relevance of heteromeric and homomeric interactions among the BMP receptors for their functional responses. TGF-β signaling depends on homocomplex and heterocomplex formation between the TGF-β receptors, and these interactions have been studied extensively (Wrana et al., 1992; Moustakas et al., 1993; Chen and Derynck, 1994; Henis et al., 1994; Gilboa et al., 1998; Huse et al., 1999; Wells et al., 1999). However, the oligomeric state of the BMP receptors was not thoroughly studied and was inferred to be similar to that of the TGF-β receptors. This may not be the case, as indicated by several observations. First, there are two BMP type I receptors versus one type I receptor for TGF-β, enabling a larger repertoire of interactions among BMP receptors. Second, the two systems differ in the basic characteristics of ligand binding: TβR-II binds ligand on its own, whereas BR-II does not, and the type I BMP receptors (but not TβR-I) bind ligand in the absence of the type II receptor (Wrana et al., 1994; Liu et al., 1995; Rosenzweig et al., 1995; Massague, 1998). These differences emphasize the need for direct studies on the oligomeric state of the BMP receptors. In the current work, we used several independent methods to investigate this issue. Our findings demonstrate that the oligomerization pattern of the BMP receptors differs from that of the TGF-β receptors, especially in homomeric complex formation, and is more flexible and susceptible to modulation by ligand.
The current studies demonstrate for the first time the formation of homomeric BMP receptor oligomers. These complexes were detected by coimmunoprecipitation studies and, on the cell surface, by both ligand–cross-linking and immunofluorescence-copatching experiments (Figures 4, 5, and 7, respectively). The latter studies enable us to evaluate the extent of receptor oligomerization. The percentage of copatching of two differently tagged forms of each receptor (BR-II, BR-Ia, and BR-Ib) was ∼20–25% in the absence of ligand, increasing to 45–50% for BR-Ia and BR-Ib (but not for BR-II) upon binding of BMP-2 (Figures 7 and 8). The simplest interpretation is that only a minor fraction of each BMP receptor type resides in homo-oligomers before ligand binding and that the ligand shifts the equilibrium strongly toward the homodimeric form. As discussed in RESULTS, the percentage of copatching underestimates the percentage of a given receptor that is in homodimers by one-third. Thus, assuming that the homomeric complexes detected are dimeric, these results suggest that 30% of each BMP receptor is in homodimers, increasing to ∼75% in the presence of ligand for the two type I receptors. This is in contrast to the type I and type II TGF-β receptors, which are essentially all in homodimers before ligand binding and whose dimerization is therefore ligand independent (Henis et al., 1994; Gilboa et al., 1998). The validity of these observations is reinforced by the lack of effect of BMP-2 on BR-II homo-oligomerization (Figures 7 and 8), in accord with the inefficient binding of the ligand to this receptor when singly expressed (Figure 5B).
The homo-oligomerization of the BMP receptors and its dependence on ligand in the case of the type I receptors may have functional relevance, because homo-oligomerization of both type I and type II TGF-β receptors was found to play important roles in TGF-β signal transduction. Thus, homodimerization of TβR-II was shown to be involved via intermolecular autophosphorylation in both positive and negative regulation of TGF-β signaling (Luo and Lodish, 1997), and homodimerization of TβR-I appears to be important for functional interactions between TβR-I subunits in the ligand-induced heterocomplex (Luo and Lodish, 1996; Weis-Garcia and Massague, 1996). The fact that BMP-2 can dramatically increase the homo-oligomerization of BR-Ia and BR-Ib raises the possibility that the homomeric interactions within these complexes may be distinct and serve to regulate functional responses. Although we did not detect signaling of type I BMP receptors when they were transfected into C2C12 cells without BR-II (Figure 9), it should be noted that the luciferase reporter construct used to measure transcriptional activation reflects activity via the Smad pathway, and it is still possible that type I BMP receptor complexes signal via another pathway. Furthermore, even in the absence of such signaling, formation of homomeric or of BR-Ia/BR-Ib complexes may modulate the pool of type I BMP receptors available for heterocomplex formation with BR-II and regulate signaling in this manner. The ability of the ligand to modulate the homomeric interactions among the BMP type I receptors (which may also vary between different ligands) allows an additional level of regulation, which is absent in the closely related TGF-β receptor system. This additional variability, manifested by multiple ligands, two type I receptors, and ligand-induced homo-oligomerization, might underlie at least part of the multiple biological activities of the BMP receptors.
Hetero-oligomeric complexes between BR-II and BR-Ia or BR-Ib were clearly detected by the various methods (coimmunoprecipitation, ligand cross-linking, and copatching) used in the current studies (Figures 1–3 and 6). Quantitation of the copatching studies performed on live cells (Figure 8) indicated that 30 and 40% of BR-Ia and BR-Ib, respectively, resided in complexes with BR-II in the absence of ligand, increasing to 50–60% upon ligand binding. These values of ligand-independent complexes are significantly higher than those observed for type I/type II heterocomplex formation among TGF-β receptors (Wells et al., 1999). These findings demonstrate that type I/type II BMP receptors have an intrinsic affinity for each other that is markedly elevated after the binding of BMP-2. It should be noted that although the outcome of ligand binding increases heterocomplex formation both in the BMP and in the TGF-β receptor systems, the ligand-binding patterns are opposite: BMP-2 binds to BR-II very weakly unless it is coexpressed with a type I BMP receptor, whereas TGF-β1 requires TβR-II to bind to TβR-I. Thus, it is plausible that BMP-2 binds first to its type I receptors, recruiting BR-II into the signaling complex. Alternatively, a higher affinity of BMP-2 to preformed BMP receptor heterocomplexes, as proposed for TGF-β2 binding to TβR-II/TβR-I (Rodriguez et al., 1995), could shift the equilibrium toward them and facilitate their formation.
It is important to note that BR-II/BR-Ia and BR-II/BR-Ib heterocomplexes were detected not only in transiently expressing COS7 cells but also in the naturally expressing cell line C3H10T1/2, which is responsive to BMP (Ahrens et al., 1993; Wang et al., 1993; Asahina et al., 1996). The heterocomplexes were detected both in the absence of ligand by coimmunoprecipitation (Figure 2A) and at the cell surface after ligand cross-linking (Figure 2B). These findings demonstrate that the oligomerization measured in COS cells is not attributable to overexpression of the transfected receptors, which is required for fluorescence imaging in the copatching experiments. This idea is further supported by the similar copatching results obtained on cells expressing as low as 15,000–20,000 receptors at the surface (evaluated by quantitative measurement of the cell-surface fluorescence intensity, using the protocol described by us previously [Henis et al., 1994], which although higher is still of the same order of magnitude as the level on naturally expressing cells).
The significant subpopulation (∼30%) of type I and type II BMP receptors that reside in heterocomplexes before ligand binding raises questions as to how spurious, ligand-independent signaling by such complexes is attenuated. A simple possibility is that heterocomplex formation per se is not sufficient for activation and that a ligand-mediated conformational change altering the relative orientation of the subunits within the complex is needed for activation. Such a mechanism was proposed for the activation of preformed high-affinity EGF receptor dimers by EGF (Gadella and Jovin, 1995) and more recently for the erythropoietin receptor, whose extracellular domain was shown to be dimeric in its unliganded form (Livnah et al., 1999) and to undergo a ligand-induced conformational change for its activation (Remy et al., 1999). Retention of a preformed heteromeric complex in an inactive conformation may also be aided by the binding of inhibitory proteins that are released after ligand binding, as proposed for the binding of the immunophilin FKBP12 to the type I TGF-β receptor (Chen et al., 1997; Huse et al., 1999). Because FKBP12 was also shown to interact with BR-Ia (Wang et al., 1996), it may play a similar role in the BMP receptor system. Other proteins that interact with type I BMP receptors, such as BRAM1 (Kurozumi et al., 1998) or XIAP (Yamaguchi et al., 1999), are also potential candidates that may be involved in suppression of ligand-independent signaling. However, the significant enhancement in the basal transcriptional activation of the reporter gene construct in cells cotransfected with BR-II together with BR-Ia or BR-Ib in the absence of ligand (Figure 9) clearly suggests that preformed BMP receptor heterocomplexes are endowed with some signaling capability.
It is notable that BMP-2 augmented heterocomplex formation not only between type II and type I BMP receptors but also between BR-Ia and BR-Ib (from 25 to 50%; Figures 6 and 8). This raises the intriguing possibility that BR-Ia/BR-Ib heterocomplexes may be functionally distinct from the homomeric BR-Ia and BR-Ib complexes, either by themselves or (more likely) when they further complex with BR-II. This is in-line with the distinct expression patterns of BR-Ib and BR-Ia during differentiation and maturation of skeletal tissues (Dewulf et al., 1995; Rosen et al., 1996; Zou and Niswander, 1996; Zou et al., 1997) and with recent reports on synergistic signaling by two BMP type I receptors in Drosophila dorsal–ventral patterning (Neul and Ferguson, 1998; Nguyen et al., 1998).
In conclusion, we have shown that oligomerization of the BMP receptors at the cell surface follows a different mode than that of the TGF-β receptors. The multiplicity of ligand-independent heterocomplexes and the induction of homo-oligomers of the type I receptors by BMP-2 are two major differences between the two related systems. Both systems use multiple ligands and downstream-signaling molecules to exert their various effects and display a measurable level of preformed complexes that is significantly enhanced by ligand binding. However, the existence of two type I BMP receptors that can interact with the type II receptor and among themselves along with the ability of the ligand to augment their homodimerization grants the BMP system a degree of flexibility that does not exist for the TGF-β receptors. Further studies are needed to elucidate the role of the various complexes in conveying the multiple effects of the BMP ligands.
ACKNOWLEDGMENTS
We thank Dr. Peter ten Dijke (Ludwig Institute for Cancer Research, Uppsala, Sweden) for the BR-Ia, BR-Ib, and pSBE–luc constructs and Dr. M. Kawabata (Cancer Institute, Tokyo, Japan) for the BR-II construct. We gratefully acknowledge helpful discussions with Dr. Ralph Schreck and Florian Neubauer and thank Wolfgang Hädelt for sequencing. This research was supported in part by a project grant from the Israel Cancer Research Fund (to Y.I.H.). L.G. is a recipient of a fellowship from the Clore Scholars Program.
REFERENCES
- Ahrens M, Ankenbauer T, Schroder D, Hollnagel A, Mayer H, Gross G. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12:871–880. doi: 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- Anders RA, Leof EB. Chimeric granulocyte/macrophage colony-stimulating factor/transforming growth factor-beta (TGF-beta) receptors define a model system for investigating the role of homomeric and heteromeric receptors in TGF-beta signaling. J Biol Chem. 1996;271:21758–21766. doi: 10.1074/jbc.271.36.21758. [DOI] [PubMed] [Google Scholar]
- Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- Cheifetz S, Bassols A, Stanley K, Ohta M, Greenberger J, Massague J. Heterodimeric transforming growth factor beta. Biological properties and interaction with three types of cell surface receptors. J Biol Chem. 1988;263:10783–10789. [PubMed] [Google Scholar]
- Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 1998;142:295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R-H, Derynck R. Homomeric interactions between type II transforming growth factor-b receptors. J Biol Chem. 1994;269:22868–22874. [PubMed] [Google Scholar]
- Chen YG, Liu F, Massague J. Mechanism of TGFb receptor inhibition by FKBP12. EMBO J. 1997;16:3866–3876. doi: 10.1093/emboj/16.13.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploin sufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997;188:235–247. doi: 10.1006/dbio.1997.8664. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadella TW, Jr, Jovin TM. Oligomerization of epidermal growth factor receptors on A431 cells studied by time-resolved fluorescence imaging microscopy. A stereochemical model for tyrosine kinase receptor activation. J Cell Biol. 1995;129:1543–1558. doi: 10.1083/jcb.129.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L, Wells RG, Lodish HF, Henis YI. Oligomeric structure of type I and type II transforming growth factor beta receptors: homodimers form in the ER and persist at the plasma membrane. J Cell Biol. 1998;140:767–777. doi: 10.1083/jcb.140.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1988. [Google Scholar]
- Henis YI, Moustakas A, Lin HY, Lodish HF. The types II and III transforming growth factor-beta receptors form homo-oligomers. J Cell Biol. 1994;126:139–154. doi: 10.1083/jcb.126.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Haerry T, Abdollah S, Stapleton M, O‘Connor MB, Attisano L, Wrana JL. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- Huse M, Chen Y-G, Massague J, Kuriyan J. Crystal structure of the cytoplasmic domain of the type I TGFb receptor in complex with FKBP12. Cell. 1999;96:425–436. doi: 10.1016/s0092-8674(00)80555-3. [DOI] [PubMed] [Google Scholar]
- Jonk LJ, Itoh S, Heldin CH, ten Dijke P, Kruijer W. Identification and functional characterization of a Smad binding element (SBE) in the JunB promoter that acts as a transforming growth factor-beta, activin, and bone morphogenetic protein-inducible enhancer. J Biol Chem. 1998;273:21145–21152. doi: 10.1074/jbc.273.33.21145. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata M, Chytil A, Moses HL. Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-beta receptor. J Biol Chem. 1995;270:5625–5630. doi: 10.1074/jbc.270.10.5625. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Liu F, Hata A, Doody J, Massague J. The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997;11:984–995. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nishita M, Yamaguchi K, Fujita T, Ueno N, Shibuya H. BRAM1, a BMP receptor-associated molecule involved in BMP signaling. Genes Cells. 1998;3:257–264. doi: 10.1046/j.1365-2443.1998.00186.x. [DOI] [PubMed] [Google Scholar]
- Lin HY, Moustakas A, Knaus P, Wells RG, Henis YI, Lodish HF. The soluble exoplasmic domain of the type II transforming growth factor (TGF)-beta receptor. A heterogeneously glycosylated protein with high affinity and selectivity for TGF-beta ligands. J Biol Chem. 1995;270:2747–2754. doi: 10.1074/jbc.270.6.2747. [DOI] [PubMed] [Google Scholar]
- Liu F, Ventura F, Doody J, Massague J. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283:987–990. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- Luo K, Lodish HF. Signaling by chimeric erythropoietin-TGF-beta receptors: homodimerization of the cytoplasmic domain of the type I TGF-beta receptor and heterodimerization with the type II receptor are both required for intracellular signal transduction. EMBO J. 1996;15:4485–4496. [PMC free article] [PubMed] [Google Scholar]
- Luo K, Lodish HF. Positive and negative regulation of type II TGF-beta receptor signal transduction by autophosphorylation on multiple serine residues. EMBO J. 1997;16:1970–1981. doi: 10.1093/emboj/16.8.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Lin HY, Henis YI, Plamondon J, O‘Connor MM, Lodish HF. The transforming growth factor beta receptors types I, II, and III form hetero-oligomeric complexes in the presence of ligand. J Biol Chem. 1993;268:22215–22218. [PubMed] [Google Scholar]
- Muramatsu M, Yan J, Eto K, Tomoda T, Yamada R, Arai K. A chimeric serine/threonine kinase receptor system reveals the potential of multiple type II receptors to cooperate with transforming growth factor-beta type I receptor. Mol Biol Cell. 1997;8:469–480. doi: 10.1091/mbc.8.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL, Ferguson EL. Spatially restricted activation of the SAX receptor by SCW modulates DPP/TKV signaling in Drosophila dorsal-ventral patterning. Cell. 1998;95:483–494. doi: 10.1016/s0092-8674(00)81616-5. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Park S, Marques G, Arora K. Interpretation of a BMP activity gradient in Drosophila embryos depends on synergistic signaling by two type I receptors, SAX and TKV. Cell. 1998;95:495–506. doi: 10.1016/s0092-8674(00)81617-7. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271:21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- Nohno T, Ishikawa T, Saito T, Hosokawa K, Noji S, Wolsing DH, Rosenbaum JS. Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors. J Biol Chem. 1995;270:22522–22526. doi: 10.1074/jbc.270.38.22522. [DOI] [PubMed] [Google Scholar]
- Okadome T, Yamashita H, Franzen P, Moren A, Heldin CH, Miyazono K. Distinct roles of the intracellular domains of transforming growth factor-beta type I and type II receptors in signal transduction. J Biol Chem. 1994;269:30753–30756. [PubMed] [Google Scholar]
- Reddi H. Bone morphogenetic proteins. Adv Dent Res. 1995;9(Suppl 3):13. doi: 10.1177/0895937495009003S0401. [DOI] [PubMed] [Google Scholar]
- Remy I, Wilson IA, Michnick SW. Erythropoietin receptor activation by a ligand-induced conformation change. Science. 1999;283:990–993. doi: 10.1126/science.283.5404.990. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Chen F, Weinberg RA, Lodish HF. Cooperative binding of transforming growth factor (TGF)-beta 2 to the types I and II TGF-beta receptors. J Biol Chem. 1995;270:15919–15922. doi: 10.1074/jbc.270.27.15919. [DOI] [PubMed] [Google Scholar]
- Rosen V, Thies RS, Lyons K. Signaling pathways in skeletal formation: a role for BMP receptors. Ann NY Acad Sci. 1996;785:59–69. doi: 10.1111/j.1749-6632.1996.tb56244.x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237:295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996;122:3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- Vivien D, Attisano L, Wrana JL, Massague J. Signaling activity of homologous and heterologous transforming growth factor-beta receptor kinase complexes. J Biol Chem. 1995;270:7134–7141. doi: 10.1074/jbc.270.13.7134. [DOI] [PubMed] [Google Scholar]
- Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9:57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- Wang T, Li BY, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. The immunophilin FKBP12 functions as a common inhibitor of the TGF beta family type I receptors. Cell. 1996;86:435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]
- Weis-Garcia F, Massague J. Complementation between kinase-defective and activation-defective TGF-beta receptors reveals a novel form of receptor cooperativity essential for signaling. EMBO J. 1996;15:276–289. [PMC free article] [PubMed] [Google Scholar]
- Wells RG, Gilboa L, Sun Y, Liu X, Henis YI, Lodish HF. Transforming growth factor-β induces formation of a dithiothreitol-resistant type I/type II receptor complex in live cells. J Biol Chem. 1999;274:5716–5722. doi: 10.1074/jbc.274.9.5716. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Niman HL, Houghten RA, Cherenson AR, Connolly ML, Lerner RA. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J. TGF-β signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, Ueno N, Nishida E, Shibuya H, Matsumoto K. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999;18:179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Zou H, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]
- Zou H, Wieser R, Massague J, Niswander L. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997;11:2191–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]